VALORIZATION OF EGYPTIAN FOOD BYPRODUCTS IN THE DEVELOPMENT OF BIOLOGICALLY ACTIVE NUTRACEUTICALS
HTML Full TextVALORIZATION OF EGYPTIAN FOOD BYPRODUCTS IN THE DEVELOPMENT OF BIOLOGICALLY ACTIVE NUTRACEUTICALS
Riham Salah El Dine 1, Tzvetomira Tzanova 2, Dalila Bousta 3, Dina R. Abou-Hussein 1, Smahane Boukhira 3, Emilie Evain-Bana 2, Nebal Darwish El-Tanbouly 1, Stéphanie Philippot 2, Latifa El Mansourai3, Denyse Bagrel 2, Reham I. Amer 4, 5 and Essam Abdel-Sattar * 1
Department of Pharmacognosy 1, Faculty of Pharmacy, Cairo University, 11562 Cairo, Egypt.
Université de Lorraine 2, UMR CNRS 7565, Structure et Réactivité des Systèmes Moléculaires Complexes, Equipe 5 (MIC), Campus Bridoux, rue du Général Delestraint, 57070 Metz Cedex, France.
Laboratory of Pharmacology and Toxicology 3, NIMAP-Taounate, USMBA of Fez, Morocco.
Department of Pharmaceutics and Industrial Pharmacy 4, Faculty of Pharmacy, Al-Azher University, Cairo, Egypt.
Faculty of Pharmacy 5, October University of Modern Science and Arts (MSA), Cairo, Egypt.
ABSTRACT: This study was aimed to maximize the benefits of the use of by-products (oil cake) of olive fruits and black seeds after extraction of their fixed oils, rather than their use as animals feed or landfilling or composting. The cake of both seeds was assessed by HPLC for their main bioactive marker compounds (thymoquinone for black seed and oleuropein for olive fruit), to choose the best method for extraction, determination of phenolic contents and in vitro antioxidant activity (DPPH and reducing powers, FRAP assays). Both by-products were also assessed for their cytotoxicity against four human breast cell lines, three of them are cancerous (MCF7, MDA-MB-213, Vcr-R), and a non-cancerous one (epithelial type) but immortalized by telomerase (hTERT-HME), in addition to a human hepatoma cell line (HepG2). Also, both wastes were subjected for in-vitro CDC25s phosphatase inhibition assay on three isoforms (A-C) and for in-vivo immunomodulatory effects. In conclusion, the results of this study showed the interest of cumulating different biological approaches exploring various physiological mechanisms and showed the utility of these extracts in different fields, the first being used as a cytoprotective agent, and the second one being promising as an anticancer agent.
| Keywords: |
Olea europea, Nigella sativa, By-products, CDC25s, Cytotoxicity, Immunomodulatory
INTRODUCTION: Food industry generates large quantities of food by-products, which are disposal causing economic and environmental problems. While most of these byproducts generally have other uses than landfilling or composting.
Therefore, advanced valorization alternatives should be developed to maximize the value derived from such an important waste source, especially in developing countries. Egypt is producing more than 30 million tons of agro-food by-products from the processing of vegetable and fruit, which represent economic and serious environmental problems. Olive cultivation and olive oil production are a part of the local heritage and rural economy throughout the North African and Mediterranean regions (MENA). In 2012, more than three million tons of olive oil was produced worldwide, the largest olive oil producers being in MENA region.
Olive is one of the major oil crops in Egypt, commonly known for its popular culinary and medicinal benefits. The production of olive oil generates two wastes: olive press cake 25% (OPC) and olive-mill wastewater 50% (OMW), in addition to olive oil (25%). The disposal of the two wastes represents a serious environmental problem characterized by large amounts and high polluting load; moreover, compounds with biostatic activity (e.g., polyphenols) are also largely present. In Egypt, there is no clear technologically feasible, economically viable, and socially acceptable solution, except for some individual trials. The problems associated with olive processing wastes are further exemplified by lack of common policy among the olive oil producing regions, funding, and infrastructure for proper treatment and disposal, and a general lack of education on the environmental and health effects caused by olive processing wastes.
Leouifoudi et al., 1 evaluated the bioactivity of the ethanolic extract of olive cake (EEOC) for its cytotoxicity effect against P815 mastocytoma murine cell line using the MTT assay. The results showed that the EEOC exerted an in vitro cytotoxic activity (IC50 20 to 40 µg/mL), no cytotoxic effect was observed on the normal human peripheral blood mononuclear cells. Furthermore, the extract showed an apoptotic effect against P815 tumor cell line. The phenol content and antioxidant activity of the methanol extract of olive extract were determined before and after hydrolysis and their possible correlation 2. Ramos et al., 3 studied the phenolic composition, antioxidant activity and antiproliferative activity against breast cancer of water and methanol/water derived extracts from olive pomace (OP) and dry olive mill residue (DOR), from Portuguese industries. DOR water extract showed the highest extraction yield; as well as the highest total phenolic content and hydroxytyrosol. DOR water extract also presented the most effective DPPH scavenging capacity and antiproliferative activity, comparatively to OP water extract.
Hashish and El-Samee 4 revealed that inclusion of the two levels of olive cake in hen diets resulted in decreases in plasma and egg yolk cholesterol, TG and LDL levels relative to the control. Study on the native olive waste extract and its nano-particles effect on oxidative stress induced by aflatoxin B1 in rat brain revealed restoration of all histochemical parameters towards the control values 5.
On the other hand, the effect of different Nigella sativa L. seed meal (NSM) on leukocytes counts and some serum immunological parameters in calves was studied by 6. The results showed that total leukocytes count, the percentage of neutrophils and lymphocytes in calves fed NSM at 11% level were higher than those fed the control diet, without a significant difference. Different extracts of Nigella sativa L. seed waste (NSW) were evaluated for their hepatoprotective activities 7. This study proved that the protein fraction of the aqueous extract of NSW exhibited a promising hepatoprotective effect in the management of different liver disorders. Reviewing literature revealed that most of the biological activities of the black seeds were due to thymoquinone (TQ) content, the major component of the essential oil, which is also present in the fixed oil 8. TQ is considered as potent antioxidant (Badary et al., 2003), anticarcinogenic and antimutagenic agent (Bourgou et al., 2008; Khader et al., 2010). Moreover, TQ is a relatively safe compound, particularly when given orally to experimental animals 9. Alpha (α)-hederin, a pentacyclic triterpene saponin isolated from the seeds of N. sativa, was also reported to have potent in vivo antitumor activity 10.
In the light of the above information, some active compounds are still present in by-products left over from oil pressing. Thus, defatted seeds (oilseed cakes) seem to be a potential source of natural bioactive substances. Therefore, we have chosen these two Egyptian waste products from the olive and black seed oil industry. We are focusing on the integrated valorization of these two wastes for the production of biologically standardized extracts of high pharmaceutical value. These products could be of promising immunotherapeutic agents in the treatment of cancer.
MATERIALS AND METHODS:
Plant Materials: Nigella sativa (black seeds) wastes (NSW) produced after oil extraction were kindly provided by Dr. Amira Abdel-Motaal (Isis food company, SEKEM, Cairo, Egypt) in July 2013 and kept at -20 °C until use. Olea europaea wastes (olive fruits, OFW) produced after oil extraction were kindly provided by Mr. Hamdy Ahmed (Al-Gefira governorate, Mersa Matruh, Egypt) in October 2012 and kept at - 20 °C until use.
Extraction: Different solvents (methanol, ethanol and 60% and 80% methanol in water) have been used for extracting active constituents from waste byproducts. Samples each of 100 g was extracted, separately 3 times each with 250 mL solvent using ultrasonication for 10 min. The sample extracts after evaporation of solvent were analyzed by HPLC to determine the concentration of thymoquinone in NSW and eleuropein in OFW to choose the best conditions for extraction. All trials were carried out in duplicates.
Extraction of Olive Fruits Waste (OFW): About 100 g of olive fruit waste was extracted with 80 % methanol (250 ml)three times to give 10.8 g of extract-1 (OFWE-1, 10.8 %,).Approximately 3 Kg of olive fruit waste was defatted using petroleum ether (4.5 L) to give 109.0 g of oily material, extract-2 (OFWE-2, 3.6 %). The defatted waste material was extracted with 80 % methanol (5L) three times to give 225.6 g extract-3 (OFWE-3, 7.5 %). Extraction was repeated three times at room temperature and using an ultrasonic bath for 10 min.
Extraction of Black Seeds Waste: About 100 g of black seed waste was extracted with methanol (250 ml) three times to give 11.9 g of extract-1 (NSWE-1, 11.9%). Approximately 2.7 Kg of black seeds waste were defatted using petroleum ether (4.5 L) to give 184.0 g oily material, extract-2 (NSWE-2, 6.8%). The defatted waste material was extracted by maceration with methanol (5 L) three times to give 132.0 g, extract-3 (NSWE-3, 4.9%) 2.3.
Chemical Assessment of Waste Products:
HPLC-UV Analysis: High-performance liquid chromatography (HPLC) was performed on an Agilent Technologies 1100 series, equipped with an Agilent 1200 series G1322A quaternary pump and degasser, and a G1314A variable wavelength detector. RP-18 Zorbax eclipse column (150 × 4.6 mm i.d., 5 μm, Merck, Germany) was used for analysis. For OFWE, the conditions were as follow: a gradient elution of 25 - 100% methanol in 0.1% acetic acid in water in 15 min, wavelength: 280 nm and flow rate 1.0 ml/ min. For NSWE, the conditions were as follow: a gradient elution of 15-100% acetonitrile in 0.1% formic acid in water in 20 min, wavelength: 254 nm and flow rate of 1.0 ml/min.
Sample Preparation: Solution of 15 mg/10 mL of OFWE and 40 mg / 10 mL of NSWE were prepared in methanol.
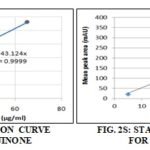
Standard Calibration Curve: It was constructed by HPLC analysis of aliquots of various standard concentrations (1 mg/ml) in the range from 10 - 65 ug/mL. Results are the mean of triplicate determinations Fig. 1S and 2S.
Determination of the Phenolic Content: Spectrophotometric determination of total phenolic of both wastes was carried out using the Folin-Ciocalteu colorimetric method 11.
Preparation of Standard Curve: Total phenolic was expressed as mg of gallic acid equivalent (GAE). The standard stock solution was prepared bydissolving25 mg of gallic acid in 100 ml distilled water at a concentration of 250 µg/ml. The stock solution was diluted serially with distilled water to the required concentrations obtained equivalent to 10 - 100 µg/ml. An aliquot (0.8 ml) of each standard solution or extract (1 mg/mL) was mixed with 0.4 ml of Folin-Ciocalteu reagent and 4 ml distilled water, then diluted to 10 ml with sodium carbonate solution (290 g/L). The absorbance of the resulting blue color was measured after 30 min at 760 nm using UV spectrophotometer. For each concentration, three replicates were carried out, and the average of the obtained absorbance was plotted versus the concentration.
Nutritional Values of Waste Materials Left After Solvent Extraction: The solid materials left after solvent extraction were analyzed for minerals content (calcium, iron, sodium, potassium, magnesium, phosphorus and manganese), total protein, total lipid, total carbohydrates, Vitamins A and C.
In-vitro Evaluation of the Antioxidant Activity: Two complementary methods were chosen for determination of the antioxidant activity: FRAP (Ferric reducing Antioxidant Power) which measures the reducing power of the tested samples and DPPH (2, 2-diphenyl-1-picrylhydrazyl) that evaluates their free radical scavenging activity. OFWE-1-3 samples were suspended in DMSO (20 mg/mL). NSWE-1-3 samples showed some solid parts, therefore some NSWE samples were treated as liquid and solid samples and suspended in the concentration of 20 mg/mL in DMSO, to give NSWE-1L, NSWE-1S, NSWE-2, NSWE-3L, and NSWE-3S.
Free Radical Scavenging Activity Measurement (DPPH Assay): The free radical-scavenging activity of the extracts was measured using assessing their DPPH (2, 2-diphenyl-1-picrylhydrazyl) spectrophotometric assay following the method reported by 12. This change in absorbance is proportional to the antioxidant capacity of the test sample and is measured by spectrophotometry. Trolox, a cell-permeable, water-soluble derivative of Vitamin E with potent antioxidant properties, was used as standard. The degree of discoloration induced by a tested sample is related to that induced by Trolox, allowing expressing its antioxidant activity as Trolox equivalents.
The stock solution of DPPH is prepared at the concentration of 2 mM and the Trolox solution at the concentration of 2.5 mM. Solution of 10 µL of the test sample (20 mg/mL) is mixed with 930 µL of methanol and 60 µL of DPPH solution. The final volume is 1 mL. The optical density is recorded after incubation in the dark for 30 minutes. Each sample was tested two times with three measures in each experiment.
Reducing Power Measurement (FRAP Assay): The antioxidant activity of the extracts was measured by assessing their Ferric Reducing Antioxidant Power (FRAP). The FRAP assay 13 depends upon the reduction of a ferric tripyridyltriazine (Fe3+–TPTZ) complex to the ferrous tripyridyltriazine (Fe2+–TPTZ) by a reductant at low pH. Fe2+–TPTZ has an intensive blue color and was monitored at 593 nm. Trolox was used as standard antioxidant. FRAP reagent was prepared by mixing 25 ml acetate buffer, 2.5 ml TPTZ solution, and 2.5 ml FeCl3. 6H2O solution. Freshly prepared FRAP reagent was warmed at 37 °C for 30 min and added to the samples every 30 s. Absorbance readings were taken after 30 min every 30 s. Each sample was tested two times with three measures in each experiment.
Biological Protocols:
Antiproliferative Activity: The MTT (3- (4, 5-di methylthiazol-2-yl)- 2, 5-diphenyl tetrazolium bromide) assay was performed on three breast cancer cell lines, a hepatoma cell line and an immortalized mammary cell line noncancerous, all of them being from human origin. Also, in vitro evaluation of CDC25 phosphatases inhibitory potential, enzymes play a primordial role in cell cycle regulation.
Cell lines and Cell Culture: The human breast adenocarcinoma cell line MCF7 (ECACC, Salisbury, UK) 14, its multidrug-resistant counterpart Vcr-R (resistant to adriamycin, vincristine, etoposide) 15 and the metastatic human breast adenocarcinoma cells MDA-MB-231 (ATCC, Manassas, USA) 16 were grown in phenol red-free RPMI-1640 medium, supplemented with 10% heat-inactivated fetal bovine serum, 10000 U/ml penicillin and 10000 µg/ml streptomycin, 2 mM L-glutamine, 1.25 mM sodium pyruvate, amino acids and Vitamins (Eurobio, France). The non-cancerous, but immortalized by telomerase, human breast epithelial cell line hTERT-HME1 (ATCC, Manassas, USA) were grown in a mixture of RPMI-1640 (90%) and mammary epithelial growth medium (10%) (MEGM) 15 (Lonza, Levallois-Perret, France). The cell lines were cultivated at 37°C in a humidified atmosphere containing 5% CO2. The hepatocellular carcinoma cell line HepG2 (ATCC, Manassas, USA) was grown in MEM medium containing 1.5 g/L sodium bicarbonate supplemented with 10% heat-inactivated fetal bovine serum at 37 °C in a humidified atmosphere of 5% CO2.
Cell Viability Determination (MTT Assay): Cell survival was determined by MTT reduction assay as described by 17. Cells were seeded in 96-well plates at a density of 1 × 104 cells/well and were incubated for 24 h. Then the medium was discarded and replaced by fresh medium containing the tested extract at the concentration of 200 µg/mL. After 48 hours exposure to test samples, the medium was removed and fresh media with MTT (final concentration of 0.5 mg/ml) was added. The incubation continued for 2 h at 37 °C. The formazan crystals were dissolved in DMSO, and the spectrophotometric determinations were done at 550 nm.
In-vitro Evaluation of CDC25s Inhibitory Activity: CDC25s phosphatases are a family of three isoforms (A, B and C) which play a primordial role in cell cycle regulation. Their physiological function consists of dephosphorylating their cellular substrates, i.e. the CDK (Cyclin-dependent kinases), allowing the progression of the cell cycle towards the mitosis step. Thus, CDC25s must be tightly regulated all through the cell cycle to maintain the adequate CDK-cyclin activity levels 18.
Deregulation of these activities is frequently observed in cancer. Indeed, numerous studies rise the relation between cancer and over-expression of CDC25 isoforms 19, and this over-expression is often related to poor prognosis. Some studies have shown the over-expression of CDC25A and B in numerous tumors, including breast, thyroid, laryngeal, esophageal, gastric, hepatocellular, ovarian, endometrial, prostate, colorectal cancers and non-Hodgkin lymphomas 18. This activity can be quantified in-vitro by fluorimetry and can be modulated by the presence of various molecules 20 - 23.
Preparation of Human Recombinant Proteins: The CDC25 proteins used in in vitro fluorimetric assays are recombinant human proteins in which CDC25 is linked to a glutathione S-transferase (GST) unit. These fusion enzymes are obtained by a method developed by 24. It requires the production of proteins in E. coli, their extraction, and purification by an affinity-chromatography process and their calibration by fluorimetric assay. Briefly, the bacteria are transformed with a pGexplasmidic vector containing the cDNA GST-CDC25 (A, B or C). Then, the bacteria were produced on a medium containing ampicillin, which only allows selection of the bacteria that have incorporated the plasmid (wearing an ampicillin resistance gene). Induction by isopropyl β-D-1-thiogalactopyranoside (IPTG) system enables the production of recombinant proteins. Proteins produced are then extracted and purified by affinity chromatography with glutathione (GSH)-agarose which specifically allow retaining the fusion proteins GST-CDC25 via a GSH-GST interaction.
Measurement of CDC25 Activity: The fluorimetric measurements of the CDC25 phosphatase activity were performed in the presence of a non-fluorescent synthetic substrate called 3- OMF- P (3-O-methylfluorescein phosphate) whose dephosphorylation leads to the emission of a fluorescent compound, the 3-OMF (3-O-methylfluorescein), which can be excited at 480 nm wavelength and then send back a fluorescent emission at 520 nm. The dephosphorylation rate of 3-OMF-P phosphatase is proportional to CDC25 activity. Thus, the in vitro activity of CDC25 on the substrate alone was measured and compared to the activity of the enzyme in the presence of potentially inhibitory compounds. The reactions are best performed at 30°C and in a buffer pH of 8.1 [50 mM Tris, 50 Mm NaCl, 1 mM EDTA, 0.1% BSA (bovine serum albumin)]. The CDC25 phosphatase activity was measured on a CytoFluor ® 4000 TC system (Applied/Per Septive Biosystems), which quantifies the soluble fluorescence.
This fluorescence is measured using a photomultiplier tube which quantifies and records the amount of fluorescence in arbitrary units of fluorescence (AFU). The device is connected to a workstation for precise adjustment of all parameters: number of measurements, time interval measurements, detector gain, number of readings detection, selection of emission and excitation wavelengths. These parameters allow automatic kinetics measurements. Experiments were performed three times and the standard deviations (r²) are calculated for each test and control. Results are expressed in percent of residual activity: naphthoquinone is used at a concentration of 10µM and serves as a positive inhibition control in these experiments. CDC25 activity that remains after incubation with the eventual inhibitor (equivalent to 100-inhibition percent). The samples were tested at a concentration of 2 mg/1mL.
In-vivo Assessment of the Waste by-products: Determination of LD50: LD50 was determined according to the procedures developed by 25.
Determination of the Immunomodulatory Activity: Determination of the immunomodulatory activity was measured for samples of olive and black seed wastes using prednisolone as immuno-suppressant standard and Echinacea purpurea extract as an immunostimulant. The immunomodulatory effect of the extracts was studied by evaluation of relative percent of leukocyte subpopulations in flow cytometer.
Animals: Male Wistar rats (100 - 150 g) were obtained from ECWP (Emirates Center for Wildlife Propagation, Missour, Morocco). They were kept in a well-ventilated environment and had free access to water and food ad libitum and were housed in a quiet room under a “12-h light: 12-h dark” cycle for 2 weeks before experimentation.
Sample Preparation: Extracts of olive and black seeds samples were dissolved in olive oil and administered them orally at a dose of 1000 mg/kg, body weight (b.w) to animals.
Flow Cytometer Experiment: The animals were anesthetized with “i.p.” sodium pentobarbital at the dose of 30 mg/kg, 24 h after treatment. The blood was collected in heparinized tubes, 24 after treatment then subjected to analysis by FCM (flow cytometer Epics-XL MCL type). FCM was used to evaluate the proportion of leukocyte subpopulations, based on cell morphology, size, and structure.
Pharmaceutical Dosage Form Preparation:
Material: Extracts of nigella seed and olive fruit wastes were prepared. Kaolin, dibasic calcium phosphate dehydrate, and talc was purchased from ADWIC, (Egypt).
Preparation of Liquisolid Granules from Extracted Nigella and Olive: Extracted nigella seed and olive fruit wastes (containing thymoquinone and oleuropein respectively) were dried by mixing separately with two different adsorbent powders which were kaolin, and dibasic calcium phosphates dehydrate respectively. In brief, the amount of extracted nigella seed and olive fruit wastes for 10 capsules were exactly weighed and pour onto the slab. The adsorbent powder, each one of them, was sprinkling onto the extracted nigella and olive and thoroughly mixed until the liquid became dump mass, then turn into the incoherent powder and eventually reach the crumbly look. The dried powder nigella and olive were consequently sieved and dried overnight at 40°C in an oven. The dried granules were stored in the desiccator until subsequent use. The amount of adsorbent powder to dry the liquid nigella and olive could be calculated by the weight difference between the starting weight of adsorbent powder and the final weight after adding into the extracted nigella and olive to dry the liquid. The amount per dose of adsorbent powder was the weight difference of adsorbent powder divided by 10.
Preparation of Nigella Tablet and Olive Capsule: The total required amount of Nigella and olive granules were mixed separately with talc powder. The mixed olive granules were packed into capsule no. 00 approximately 500 mg per capsule. The mixed nigella granules were compressed into approximate 1 g tablets in a single punch tablet machine with 12 mm round flat faced punch. Each olive capsule contains 30 mg active ingredient (oleuropein). Each nigella tablet contains 3.6 mg active ingredient (thymoquinone).
Statistical Analysis: Data were expressed as the mean ± SEM. Comparisons of means were performed by using the student t-test. The level of statistical significance was set at p<0.05.
RESULTS:
Chemical Analysis of Olive Fruit and Nigella Seed Wastes Extracts:
HPLC Analysis: To determine the suitable solvent for extraction of plant material, a preliminary HPLC analysis of OFW and NSW samples extracted with different solvents viz. (ethanol, methanol and 60% and 80% MeOH in water by ultrasonication at room temperature for 10 min. The 80% MeOH extract showed the highest amount of oleuropein (0.21 g% by-product of the dry fruit) while the methanol (100%) showed the highest amount of thymoquinone (0.24 g% byproduct of the dry seed). Therefore, 80% methanol and 100% methanol were selected for extraction of olive fruits and black seed waste samples, respectively for analysis and biological studies.
Linearity: The presented method exhibited strict linearity between the peak area and concentration covering the range from 0.1-1.4 mg/ml for thymoquinone Fig. 1S and oleuropein Fig. 2S. The linear regression equations were found to be y =43.124 x with correlation coefficient (r2 = 0.9999) and y = 5.347 x with correlation coefficient (r2 = 0.999) for Nigella and olive wastes, respectively, where y is the spot area and x is the concentration in mg/ml.
Total Phenolics in Olive and Nigella Wastes: Phenolic substances are responsible for the antioxidant activity of many plants. Therefore, the amounts of total phenolics present in a standardized olive and nigella waste extracts were investigated. The content of total phenolics is expressed as mg gallic acid/g of extract (GAE) and calculated from the regression equation (y=0.0081x, r2=0.9905). The total phenol in olive and nigella wastes is 15.76 ug and 2.96 ug, respectively at a concentration of 1 mg/ml each.
Nutritional Values of Waste Materials Left After Solvent Extraction: Chemical composition of olive fruits and nigella seed wastes left after solvent extraction are given in Table 1.
TABLE 1: DETERMINATION OF THE LIPIDS, MINERALS, CARBOHYDRATES AND VITAMINS CONTENT IN THE OLIVE FRUITS AND NIGELLA WASTES
| Element | OLV | NGL |
| Calcium (Ca), g/100 g | 0.62 | 0.75 |
| Iron (F), g/100 g | 0.05 | 0.01 |
| Sodium (Na), g/100 g | 0.08 | 0.09 |
| Potassium (K), g/100 g | 0.81 | 0.68 |
| Magnesium (Mg), g/100 g | 0.09 | 0.35 |
| Phosphorus (P), g/100 g | 0.07 | 0.87 |
| Manganese (Mn), ppb | 1.0 | 3.0 |
| Total lipid % | 6.37 | 28.83 |
| Total carbohydrate % | 20.1 | 22.03 |
| Total protein % | 5.0 | 28.1 |
| Vitamin A (mg/g) | 1.07 | 0.59 |
| Vitamin C (mg/g) | 0.14 | 0.22 |
OLV: Olive waste; NGL: Nigella waste
The results indicated nigella waste had higher fat (28.82%) and protein (28.1%) contents of than that of olive waste samples. Also, nigella waste showed slight higher carbohydrates content than that of olive waste. The mineral contents of the two wastes under investigation Table 1 showed that Nigella and olive wastes contain higher levels of potassium followed by calcium, phosphorus, magnesium, iron, and sodium. The two waste products contain Vitamin A and C in the range of 0.59-1.07 mg/g and 0.14-0.22 mg/g, respectively.
In-vitro Evaluation of the Antioxidant Activity:
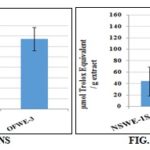
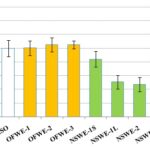
Free Radical Scavenging Activity Measurement (DPPH Assay): The antioxidant activity of the OFW Fig. 3SA and NSW Fig. 3SB fractions were examined by exploring scavenging activity of the stable 1,1-diphenyl-2- picrylhydrazyl (DPPH) free radical. The reduction capability of DPPH radical was determined by the decrease in absorbance at 515 nm induced by antioxidants. OFWE could reduce the stable radical DPPH to the yellow colored diphenyl-picrylhydrazine. OFWE-1 (80% alcohol extract) and OFWE -3 (defatted 80% alcohol extract) Fig. 3SA showed a comparable antioxidant activity.
They exert a strong neutralization of DPPH radical, respectively corresponding to 139.7 ± 14.4 and 134.4 ± 22.5 µmol Trolox equivalent/g extract. OFWE-2 exhibits a weak antiradical activity (17 ± 5.5 µmol Trolox equivalent/g extract).
The liquid part of NSWE fractions (NSWE-1L and NSWE-3L) Fig. 3SB showed antiradical properties being in the 15.8 to 21.6 µmol Trolox equivalent/g extract values range. The solid part fractions (NSWE-1S and 3S) varied between 30.3 and 51.8 µmol Trolox equivalent/g extract.
FIG. 3S: FREE RADICAL-SCAVENGING ACTIVITY OF THE WASTE BYPRODUCT EXTRACTS USING THE DPPH (2, 2-DIPHENYL-1-PICRYL-HYDRAZYL) METHOD USING TROLOX AS A STANDARD
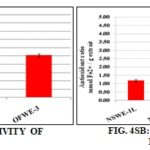
Reducing Power Measurement (FRAP Assay): A strong reducing power was exerted by the OFWE-1 (2.6 ± 0.2 mmol Fe2+/g extract) and OFWE-3 (2.5 ± 0.1 mmol Fe2+/g extract) Fig. 4SA. OFWE-2 exhibited a weak ferric reducing power (0.5 ± 0.02 mmol Fe2+/g extract). NSWE-1, -2 and -3 fractions Fig. 4SB displayed a comparable reducing power being in the range of 0.8 to 1.2 m mol Fe2+/g extract.
Biological Assessment:
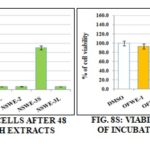
The Antiproliferative Activity: Determination of Cell viability (MTT assay). The impact of the extracts on cell viability was evaluated on four human breast cell lines, three of them originating from tumors (MCF7, MDA-MB-213, Vcr-R), and one coming from epithelial cells, not cancerous, but immortalized by telomerase (hTERT-HME1). Moreover, a human hepatoma cell line (HepG2) was also tested owing to its high metabolic capacities. Different concentrations were tested (data not shown) and the concentration of 200µg/mL was selected because of the most pronounced biological activity. The cell line hTERT-HME1 Fig. 5S was very sensible to NSWE-1L, NSWE-2, and NSWE-3L fractions with around 95% loss of cell viability. These three NSWE fractions also displayed a strong cytotoxic effect on MCF7 and MDA-MB231 cells Fig. 6S and 8S and on the multidrug resistant cell line Vcr-R Fig. 7S with a loss of about 93% of cell viability.
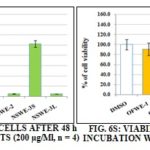
The hepatocellular carcinoma cells Fig. 9S was less sensitive to NSW fractions than mammary cancer cell lines. The most toxic was NSWE-3L. It killed 90% of cancer cells. NSWE-3S exhibited moderate toxicity, ~ 62% loss of live cells was observed.
FIG. 9S: VIABILITY OF HEPG2 CELLS AFTER 48 h OF INCUBATION WITH BOTH EXTRACTS (200µg/Ml, n = 4)
On the contrary, the olive waste fractions did not show any cytotoxicity against all the tested cell lines. The percent of live cells varied between 81 ± 2.7 % and 96.4 ± 4.6 %.
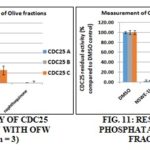
Measurement CDC25 Phosphatase Inhibitory Activity: All samples were tested at 2 mg/mL. The three fractions of olive waste (OFWE-1-3) Fig. 10S showed an important inhibitory effect against the three CDC25 isoforms (A, B and C), specially OFWE-2. CDC25A is the most sensitive to these fractions. All the liquid part of the three fractions of NSW (NSWE-1L, -2L and -3L) and NSWE-2S Fig. 11S induced strong CDC25 inhibition of the three isoforms
In-vivo Assessment of the Waste by-products:
Determination of LD50: No toxic sign and symptoms and mortalities were observed in any of the tested samples up to a dose of 7000 mg/kg.
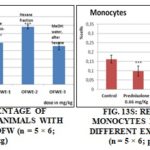
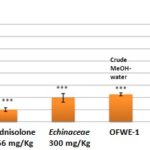
Immunomodulatory Activity: Generation of effective immunity requires both innate and adaptive immunity that respectively recognizes pathogen-associated molecular patterns and specific antigens. Innate immunity consists of non-specific cells, including macrophages, granulocytes, NK cells, and dendritic cells. Adaptive immunity is comprised of a humoral arm mediated by B cells that secrete antigen-specific antibodies, and cellular pathway mediated by CD4+ (helper) and CD8+ (cytolytic) T cells. CD4+ T helper cells are responsible for orchestrating an immune response, whereas cytolytic CD8+ T cells are the killer cells that traffic to sites of infection or cancer and lyse infected or tumor cells. Together, these two types of effect or T lymphocytes play critical roles in eliminating infections and controlling cancer. One of the precious properties of N. sativa is the immunomodulatory effects of its constituents. Studies begun just over a decade ago suggested that if it is used on an ongoing basis, N. sativa can enhance immune responses in human. The majority of subjects who treated with N. sativa oil for 4 weeks showed a 55% inhibition. The immunomodulatory effect of extracts was studied by evaluation of relative percent of leukocyte subpopulations in flow cytometer. An immunostimulant effect of lymphocyte Fig. 12S and monocytes Fig. 13S subpopulations at 1000 mg/kg, b.w. was observed by testing of OFWE-1, and OFWE-2in comparison with Echincea aqueous extract treated group (27%, p<0.01). For granulocytes Fig. 14S, all the olive waste extracts induced an immunosuppressive effect after comparison with the prednisolone group (6.23%; p<0.001).
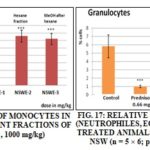
FIG. 14S: RELATIVE PERCENTAGE OF GRANULOCYTES (NEUTROPHILES, EOSINOPHILES AND BASOPHILES) IN TREATED ANIMALS WITH DIFFERENT FRACTIONS OF OFW (n = 5x 6; p<0.001, 1000 mg/kg)
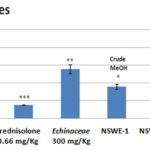
A significant enhancement of lymphocytes Fig. 15S and monocytes Fig. 16S sub-populations after treatment with 1000 mg/kg of NSWE-1, NSWE-2and NSWE-3 compared to control and to Echinacea treated groups. However, an immunosuppressive activity on granulocytes Fig. 17S sub-population was observed after treatment with all extracts of NSW.
FIG. 15S: RELATIVE PERCENTAGE OF LYMPHOCYTES IN TREATED ANIMALS WITH DIFFERENT FRACTIONS OF NSW (n = 5 × 6; p<0.001; p<0.01; p<0.05, 1000 mg/kg)
DISCUSSION:
In-vitro Evaluation of the Antioxidant Activity: The role of oxidative stress in the etiology of inflammatory diseases and cancer is well-known. Deleterious effects of this stress are due to an increasing amount of cellular free radicals leading to the activation of free radical-stimulated pathways. The results showed that some fractions of OFW and NSW waste extracts displayed an antiradical activity and a reducing power in-vitro. The antiradical and antioxidant potential of OFW products.
The most active fractions with strong antiradical and antioxidant potential were OFW fractions OFWE-1 and OFWE-3. These results were by the antioxidant properties of the olive oil and can be attributed to the polyphenolic content of the oil 26 - 28. Moreover, the virgin olive oil is not only rich in phenolic compounds, but also antioxidant substances like alpha-tocopherol, carotenoids (Cicerale et al., 2010). The obtained results also matched with those reported by 29. Therefore the Egyptian olive oil by-products, not investigated previously, are an interesting source of phytochemicals and natural antioxidants.
The antioxidant potential of NSW fractions was much lower than those of OFW fractions Fig. 3SB and Fig. 4SB. However, the polyphenolic profile and the antioxidant activities of NSW have been reported 30 leading to suggest its use in some pathologies 31, 32. Recently, it was shown that the protein fraction of the extract of NSW exhibited a promising hepatoprotective effect 7. At first glance, OFW seems to be more attractive than NSW. Our results showed how this waste could be valorized in the domain of the protection of cells attacked by oxidative substances. For that, it would be interesting to pursue this study by testing this activity “in cellulo” by protecting cells against deleterious oxidant stress.
Cytotoxic Activity of Waste Byproduct Extracts: In-vitro cytotoxicity of the extracts was performed on cancerous (MCF7, its multidrug-resistant counterpart Vcr-R, the metastatic MDA-MB231) and non-cancerous (hTERT-HME1) mammary cell lines and on hepatocellular carcinoma cells (HepG2). At a concentration of 200µg/mL and after 48h of incubation, it was observed that the liquid parts of NSW fractions and NSWE-2 showed the strongest cytotoxic effect. These fractions caused around 95% inhibition of cell viability in all mammary cell lines, even in the drug-resistant Vcr-R Fig. 7S. NSW has been shown to possess anti-carcinogenic activity in-vitro on mammary cancer cell lines 33, 34. The studies suggested that the anti-cancer properties of NSW fractions occurred via induction of apoptosis, decrease in the expression levels of antiapoptotic - Bcl-2 and DNA fragmentation.
It is interesting to notice that breast cancer cells (MCF-7, MDA-MB-231, Vcr-R) are more sensitive to the anticancer activity of MSW fractions Fig. 6S, 7S, and 8S when compared to hepatoma cancer cell line (HepG2) Fig. 9S. This phenomenon was recently reported by 34. On the contrary, OFW fractions did not display any cytotoxicity towards the cell lines used in this study. These results confirm the general reputation of this plant. Our results show the interest of NSW by-products in the cancer field. To the best of our knowledge, it is the first time that such activity on a pluriresistant cell line (Vcr-R) is reported. These results have to be confirmed by assays performed with the major molecules identified in these extracts.
Inhibitory Potential of Waste Byproduct Extracts on CDC25s: A deregulation of these activities is frequently observed in cancer. Previous studies rise the relation between cancer and over-expression of CDC25 isoforms, and this over-expression is often related to poor prognosis. Some studies have shown the over-expression of CDC25A and B in numerous tumors, including breast, thyroid, laryngeal, esophageal, gastric, hepatocellular, ovarian, endometrial, prostate, colorectal cancers and non-Hodgkin lymphomas.
All the plant extracts contain substances able to inhibit CDC25 phosphatase activity. Considering the two waste extracts, NSW fractions Fig. 11S seems to be the most potent for CDC25 inhibition (except NSW-1S and NSW-3S) with an equivalent inhibition of the three isoforms. These profiles CDC25 inhibition versus MTT test are thus parallel in NSW fractions. The two waste products contain substances able to inhibit CDC25 phosphatase activity. NSW fractions (Fig. 11S) were found to be the most potent on CDC25s inhibition than the OFW fractions Fig. 10S with an equivalent inhibition of the three isoforms.
Immunomodulatory Activity: The immuno-stimulant effect of OFW and NSW extracts shown in this study could be related to the effect of various substances such as derivatives of phenolic acid, alkamids, and polysaccharides that was earlier demonstrated with Echinacea purpurea on the immune system. However, the immunosuppressive effect on granulocytes is probably related to the effect of flavonoids. In this context, it was earlier demonstrated that genistein, an isoflavone isolated from Genista tinctoria showed a potential inhibition of T lymphocytes proliferation, IL-2 synthesis and IL-2 receptor expression and also an another flavonoid, inhibited the growth of T-lymphoid leukemia cells and reduced the Protein Kinase C activity. The suggestion of the implication of tannins in immunosuppressive activity, such as methyl gallate isolated from Acacia nilotica, showed an immunosuppressive activity. It was shown also that most of the flavonoids are immunosuppressive and have an important phagocytosis power on humoral, cellular immune and also on the development of immunological memory after the antigenic stimulation 35.
Other work was demonstrated that Quercetin had an excellent immunosuppressive on Dendritic cells, regarded as a major target of immunosuppressant on the immune response. This flavonol could be used on autoimmune diseases and transplantation 36.
CONCLUSION: The extraction of bioactive organic compounds from OFW and NSW fractions present a double interest. Firstly, they are a promising source of substances of high value and secondly, the recycling and upgrading of residues from plant food processing could be a solution to the potentially severe environmental problem of the industry.
The lack of toxicity of OFW fractions and their strong antiradical and antioxidant activity could be interesting for the needs of chemoprevention. Particularly the chemoprotective potential of these extracts against oxidative stress induced by therapeutic molecules could be checked (Beillerot et al., 2008). Moreover, the cytotoxicity of NSW fractions were confirmed in five cell lines and, to our knowledge, it is the first time that such activities are reported on a pluriresistant cell line (Vcr-R).
The main bioactive compounds were identified as oleuropein for OFW fractions and thymoquinone for NSW fractions. At this step of research, it will be important to test these compounds directly and to compare them to those obtained with extracts in order to see if extracts contain synergistic or inhibitory compounds for the tested activities.
ACKNOWLEDGEMENT: The authors are grateful to the Agence Universitaire de la Francophonie (AUF), Alexandria, Egypt, for funding this project. The authors also thank the staff members of the Department of Taxonomy, Faculty of Science, Cairo University, Cairo, Egypt, for the identification of the plant materials, to Mrs. Mayy Moataz, Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Cairo Egypt, for technical assistance and to Ghada H. El-Osaily, Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Al-Azher University, Cairo, Egypt and, Faculty of Pharmacy, Modern University for Technology and Information (MTI) for helping in the preparation of the pharmaceutical dosage forms.
CONFLICT OF INTEREST: The authors report no conflict of interest.
REFERENCES:
- Leouifoudi I, Mbarki M, Tilaoui M, Amechrouq A, Rakib EM, Mouse HA and Zyad A: Study of the in-vitro anticancer activity of Moroccan phenolic olive cake extracts. Journal of Pharmacognosy and Phytochemistry 2014; 2(6): 154-65.
- Alu’datt MH, Alli I, Ereifej K, Alhamad M, Al-Tawaha AR and Rababah T: Optimisation, characterization and quantification of phenolic compounds in the olive cake. Food Chemistry 2010; 123(1): 117-22.
- Ramos P, Santos SA, Guerra ÂR, Guerreiro O, Felício L, Jerónimo E, Silvestre AJ, Neto CP and Duarte M: Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Industrial Crops and Products 2013; 46: 359-68.
- Hashish S and El-Samee LA: Effects of Feeding Olive Cake and Barley Radicle as fiber sources on lipids, cholesterol and fatty acids in Hen Eggs. In: Proceedings of the 15th European Symposium on poultry nutrition, Balatonfüred, Hungary, 25-29 September 2005; World's Poultry Science Association (WPSA) 2005; 628-30.
- Mohamed SS, Mohamed SR, Abou-Arab AA, Naguib KM, Helmy MH and Owiss NA: Comparison study on the native olive waste extract and its nano-particles effect on oxidative stress induced by aflatoxin B1 in rat brain. Int J Curr Microbiol App Sci 2014; 3(4): 141-52.
- Mansour RS, Nasser, AK and Abo NY: The Effect of different Nigella sativa seed (cake) concentrations on leukocytes counts and some serum immunological parameters in calves. Tikrit Journal of Pure Science 2013; 18: 31-35.
- Michel CG, El-Sayed NS, Moustafa SF, Ezzat SM, Nesseem DI and El-Alfy TS: Phytochemical and biological investigation of the extracts of Nigella sativa seed waste. Drug testing and analysis 2011; 3(4): 245-54.
- Ali B and Blunden G: Pharmacological and toxicological properties of Nigella sativa. Phytotherapy Research 2003; 17(4): 299-05.
- Al-Ali A, Alkhawajah AA, Randhawa MA and Shaikh NA: Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J Ayub Med Coll Abbottabad 2008; 20(2): 25-27.
- Huat BTK and Swamy SMK: Intracellular glutathione depletion and reactive oxygen species generation are important in α-hederin-induced apoptosis of P388 cells. Molecular and Cellular Biochemistry 2003; 245(1-2): 127-39.
- Suárez B, Álvarez ÁL, García YD, del Barrio G, Lobo AP and Parra F: Phenolic profiles, antioxidant activity and in-vitro antiviral properties of apple pomace. Food Chemistry 2010; 120(1): 339-42.
- Molyneux P: The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004; 26(2): 211-19.
- Benzie IF and Strain J: The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry 1996; 239(1): 70-76.
- Soule H, Vazquez J, Long A, Albert S and Brennan M: A human cell line from a pleural effusion derived from breast carcinoma. Journal of the National Cancer Institute 1973; 51(5): 1409-16.
- Whelan RD, Waring CJ, Wolf CR, Hayes JD, Hosking LK and Hill BT: Over‐expression of P-glycoprotein and glutathione S-transferase PI in MCF-7 cells selected for vincristine resistance in-vitro. International Journal of Cancer 1992; 52(2): 241-46.
- Cailleau R, Young R, Olive M and Reeves W: Breast tumor cell lines from pleural effusions. Journal of the National Cancer Institute 1974; 53(3): 661-74.
- Mosmann T: Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 1983; 65(1-2): 55-63.
- Boutros R, Lobjois V and Ducommun B: CDC25 phosphatases in cancer cells: key players? Good targets? Nature Reviews Cancer 2007; 7(7): 495-07.
- Boutros R, Dozier C and Ducommun B: The when and where of CDC25 phosphatases. Current Opinion in Cell Biology 2006; 18(2): 185-91.
- Bana E, Sibille E, Valente S, Cerella C, Chaimbault P, Kirsch G, Dicato M, Diederich M and Bagrel D: A novel coumarin-quinone derivative SV37 inhibits CDC25 phosphatases, impairs proliferation, and induces cell death. Molecular Carcinogenesis 2015; 54(3): 229-41.
- Brault L, Denancé M, Banaszak E, El Maadidi S, Battaglia E, Bagrel D and Samadi M: Synthesis and biological evaluation of dialkylsubstituted maleic anhydrides as novel inhibitors of Cdc25 dual specificity phosphatases. European Journal of Medicinal Chemistry 2007; 42(2): 243-47.
- Valente S, Bana E, Viry E, Bagrel D and Kirsch G: Synthesis and biological evaluation of novel coumarin-based inhibitors of Cdc25 phosphatases. Bioorganic and Medicinal Chemistry Letters 2010; 20(19): 5827-30.
- Viry E, Anwar A, Kirsch G, Jacob C, Diederich M and Bagrel D: Antiproliferative effect of natural tetrasulfides in human breast cancer cells is mediated through the inhibition of the cell division cycle 25 phosphatases. International Journal of Oncology 2011; 38(4): 1103-11.
- Brault L and Bagrel D: Activity of novel Cdc25 inhibitors and preliminary evaluation of their potentiation of chemotherapeutic drugs in human breast cancer cells. Life Sciences 2008; 82(5): 315-23.
- Reed LJ and Muench H: A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology 1938; 27(3): 493-97.
- Frankel E, Bakhouche A, Lozano-Sánchez Js, Segura-Carretero A and Fernández-Gutiérrez A: Lita erature review on prothe duction process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. Journal of Agricultural and Food Chemistry 2013; 61(22): 5179-88.
- Rafehi H, Ververis K and Karagiannis TC: Mechanisms of action of phenolic compounds in olive. Journal of Dietary Supplements 201; 9(2): 96-09.
- Visioli F: Olive oil phenolics: Where do we stand? Where should we go? Journal of the Science of Food and Agriculture 2012; 92(10): 2017-19.
- Pazos M, Alonso A, Fernández-Bolaños J, Torres JL and Medina I: Physicochemical properties of natural phenolics from grapes and olive oil by-products and their antioxidant activity in frozen horse mackerel fillets. Journal of Agricultural and Food Chemistry 2006; 54(2): 366-73.
- Meziti A, Meziti H, Boudiaf K, Mustapha B and Bouriche H: Polyphenolic profile and antioxidant activities of Nigella sativa seed extract in-vitro and in-vivo. World Academy of Science, Engineering and Technology 2012; 64(6): 24-32.
- Ahmad A, Husain A, Mujeeb M, Khan SA, Najmi AK, Siddique NA, Damanhouri ZA and Anwar F: A review on the therapeutic potential of Nigella sativa: A miracle herb. Asian Pacific Journal of Tropical Biomedicine 2013; 3(5): 337-52.
- Hasani-Ranjbar S, Larijani B and Abdollahi M: A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflammation and Allergy-Drug Targets (Formerly Current Drug Targets -Inflammation and Allergy) 2009; 8(1): 2-10.
- Dilshad A, Abulkhair O, Nemenqani D and Tamimi W: Antiproliferative properties of methanolic extract of Nigella sativa against the MDA-MB-231 cancer cell line. Asian Pacific Journal of Cancer Prevention 2012; 13(11): 5839-42.
- Neha R, Vali V, Gunaseelan V and Perinbam K: Antioxidant and Antiproliferative Activity of the Methanolic Extract from Nigella sativa Asian Journal of Biological Sciences 2014; 7(3): 122-30.
- Kim CJ and Cho SK: Pharmacological activities of flavonoids (III) structure-activity relationships of flavonoids in immunosuppression. Archives of Pharmacal Research 1991; 14(2): 147-59.
- Huang RY, Yu YL, Cheng WC, Ou-Yang CN, Fu E and Chu CL: Immunosuppressive effect of quercetin on dendritic cell activation and function. The Journal of Immunology 2010; 184(12): 6815-21.
How to cite this article:
El Dine RS, Tzanova T, Bousta D, Abou-Hussein DR, Boukhira S, Evain-Bana E, El-Tanbouly ND, Philippot S, El Mansourai L, Bagrel D, Amer RI and Abdel-Sattar E: Valorization of Egyptian food byproducts in the development of biologically active nutraceuticals. Int J Pharmacognosy 2018; 5(3): 144-57. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(3).144-57.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
144-157
914
947
English
IJP
R. S. El Dine, T. Tzanova, D. Bousta, D. R. Abou-Hussein, S. Boukhira, E. Evain-Bana, N. D. El-Tanbouly, S. Philippot, L. El Mansourai, D. Bagrel, R.I. Amer and E. Abdel-Sattar *
Department of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo, Egypt.
essam.abdelsattar@pharma.cu.edu.eg
23 October 2017
12 November 2017
18 November 2017
10.13040/IJPSR.0975-8232.IJP.5(3).144-57
01 March 2018