PHYTOCHEMICAL STUDIES AND ANTIOXIDANT ACTIVITY OF A HEMIPARASITIC PLANT (TAPINANTHUS BANGWENSIS ENGL. & KRAUSE, LORANTHACEAE) AND ITS HOST (COMBRETUM MICRANTHUM G. DON, COMBRETACEAE)
HTML Full TextPHYTOCHEMICAL STUDIES AND ANTIOXIDANT ACTIVITY OF A HEMIPARASITIC PLANT (TAPINANTHUS BANGWENSIS ENGL. & KRAUSE, LORANTHACEAE) AND ITS HOST (COMBRETUM MICRANTHUM G. DON, COMBRETACEAE)
Abdou Sarr *, Serigne Ibra Mbacké Dieng, Amadou Ibrahima Mbaye, Yacine Diouf, Mairame Diop, Kady Diatta-Badji, William Diatta and Alioune Dior Fall
Laboratory of Pharmacognosy and Botany, Faculty of Medicine, Pharmacy and Odontology, Cheikh Anta Diop University, Dakar, Senegal.
ABSTRACT: Tapinanthus bangwensis is a hemiparasitic plant of the Senegalese flora found in several host plant species such as Combretum micranthum. These two species are used in traditional medicine against several diseases. The aim of this study was to conduct a comparative study between the parasitic plant (Tapinanthus bangwensis) and its host (Combretum micranthum) with regard to their chemical composition and antioxidant activity. Phytochemical screening of the two plants leafy stems were carried out by coloring and/or precipitation reactions. The total polyphenol contents were evaluated by the Folin-Ciocalteu reagent and the flavonoid content by a method using aluminium chloride (AlCl3) and sodium nitrite (NaNO2). As for the antioxidant activity, it was evaluated by DPPH and FRAP methods. The same chemical groups were identified in both plants. However, T. bangwensis was richer in total polyphenols than C. micranthum with respective contents of 145.85±6 and 120.08±2.62mgEAG/g of dry extract. Thus, T. bangwensis extract showed more antioxidant activity than C. micranthum extract with respective IC50 of 3.49±0.06 µg/ml and 6.32±0.05 µg/ml. The parasitic plant has a better antioxidant activity possibly due to its higher concentration in secondary metabolites, in particular, polyphenols.
Keywords: Host plant, Hemi-parasitic plant, Tapinantus bangwensis, Combretum micranthum, Polyphenols, Antioxydant activity
INTRODUCTION: Very early on, humans used plants to treat the various diseases they faced. These plants represent a huge potential source of bioactive molecules 1. Among these molecules, polyphenols represent a significant proportion. Indeed, more than 8,000 phenolic compounds, including 5,000 in the flavonoid subfamily, have been identified 2.
Parasitic plants possess a specialized structure called a haustorium (haustoria in the plural) that penetrates the stem or root of their host and form a vascular connection with it. Through the haustorium, they extract water and nutrients from their host. Hemiparasites are content to use the water and minerals that their host have drawn from the soil, but will still need to photosynthesize 3.
Tapinanthus bangwensis is a hemiparasitic plant of the Senegalese flora found in several plant species such as Kinkéliba (Combretum micranthum G. Don). It is used in traditional medicine for epilepsy 4 and asthma 5. A decoction of the fruits is administered orally in the treatment of mucosal candidiasis 6. As for Combretum micranthum, well known for its leaf tea named Kinkeliba 7, it is also used for coughs, bronchitis, malaria, and hepatobiliary disorders as an adjunct medication 8.
The objective of this study was to conduct a comparative study between the parasitic plant (Tapinanthus bangwensis) and its host (Combretum micranthum) with regard to their chemical composition and antioxidant activity.
MATERIALS AND METHODS:
Plant Material: The plant material consisted of leafy stems of Combretum micranthum (Combretaceae) and Tapinanthus bangwensis (Loranthaceae). These drugs were harvested from the same plant in Sindia, Mbour Department (Thiès Region, Senegal). Both plants were identified at the Pharmacognosy and Botany Laboratory of the Faculty of Medicine, Pharmacy, and Odontology (FMPO) at Cheikh Anta Diop University of Dakar (UCAD). The leafy stems were dried in a well-ventilated room away from light before being ground into powder using an electric grinder equipped with a medium-sized sieve. The resulting powders were used for extractions.
Extraction: Twenty-five (25) grams of leafy stem powder of Combretum micranthum and Tapinanthus bangwensis were separately boiled under reflux in 500 ml of ethanol/water mixture (80/20; v/v) for 30 minutes. To stabilize the boiling, pumice stone was added.
After filtration, the hydro-ethanolic solution thus obtained was evaporated using a rotary evaporator yielding a concentrate that was subsequently dried in a desiccator. This results in a hydro-ethanolic dry extract (HEE) for each plant. This extract was used for characterization tests, determination of total polyphenols and flavonoids and for the evaluation of antioxidant activity.
Phytochemical Screening: The main phytochemical families were searched for using colorimetric and precipitation reactions, referring to the tests described by Bassène 9, Bekro et al. 10 and Longanga et al. 11. The aim was to highlight the presence of some chemical compounds in the hydro-ethanolic extract of the leafy stem hydro-ethanolic extract of C. micranthum and T. bangwensis.
Total Polyphenol Contents: The total polyphenol (TP) contents of the HEE of both plants were evaluated by the colorimetric method using the Folin-Ciocalteu reagent according to the protocol of Magalhaes et al. 12. Thus, the samples were treated in triplicate (n=3) by mixing in each test tube, 200 µl of sample at 200 mg/l, 100 µg of Folin-Ciocalteu reagent and 1700 µl of Na2CO3 at 2.36%. The tubes were then vortexed for 10 seconds and incubated at 450 C for 45 min. Absorbances were measured at 760 nm against a methanol blank using an Évolutive 300 UV-Visible spectrophotometer. A calibration range made with gallic acid at different concentrations (0-2 4-6-8-10-12-14 µg/ml) was treated in the same way as the samples in order to obtain a calibration line. The results are expressed in mg gallic acid equivalent per gram of dry extract (mg GAE/g) and presented as the mean plus or minus the standard deviation from the mean (SEM).
Flavonoïds Contents: The method described by Zhishen et al., 13 was used with some modifications. For this, 400 µl of sample (or standard or distilled water for the control) was placed in a glass haemolysis tube with 120 μl of 5% NaNO2. After 5 minutes of incubation, 120 μl of 10% AlCl3 was added and mixed in the vortex. Then 800 μl of 1 M NaOH was added 6 minutes later. After homogenisation, the absorbance was read immediately at 510 nm against the control. The test was repeated 3 times for each sample (n = 3). A calibration range performed with rutin at different concentrations (0; 5,54; 11,08; 16,62; 22,16; 22,7; 33.24; 38,78; 44,32 µg/ml), was treated in the same way as the samples. The results were expressed in milligram rutin equivalent per gram of dry extract (mg RE/g).
Antioxydant Activity:
Radical Scavenging Assay: The determination of the DPPH free radical scavenging activity of samples was done using the described method 14. An ethanol solution of DPP. Was prepared by dissolving 4 mg of DPPH. In 100 ml of ethanol, followed by a cool incubation between 4-8° for at least 16 hours. An aliquot of each sample (0.8 ml) at appropriate concentration was added to 3.2 ml of ethanol solution of DPPH. The extracts and ascorbic acid were tested at different concentrations.
The absorbance of each sample was measured at 517 nm after 30 min. Each experiment was done in triplicate. The antioxidant activity related to the DPPH free radical scavenging effect was expressed as IC50 (concentration of sample required to scavenge 50% of free radicals).
FRAP Assay: The ferric reducing power was determined according to the described method 9. An aliquot of 0.20 ml of each sample at appropriate concentration was mixed with 0.5 ml of phosphate buffered saline (0.2 M; pH 6.6) and 0.5 ml of 1% potassium ferricyanide (K3Fe(CN)6). The mixture was incubated at 50 °C for 30 min and 0.5 ml of 10% trichloroacetic acid was added. After centrifugation for 10 minutes at 3000 rpm, the supernatant (0.5 ml) was mixed with distilled water (0.5 ml) and 0.1% ferric chloride (0.1 ml). The experiments were done in triplicate. Absorbance was measured at 700 nm. Ascorbic acid was used as positive control. Absorbance increasing relatively to that of concentration represented the reducing capacity of tested sample.
Statistical Analyses: Data were expressed as mean ± SD. Statistical analysis were done by Stat view 4.5 software 0,05.< using the Fischer test. The difference was considered as significant when p. < 0,05.
RESULTS:
Extraction Yields: Extraction of 25 g of leafy stem powder from the two plants under study yielded 3.95 g and 6.39 g for C. micranthum and T. bangwensis, respectively. The extraction yields were 15.18% for the host plant (C. micranthum) and 25.26% for the parasitic plant (T. bangwensis).
Phytochemical Screening: Phytochemical characterisation tests, carried out on the hydro-ethanolic extracts of the plant's leafy stem, gave the results mentioned in Table 1.
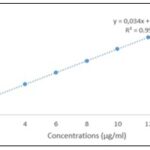
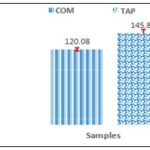
Total Polyphenol Contents: The total polyphenol contents were calculated using the equation y=0,034x+0,0367; R2=0,9994. They are deduced from the equation of the straight line illustrated in Fig. 1. The hydro-ethanolic extract of the leafy stems of the parasitic plant had a higher total polyphenol contents Fig. 2 than that of the host plant with respective values of 145.85±6 and 120.08 ± 2.62 mg EAG /g of dry extract (significant difference, p < 0.0025).
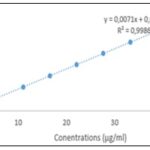
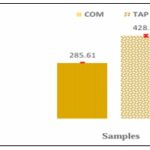
Flavonoids Contents: The calibration line of equation y=0.0071x+0.0071, R²=0.9986, obtained with rutin is illustrated in Fig. 3. The flavonoid contents of the two samples, deduced from this equation, are represented in Fig. 4. The hydro-ethanolic extract of the leafy stems of T. bangwensis had a higher flavonoid content than that of C. micranthum with respective values of 428.10 ± 5.50 and 285.61 ± 2.93 mg ER/g of dry extract (significant difference, p<0.0001).
Antioxidant Activity:
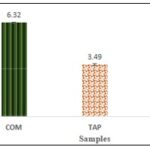
DPPH Assay: The leafy stem hydro-ethanolic extract of C. micranthum and T. bangwensis significantly inhibit the DPPH radical (p<0.05 versus negative control) at all concentrations tested and, in a concentration dependent manner. The concentrations of sample required to scavenge 50% of free radicals (IC50) was 6,32±0,05 and 3,49 ± 0,06 μg/ml respectively for C. micranthum and T. bangwensis. The ascorbic acid solution used as reference, had an IC50 value of 1,13 ± 0.01 μg/ml Fig. 5.
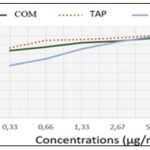
FRAP Assay: The hydro-ethanolic extract reducing activities of the leafy stem of C. micranthum and T. bangwensis are illustrated in Fig. 6. The results of the FRAP test confirmed those of the DPPH test. Indeed, T. bangwensis had a greater reducing power than C. micranthum at all tested concentrations. reducing power exceeded 50% for all samples at any concentration.
TABLE 1: CHEMICAL GROUPS IDENTIFED IN THE HYDRO-ETHANOLIC EXTRACTS OF C. MICRANTHUM AND T. BANGWENSIS LEAFY STEM
| Chemical groups | Samples | ||
| Combretum micranthum | Tapinanthus bangwensis | ||
| Alcaloids | + | + | |
| Saponines | + | + | |
| Phenol compounds | + | + | |
| Condensed | + | + | |
| Tannins | Hydrolyzables | + | + |
| Flavonoids | + | + | |
| Anthracene glycosides | _ | _ | |
| Cardiotonic glycosides | _ | _ | |
| Sterols and triterpenes | + | + | |
FIG. 1: CALIBRATION LINE OBTAINED WITH GALLIC ACID
FIG. 2: TOTAL POLYPHENOL CONTENTS OF SAMPLES. COM: Combretum micranthum Extract; Tap: Tapinanthus bangwensis extract.
FIG. 3: CALIBRATION LINE OBTAINED WITH RUTIN
FIG. 4: FLAVONOIDS CONTENTS. COM: Combretum micranthum extract; TAP: Tapinanthus bangwensis extract.
FIG. 5: IC50 OF SAMPLES ON DPPH TEST. COM: Combretum micranthum extract; TAP: Tapinanthus bangwensis extract; AA: Ascorbic acide solution.
FIG. 6: REDUCING PERCENTAGE OF DIFFERENT SAMPLES IN FRAP TEST. COM: Combretum micranthum extract; TAP: Tapinanthus bangwensis extract; AA: Ascorbic acide solution.
DISCUSSION: The aim of this study was to conduct a comparative phytochemical study between a hemiparasitic plant (T. bangwensis) and its host (C. micranthum). The drugs studied consisted of the leafy stems of both plants. The extraction of the leafy stem powder from both plants was carried out with an ethanol/water mixture (80/20; v/v). The choice of this solvent system is based on the fact that ethanol, being polar, has the capacity to extract hydrophilic compounds such as polyphenols but also certain non-heterosidic compounds such as alkaloids and certain lipophilic constituents 15. Water, on the other hand, is a good extraction solvent for polar phytochemical compounds such as flavonoids and tannins, which are polyphenolic compounds. The combination of these two solvents then allows for a broader extraction spectrum. Indeed, it has been noted that polymers insoluble in ethanol and water could be dissolved in an ethanol/water mixture 16.
The results obtained show that the parasitic plant (T. bangwensis) had a higher extraction yield than the host plant (C. micranthum) with respective values of 25.56 and 15.80%. This may be attributed to increased metabolic activity, or an adaptive strategy aimed at producing more secondary metabolites to ensure its survival as a hemiparasitic organism 17.
Phytochemical studies carried out on the samples revealed the presence of several chemical families. Except for saponins, which were detected only in the host plant”, the same chemical groups were identified in both plants. It was about flavonoids, tannins, alcaloids, sterols and triterpenes. The difference may lie in the active ingredient levels. Thus, total polyphenols and flavonoids were quantified for extracts from the host plant and its parasite. It was noted that the HEE of the leafy stems of T. bangwensis was richer in total polyphenols than the EHE of the leafy stems of C. micranthum) with respective contents of 145.85 ± 6 and 120.08 ± 2.62 mg EAG/g of dry extract. The quantification of flavonoids, a subfamily of polyphenols, corroborates the results of the dosage of total polyphenols with contents of 428.10±5.5 mg ER/g and 285.61±2.93 mg ER/g for T. bangwensis and C. micranthum, respectively. Regarding the evaluation of the antioxidant activity of the hydro-ethanolic extracts of the two plants studied, two methods were used: DPPH and FRAP. The DPPH method is based on the ability of an antiradical to stabilize the purple DPPH free radical by transforming it into pale yellow DPPH, H+, by trapping a proton 18. As for the FRAP method, it is based on the ability of a compound to reduce the ferric ion (Fe3+) to the ferrous one (Fe2+). The reducing capacity of ferric ion seems to be related to the degree of hydroxylation and extend to the conjugation in phenolic compounds 19. The results obtained showed that T. bangwensis significantly inhibited the DPPH free radical more than C. micranthum, with IC50 of 3.49±0.06 and 6.32±0.05 µg/ml, respectively. The FRAP test confirm results obtained with the DPPH method. Thus, at all concentrations tested, the hemiparasitic plant exhibited better reducing powers than the host plant.
These results corroborate those of Sarr et al. 20. Indeed, the leafy stems of T. bangwensis harvested from C. micranthum, had shown a greater richness in secondary metabolites, in particular polyphenols, resulting in a better antioxidant activity of the parasitic plant compared to the host plant. It was also noted that the secondary metabolite content of T. bangwensis varies depending on the parasitized species. Thus, for total polyphenols, the hydro-ethanolic extract of the leafy stems of T. bangwensis harvested from Guiera senegalensis with a content of 184.27 ± 3.21 mg GAE/g 20, was richer than the sample from C. micranthum (145.85 ± 6 mg GAE/g) with a significant difference (p < 0,05). This confirms the hypothesis of the adaptive strategy of hemiparasitic plants by synthesizing more secondary metabolites 17.
Antioxidant activity (anti-radical and reducing power) appears to be linked to the content of phenolic compounds, the levels of which are higher in the HEE of T. bangwensis. Flavonoids, which are 1.5 times higher in the HEE of the leafy stems of T. bangwensis than in the HEE of those of C. micranthum, could have a large part of this antioxidant activity. Oxidative stress is widely recognized as a key factor in the onset of several pathologies such as cardiovascular diseases 21. The use of plant-based antioxidants could therefore prevent the onset of these diseases. In view of these results, parasitic plants could be used in herbal medicine to reduce the pressure on the resource made up of host plants, some of which are classified as endangered plants. However, toxicity studies are needed to verify the safety of the parasitic plant.
CONCLUSION: These studies showed that the hydro-ethanolic extract of C. micranthum (host plant) and T. bangwensis (parasitic plant) contain similar chemical constituents. However, when it comes to polyphenols, the parasitic plant is richer in them, resulting in greater antioxidant activity for T. bangwensis.
ACKNOWLEDGEMENTS: We wish to acknowledge the contribution of all members of this article.
CONFLICTS OF INTEREST: Authors have declared that no competing interests exist.
REFERENCES:
- Bourmita Y, Belboukhari N, Cheriti A and Ould El Hadj MD: Recherche préliminaire des sources végétales sahariennes à alcaloïdes pour usage bio-insecticides. Algerian Journal of Arid Environment 2013; 3(1): 98-102
- Tabet A: Etude phytochimique et substances bioactives de la plante médicinale dans la région d’El Oued. Thèse Doct. en chimie organique Annaba 2019; 122.
- Brisson J: Les plantes parasites, ces merveilleuses méconnues. Quatre-temps2021; 45(1): 23-27
- Elusiyan CA, Faria ALG, Mendes AEQ, Silva IO, Martins JLR, Rosa DA, Pedrino GR, Costa EA, Ibrahim MA, Zjawiony JK and Fajemiroye JO: Involvement of the Benzodiazepine Site in the Anticonvulsant Activity of Tapinanthus globiferus against Pentylenetetrazole-induced Seizures in Mice. Planta Med 2020; 86(16): 1204 1215. doi: 10.1055/a-1209-1254.
- Ould Ismaïl BA: Contribution a l'étude taxonomique et botanique de quelques phanérogames parasites de Mauritanie: Tapinanthus Blume (Loranthacées), Cynamorium Micheli (Cynamoriacees), Striga lour. (Scrofulariacées), et Cistanche phalypaea hoff. Et Link: (Orobanchacées). Thèse de Doctorat de 3eme Cycle de Biologie végétale, Dakar 2000; 131.
- Le Grand A: Anti-infectious phytotherapies from the savannah forest, Senegal (West Africa) III: A summary of phytochemicals and antimicrobial activity of 43 species. Journal of Ethnopharmacology 1989; 25(3): 315-338.
- Baumer M: Arbres, arbustes et arbrisseaux nourricières en Afrique Occidentale. Enda Edition Dakar 1995; 260
- Fortin D, Lo M and Maynart G: Plantes médicinales du Sahel. Enda-edition, Dakar 2000; 280.
- Bassène E: Initiation à la recherche sur les substances naturelles: Extraction – analyses – essais biologiques. Presses Universitaires De Dakar 2012; 150.
- Békro YA, Mamyrbekova JA, Boua BB, TRA BI FH and Ehilé EE: Etude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend. et Zarucchi (Caesalpiniaceae) Rev Sci Nat 2007; 4(2): 217-225. DOI: 10.4314/scinat.v4i2.42146
- Longaga A, Otshudi Vercruysse A and Foriers A: Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plants in the treatment of dysentery and diarrhoea in Lomola area, Democratic Republic of Congo (RDC). J. Ethnopharmacol 2000; 71: 411 423. DOI: 10.1016/s0378-8741(00)00167-7
- Magalhaes L, Segundo M, Reis S, Lima JLFC and Rangel AOSS: Automatic Method for the Determination of Folin−Ciocalteu Reducing Capacity in Food Products. J of Agricultural and Food Chemistry 2006; 54(15): 5241-6
- Zhishen J, Mengcheng T and Jianming W: The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry 1999; 64: 555-559. DOI: 10.12691/jnh-5-2-4
- Nunes PX, Silva SF, Guedes JR, Tolentino J, Augusto L, Quintans Junior LJ and Almeida S: Biological oxidations and antioxidant activity of natural products. Phytochemicals as nutraceuticals - Global Approaches to Their Role in Nutrition and Health 2012; 278: DOI: 10.5772/26956
- Fall AD, Dieng SIM, Sarr A and Dieng M: Phytochemical screening and antioxidant effect of ethanol leaf and trunk bark extracts of Cordyla pinnata (Lepr. Ex A. Rich.) Milne-Redh. (Caesalpiniaceae) Pharmacognosy Journal 2019; 11(6) 1415 1418. DOI: 10.5530/pj.2019.11.219.
- Hoogenboom R, Hanneke ML, Thijs DW, Hoeppener S and Schubert US: Tuning Solution Polymer Properties by Binary Water – Ethanol Solvent Mixtures. Soft Matter 2008; 4(1): 103-7. https://doi.org/10.1039/B712771E.
- Yoder JI and Scholes JD: Host plant resistance to parasitic weeds; recent progress and bottlenecks. Current Opinion in Plant Biology 2010; 13(4): 478-484
- Kumaran A and Karunakaran RJ: In-vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Leben Wissen Technol 2007; 40: 344-52.
- Yen GC and Chen HY: Antioxidant activity of various tea extracts in relation to their antimutagenicity, Journal of Agricultural and Food Chemistry 1995; 43(1): 27-32.
- Sarr A, Dieng SIM, Dione A, Kebe F. Diatta-Badji K, Diatta W and Fall AD: “Comparative Phytochemical Study and Antioxidant Activity of a Hemiparasitic Plant (Tapinanthus bangwensis Engl. & Krause, Loranthaceae) and Its Host (Guiera senegalensis, Combretaceae)”. European Journal of Medicinal Plants 2025; 36(5): 56–64. https://doi.org/10.9734/ejmp/2025/v36i51294.
- Haleng J, Pincemail J, Defraigne JO, Charlier C and Chapelle JP: Oxidative stress. Revue Medicale de Liege 2007; 62(10): 628–638.
How to cite this article:
Sarr A, Dieng SIM, Mbaye AI, Diouf Y, Diop M, Diatta-Badji K, Diatta W and Fall AD: Phytochemical studies and antioxidant activity of a Hemiparasitic plant (Tapinanthus bangwensis engl. & krause, loranthaceae) and its host (Combretum micranthum g. don, combretaceae). Int J Pharmacognosy 2025; 12(10): 800-06. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.12(10).800-06.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
800-806
613 KB
18
English
IJP
Abdou Sarr *, Serigne Ibra Mbacké Dieng, Amadou Ibrahima Mbaye, Yacine Diouf, Mairame Diop, Kady Diatta-Badji, William Diatta and Alioune Dior Fall
Laboratory of Pharmacognosy and Botany, Faculty of Medicine, Pharmacy and Odontology, Cheikh Anta Diop University, Dakar, Senegal.
abdou.sarr@ucad.edu.sn
01 October 2025
29 October 2025
30 October 2025
10.13040/IJPSR.0975-8232.IJP.12(10).800-06
31 October 2025