TRADITIONAL MEDICINAL USES, PHYTOCHEMICAL PROFILE AND PHARMACOLOGICAL ACTIVITIES OF LUFFA ACUTANGULA LINN.
HTML Full TextTRADITIONAL MEDICINAL USES, PHYTOCHEMICAL PROFILE AND PHARMACOLOGICAL ACTIVITIES OF LUFFA ACUTANGULA LINN.
J. Anitha * and S. Miruthula
Department of Biotechnology, Arunai Engineering College Thiruvannamalai - 606603, Tamil Nadu, India.
ABSTRACT: Inflammation is a body defence reaction to prevent the spread of injurious agent and to remove the necrosed cells and tissues. Inflammatory abnormalities are a large group of disorders which underlie a vast variety of human diseases. During treatment of inflammatory diseases, many conventional therapies (non-steroidal anti-inflammatory drugs) used to relief pain and inflammation. Continuous use of the intended drugs is frequently associated with serious side effects, whereas plants still hold their unique place, by way of having no side effects. Therefore, a systematic approach should be made to find out the efficacy of plants against inflammation to exploit them as herbal anti-inflammatory agents with a better safety profile. Luffa acutangula Linn. (Cucurbitaceae) has many therapeutic uses mentioned in Ayurveda and therefore we aimed to study its anti-inflammatory activity.
| Keywords: |
Anti-inflammatory activity, Luffa acutangula, Ethanol extract
INTRODUCTION: Inflammation is defined as the local response of living mammalian tissues to injury due to any agent. It is a body defense reaction to prevent the spread of injurious agent and to remove the necrosed cells and tissues1-2. The development of non-steroids in overcoming human sufferings such as Rheumatoid arthritis has evoked much interest in the extensive search for new drugs with this Property 3. Inflammation can be classified as either acute or chronic. Acute inflammations the initial response of the body to harmful stimuli and is achieved by the increased movement of plasma and leukocytes (especially granulocytes) from the blood into the injured tissues 4-5.
A cascade of biochemical events propagates and matures the inflammatory response, involving the local vascular system, the immune system, and various cells within the injured tissue 6-7. Five cardinal signs characterize it: The acronym that may be used for this is "PRISH" for Pain, Redness, Immobility (loss of function), Swelling and Heat 8.
The traditional names for signs of inflammation come from Latin-
Dolor (pain)
Calor (heat)
Rubor (redness)
Tumor (swelling)
Functio laesa (loss of function).
Prolonged inflammation, known as chronic inflammation, leads to a progressive shift in the type of cells present at the site of inflammation and is characterized by simultaneous destruction and healing of the tissue from the inflammatory process.
Morphologic Patterns: Specific patterns of acute and chronic inflammation are seen during particular situations that arise in the body, such as when inflammation occurs on an epithelial surface, or pyogenic bacteria are involved.
Granulomatous Inflammation: Characterized by the formation of granulomas, they are the result of a limited but diverse number of diseases, which include among others tuberculosis, leprosy, sarcoidosis, and syphilis 9-10.
Fibrinous Inflammation: Inflammation resulting in a large increase in vascular permeability allows fibrin to pass through the blood vessels. If an appropriate procoagulative stimulus is present, such as cancer cells, a fibrinous exudate is deposited. This is commonly seen in serous cavities, where the conversion of fibrinous exudate into a scar can occur between serous membranes, limiting their function.
Purulent Inflammation: Inflammation is resulting in a large amount of pus, which consists of neutrophils, dead cells, and fluid. Infection by pyogenic bacteria such as staphylococci is characteristic of this kind of inflammation. Large, localized collections of pus enclosed by surrounding tissues are called abscesses.
Serous Inflammation: Characterized by the copious effusion of non-viscous serous fluid, commonly produced by mesothelial cells of serous membranes, but may be derived from blood plasma. Skin blisters exemplify this pattern of inflammation.
Ulcerative Inflammation: Inflammation occurring near an epithelium can result in the necrotic loss of tissue from the surface, exposing lower layers. The subsequent excavation in the epithelium is known as an ulcer.
Inflammatory Disorders: Inflammatory abnormalities are a large group of disorders which underlie a vast variety of human diseases. The immune system is often involved with inflammatory disorders, demonstrated in both allergic reactions and some myopathies, with many immune system disorders resulting in abnormal inflammation. Non-immune diseases with etiological origins in inflammatory processes include cancer, atherosclerosis, and ischemic heart disease. A large variety of proteins are involved in inflammation, and any one of them is open to a genetic mutation which impairs or otherwise deregulates the normal function and expression of that protein.
Examples of disorders associated with inflammation include:
- Acne vulgaris
- Asthma
- Autoimmune diseases
- Celiac disease
- Chronic prostatitis
- Glomerulonephritis
- Hypersensitivities
- Inflammatory bowel diseases
- Pelvic inflammatory disease
- Reperfusion injury
- Rheumatoid arthritis
- Sarcoidosis
- Transplant rejection
- Vasculitis
- Interstitial cystitis
Luffa acutangula:
FIG. 1: LUFFA ACUTANGULA
Luffa acutangula is a species of Luffa. It is commercially known for its unripe fruits as a vegetable. Mature fruits are used to make cleaning sponges. Its fruit slightly resembles a cucumber with ridges. It ranges from central Asia and eastern Asia to southeastern Asia. Monoecious, annual, climbing or trailing herb, with an acutely 5-angled stem; tendrils up to 6-fid, hairy. Leaves alternate, simple; stipules absent; petiole up to 15 cm long; blade broadly ovate to kidney-shaped in outline, 10–25 cm × 10–25 cm, shallowly palmately 5–7-lobed with broadly triangular to broadly rounded lobes, cordate at base, shallowly sinuate-dentate, pale green, scabrous, palmately veined. Male inflorescence is racemose with 15–35 cm long peduncle. Flowers unisexual, regular, obconic below, expanded above, c. 0.5 cm long, lobes triangular, 1–1.5cm long; petals free, pale yellow; male flowers with 3 free stamens inserted on the receptacle tube, connectives broad; female flowers solitary, on pedicels 2–15 cm long, with the inferior, densely pubescent, longitudinally ridged ovary, stigma 3-lobed. Fruit a club-shaped, dry and fibrous capsule 15–50 cm × 5–10 cm, acutely 10-ribbed, brownish, dehiscent by an apical operculum, many-seeded. Seeds broadly elliptical in outline, compressed, up to 1.5 cm long, smooth, dull black.
Scientific Classification:
Kingdom : Plantae
(Unranked) : Angiosperms
(Unranked) : Eudicots
(Unranked) : Rosids
Order : Cucurbitales
Family : Cucurbitaceae
Genus : Luffa
Species : L. acutangula
Vernacular Name:
- Assamese : Jeeka
- Bengali: Jhingge or Jhinga
- Hindi : Turai, Tori
- Gujarati: Turiya
- Kannada: Eere kay
- Lao: mark noy
- Vietnamese: mướp khía.
- Tamil: Pirkanga
- Telugu: beera kaaya
- Thai: Buap liyam
- Marathi: dodaki
- Konkani: Gossale
- Indonesian: gambas, oyong
- Javanese: oyong
- Malayalam: Peechinga
- Malay: Petola segi
- Sinhalese: Watakolu
Traditional Medicinal Uses: Ayurveda has attributed ride gourd with some health benefits which current clinical research is supporting as well. The ridge gourds are rich in minerals and are very alkaline for the body, and hence they have a cooling effect on the body. From Ayurveda point of view, ridge gourd increases vata and kapha, but it cools down and pacifies the dosha pitta in the body. All parts of the ridge gourd plant, fruits, leaves, seeds, and even roots are used for their medicinal value.
In spite of their bland taste, ridge gourds have many health benefits:
The most common use of the ridge gourd fruit is to be cooked as a vegetable. It is a very nutritive plant and has a bitter taste if taken raw. Ridge gourd acts as an appetizer. The ridge gourd is a healthy diet and contains a good amount of fiber, vitamins, and minerals including vitamin B2, vitamin C, carotene, niacin, calcium, phosphorus, iron and small quantities of iodine and fluorine. They form a low-calorie diet, which is considered good for diabetes. Ridge gourd is used as an expectorant and hypoglycemic, bitter tonic, enlargement of the spleen and also prevention of premature greying of hair. The roots of the ridge are helpful in the removal of kidney stones, swelling of the lymph glands. The leaves of the ridge gourd are useful in the treatment of dysentery conditions, dressing in diseases such as inflammation of spleen, ringworms, piles and even leprosy. Pounded leaves mixed with garlic are applied locally for relief in leprosy. Seeds of ridge gourd are used as a laxative and purgative. Oil is extracted from the seeds of ridge gourd which is used in the treatment of skin diseases.
Phytochemical Constituents: Chemical constituents of Luffa acutangula mainly include carbohydrates, carotene, fat, protein, phytin, amino acids, alanine, arginine, cysteine, glutamic acid, glycine, hydroxyproline, leucine, serine, tryptophan, pipecolic acid, flavonoids, and saponins. The fruit contains an amorphous bitter principle, luffeine. The seeds contain a fixed oil which consists of the glycerides of palmitic, stearic, and myristic acids. Lectin specific for chito-oligosaccharides was isolated from Luffa acutangula and has been purified to homogeneity by affinity chromatography and its macromolecular properties and combining affinity with different sugars was studied. The studies revealed that lectin has a molecular weight of 48,000 and Stokes radius of 2.9 nm. When sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed, an only single band corresponding to molecular weight of 24,000 was observed both in the presence as well as the absence of 2-mercaptoethanol. The subunits in this dimeric lectin are therefore held by non-covalent interactions alone. The lectin is not a glycoprotein and circular dichroism spectral studies indicate that this lectin has 31% α-helix and no β-sheet. The lectin is found to bind specifically tochito oligosaccharides, and the affinity of the lectin increases with increasing oligosaccharide chain length as monitored by near ultra-violet circular dichroism and intrinsic fluorescence titration. The thermodynamic data revealed that binding site in lectin accommodates a tetrasaccharide and the values of G, Η, and S for the binding process showed a pronounced dependence on the size of the oligosaccharide.
A chito oligosaccharide specific lectin (Luffa acutangula agglutinin) has been purified from the exudate of ridge gourd fruits by affinity chromatography on soybean agglutinin glycopeptides coupled to Sepharose-6B. The affinity purified lectin was found homogeneous by polyacrylamide gel electrophoresis. Based on the thermodynamic data, blue shifts, and fluorescence enhancement, the spatial orientation of chitooligosaccharides in the combining site of the lectin were studied. Luffangulin, a novel ribosome inactivating peptide with an N-terminal sequence, was isolated from seeds of Luffa acutangula. The 5.6 k Da-peptide designated luffangulin inhibited cell-free translation with an IC50 of 3.5 nM but lacked inhibitory activity toward HIV-1 reverse transcriptase. A bitter principle, Cucurbitacin B, an acidsapogenin, oleanolic acid were isolated from the seeds of Luffa acutangula. The study of nutritional and oil characteristics of the Luffa acutangula seeds showed that it has Iodine value, saponification value and acid value as 99.5, 190.8 and 10.5 respectively. The maximum melting and freezing points were found to be -3 °C and -10 °C respectively.
Nutritional Composition: The seeds of Luffa acutangula were studied for potential nutritional and oil characteristics. The fatty acid profile indicates that the glycerides of oleic and linoleic acid constitute 68% of the total kernel oil. The seeds were also found to be a good source of certain amino acids, phosphorous, iron, and magnesium.
Ethnomedical Properties: The ethno-medico botanical survey of the hilly areas in Maharashtra revealed that fruits of Luffa acutangula are used to protect from jaundice when taken in the form of very fine powder through the nose.
MATERIALS AND METHODS: The present study was carried out to evaluate the anti-inflammatory activity of Luffa acutangula. Qualitative and quantitative analysis was done, and the ethanolic extracts of the two plant species were used for GC-MS studies. The details of the material used and methods followed are described below.
Collection of Plant Materials: Fruit pulp of Luffa acutangula was collected in the month of Jan-Feb, 2013 from local areas of Thiruvannamalai (Ramanaashramam). Fruit pulp of Fresh fruits of Luffa acutangula was crushed and used for the study.
Chemicals and Reagents: Ethanol, Fehling’s reagent, Hydrochloric acid, sulphuric acid, Ferric chloride, acetic anhydride, chloroform, Mayer’s reagent, glacial acetic acid, ammonia, magnesium, Anthrone reagent, Bradford reagent.
Extraction: The plant materials were powdered, and 30 gm of powder sample was extracted with 150 ml of ethanol (1:5) by using soxhlet apparatus. The whole apparatus was kept over a heating mantle and was heated continuously for 24 h at the boiling point of the solvent. The extract was concentrated to dryness, and the residues were transferred to a preweighed sample bottle and were stored in a desiccator for further studies.
FIG. 5: SOXHLET APPARATUS AND EXTRACT
Qualitative Analysis: Different biochemical parameters like reducing sugar, Flavonoid, Terpenoid, Tannin, Saponin, Anthraquinone, glycosides, alkaloids, etc. were tested.
Test for Reducing Sugars: The aqueous extract was added to boiling Fehling solution in a test tube; a brick red color indicates the presence of reducing sugars.
Test for Flavonoids: The extract and add a few magnesium turnings, followed by the addition of con. HCl drop by drop. A pink color indicates the presence of flavonoids.
Steroids and Terpenoids: Extract, dry and dissolve in chloroform. Add a few drops of acetic anhydride and conc. H2SO4 and keep undisturbed for few minutes. Formation of green color indicates the presence of steroids, while pink color indicates the presence of terpenoids.
Test for Tannins: To extract, add 2 drops of 5% FeCl3. Presence of dirty green precipitate indicates the presence of tannin.
Test for Saponin: To extract was shaken with 5ml of distilled water and was heated to the boiling point. Frothing indicates the presence of saponin.
Test for Anthraquinones: To powdered material add 10 ml of 1% HCl and boiled for 5 min. Filter the sample and allowed to cool. Partition the cool filtrate against an equal volume of chloroform. Carefully transfer the chloroform layer into clean test tubes. Shake with an equal volume of 10% ammonia solution and allow the layer to separate. Presence of delicate rose pink color indicates the presence of combined anthraquinones.
Glycosides: To 0.5 gm of extract diluted to 5ml with distilled water and add 2 ml of glacial acetic acid and containing one drop of ferric chloride solution. This was underplayed with 1ml of conc. H2SO4. Brown ring at the interface the presence of glycosides.
Test for Alkaloids: 5 ml of extract evaporated to dryness. Residue heated on a boiling water bath with 2% HCl. Then filtered, treated Mayer’s reagent. Yellow precipitate is indicating the presence of alkaloid.
TABLE 1: PHYTOCHEMICALS OF LUFFA ACUTANGULA
| Test | Test method | Test Result |
| Steroid | Libermann-Burchard Test | + |
| Tannin | Ferric chloride test | + |
| Flavanoid | Shinoda test | + |
| Alkaloid | Mayer’s test | - |
| Glycoside | Killer-Killiani Test | - |
| Anthraquinone | + |
(+) –Presence, (-)-Absence
A Quantitative Test for Luffa acutangula:
Determination of Moisture: 5 gm of material was taken in a pre-weighed Petridis. The Petri dish was placed without lid into an oven at 110 ºC for three hours. The Petri dish was taken out and closed immediately with a lid. The dish was cooled in a desiccator and weighed. The amount of moisture of the material was calculated from the difference in weight.
Total Carbohydrate: A weighed amount of fresh tissue was homogenized with distilled water. The homogenate was filtered using a two-layered cheesecloth. The filtrate was then centrifuged at 10,000 rpm for 15 min. The supernatant was collected, and the volume was made up to 25ml using distilled water. An aliquot of the sample was pipetted out, and 4 ml Anthrone reagent added. It was then kept in a boiling water bath for 10 min. The tubes were cooled, and the absorbance was measured at 530 nm. The amount of total carbohydrate present was determined using the standard graph of glucose.
Estimation of Protein: A total protein present in the plant was estimated by Lowrey’s method. 1 gm powdered plant material was homogenized in 5 ml of 0.1 M PO4 buffer. The homogenate was filtered through double layered cheesecloth and centrifuged at 10,000 rpm for 10 min. The supernatant was collected, and the volume was made up to 1.5 ml by PO4 buffer. After that 1.5 ml of Bradford reagent was added and kept it for 5 min. The absorbance was recorded spectrophotometrically by using appropriate blank at 595 nm. The protein content was calculated from the standard graph of BSA or Bovine Serum Albumin.
GC-MS Analysis:
GC-MS Gas chromatography-mass spectrometry (GC-MS) is a method that combines the features of gas-liquid chromatography and mass spectrometry to identify different Substances within a test sample. The primary goal of instrument analysis is to quantify an amount of substance. This is done by comparing the relative concentrations among the atomic masses in the generated spectrum. Two kinds of analysis are possible, comparative and original. Comparative analysis essentially compares the given spectrum to a spectrum library to see if its characteristics are present for some sample in the library. This is best performed by a computer because there is a myriad of visual distortions that can take place due to variations in scale. Computers can also simultaneously correlate more data (such as the retention times identified by GC), to more accurately relate certain data.
Another method of analysis measures the peaks about one another. In this method, the tallest peak is assigned 100% of the value, and the other peaks being assigned proportionate values. All values above 3% are assigned. The parent peak normally indicates the total mass of the unknown compound. The value of this parent peak can be used to fit with a chemical formula containing the various elements which are believed to be in the compound. The isotope pattern in the spectrum, which is unique for elements that have many isotopes, can also be used to identify the various elements present. Once a chemical formula has been matched to the spectrum, the molecular structure and bonding can be identified and must be consistent with the characteristics recorded by GCMS. Typically, this identification done automatically by programs which come with the instrument, given a list of the elements which could be present in the sample.
Preparation of Extract: 2 μl of the ethanolic extract of Cassia fistula and Luffa acutangula was employed for GC/MS analysis.
Instruments and Chromatographic Conditions: GC-MS analysis was carried out on a GC-MS analysis was carried out on a GC Clarus 500 Perkin Elmer system comprising an AOC-20i autosampler and Gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions:
GC Programme:
- Column: Elite-5MS (5% Diphenyl / 95% Dimethyl polysiloxane), 30 × 0.25 mm × 0.25 µm df
- Equipment: GC Clarus 500 Perkin Elmer
- Carrier gas: 1 ml per min, Split: 10:1
- Detector: Mass detector Turbo mass gold-Perkin Elmer
- Software: Turbomass 5.2
- Sample injected: 2 µl
Oven temperature Programme:
- 110 °C -2 min hold
- Up to 200 °C at the rate of 10 °C/min-No hold
- Up to 280 °C at the rate of 5 °C / min-9 min hold
- Injector temperature 250 °C
- Total GC running time 36 min
MS Programme:
- Library used NIST Version-Year 2005
- Inlet line temperature 200 °C
- Source temperature 200 °C
- Electron energy: 70 eV
- Mass scan (m/z): 45-450
- Solvent Delay: 0-2 min
- Total MS running time: 36 min
RESULTS:
Extraction: The phytochemicals present in the plant material was extracted by the distillation method using sox let apparatus. The solvent, ethanol was used for the separation of the chemical component.
Phytochemical Analysis: Standard phytochemical screening for flavonoid (Ferric chloride test), glycosides (Fehling’s test), alkaloids (Mayer’s test), tannin (Ferric chloride test), saponins (foam test) were done 11-12. The qualitative phytochemical investigations of Cassia fistula Linn extract showed the presence of steroids, flavonoids, saponins, alkaloids, and tannin in the ethanol extracts. Results showed the moisture content of plant was found to be 90%, while the least content was found to be phenol which was only about 0.002 mg/g fresh tissue.
The carbohydrate content is 8.35mg/g and phenol, protein; content is 0.0024, 1.94 mg/g respectively. Biochemical parameters such as protein, carbohydrate, phenol were analyzed, and the results were given
GC-MS Results:
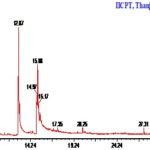
Compounds Identified: Luffa acutangula: GC-MS chromatogram analysis of the ethanolic extract of Luffa acutangula showed ten peaks which indicate the presence of ten phytochemical constituents. On comparison of the mass spectra of the constituents with the help of Dr. Duke’s Phytochemical and Ethnobotanical databases, the ten phytocompounds were characterized and identified. The various phytochemicals which contribute to the medicinal activities of the plant were shown. The mass spectra of all the phytochemicals identified in the whole plant ethanolic extract of Luffa acutangula were shown. Of the ten compounds identified, the most prevailing compounds were4H-Pyran-4-one, 2, 3-dihydro-3, 5-dihydroxy-6-methyl, 9, 12, 15-Octadecatrienoic acid, (Z,Z,Z), 9, 12, 15-Octadecatrienoic acid, (Z,Z,Z), 1, 6, 10, 14-Hexadecatetraen-3-ol, 3, 7, 11, 15-tetramethyl-, (E,E). Among the compounds, three compounds were reported to have 5 Alpha-reductase inhibitor activity, and other three compounds were reported to have anti-microbial activity in general in general, and no activity was reported in Bicyclo [4.4.0]dec-2-ene-4-ol, 2-methyl-9-(prop-1-en-3-ol-2-yl), Spiro [androst- 5- ene- 17, 1'- cyclobutan]- 2'- one, 3-hydroxy-, (3á,17á) of the sample.
Components Identified in the Sample - Luffa acutangula:
TABLE 2: GC MS STUDY
| S. no. | RT | Name of the compound | Molecular formula | MW | Peak Area % |
| 1 | 3.62 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144 | 3.10 |
| 2 | 12.87 | n-Hexadecanoic acid | C16H32O2 | 256 | 36.91 |
| 3 | 14.97 | 9-Octadecynoic acid | C18H32O2 | 280 | 14.49 |
| 4 | 15.08 | 9,12,15-Octadecatrienoic acid, (Z,Z,Z)- | C18H30O2 | 278 | 19.85 |
| 5 | 15.17 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 292 | 9.55 |
| 6 | 20.25 | 1,2-Benzenedicarboxylic acid, diisooctyl ester | C24H38O4 | 390 | 1.37 |
| 7 | 27.31 | Bicyclo[4.4.0]dec-2-ene-4-ol, 2-methyl-9-(prop-1-en-3-ol-2-yl)- | C15H24O2 | 236 | 1.51 |
| 8 | 31.27 | Spiro[androst-5-ene-17,1'-cyclobutan]-2'-one, 3-hydroxy-, (3á,17á)- | C22H32O2 | 328 | 6.45 |
| 9 | 32.21 | Diazoprogesterone | C21H30N4 | 338 | 2.18 |
| 10 | 35.17 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl, (E,E)- | C20H34O | 290 | 4.61 |
FIG. 6: GC-MS CHROMATOGRAM –LUFFA ACUTANGULA
Structure of Compounds having Anti-inflammatory Property:
DISCUSSION: Inspite of tremendous develop-ment in the field of synthetic drugs during the recent era, they are found to have some or other side effects, whereas plants still hold their unique place, by way of having no side effects. Therefore, a systematic approach should be made to find out the efficacy of plants against inflammation to exploit them as herbal anti-inflammatory agents. The potential effect of the ethanolic extract of Luffa acutangula was investigated.
Recent studies suggest that the inflammatory tissue damages are due to the liberation of reactive oxygen species from phagocytes invading the inflammation sites 13-14-15. In addition to this, nitric oxide is also implicated in inflammation, cancer, and other pathological conditions 16. Interactions between superoxide and nitric oxide regulate the vascular tone or inflammation 17-18.
Luffa acutangula contains alkaloids, tannins, flavonoids, terpenes, sugars, and glucosides. Flavonoids have been shown to possess various biological properties related to antioxidant, antinociceptive, and anti-inflammatory mechanisms by targeting reactive oxygen species and prostaglandins which are involved in the late phase of acute inflammation and pain perception.
Using the results obtained from GC-MS, we found the presence of compounds which are active against inflammation.
CONCLUSION: In the present study, we carried out several tests to evaluate the anti-inflammatory activity of Luffa acutangula. Qualitative and Quantitative phytochemical analysis was done. From the results, we found that our plant species contains many effective compounds like flavonoids, alkaloids, tannin, anthroquinone, etc. Further, we analyzed our samples using gas chromatography and mass spectrometry (GC-MS). Based on the GC-MS results obtained we conclude that Luffa acutangula has anti-inflammatory activity.
Further, we planned to do in-vitro studies using animals. There are certain problems associated with the use of animals in experimental pharmacological research such as ethical issues and the lack of rationale for their use.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCE:
- Perumal-Samy RS, Gnacirnuthu I and Sen H: Screening of 34 Indian medicinal plants for antibacterial properties. J Etlmophannacol 1998; 62: I73-182.
- Bhakta T, Mukherjee PK, Saha K, Paland M and Saha BP: Hypoglycemic activity of Cassia fistula (Leglllllinosae) Leaf (Methanol extract) in alloxan induced diabetic rats. J Ethnobot 1997; 9: 35-.38
- Verpoorte R, Heijden RV, Hoopen HJGT and Memelink J: Metabolic engineering of plant secondary metabolite pathways for the production of new chemicals. Biotechnol Lett 1999; 21: 467-479.
- Joshi KP, Chavan D and Patwardhan BW: Molecular markers in herbal drug technology. Cwr Sci 2004; 87: 159-165.
- Chopra NC, Nayar SL and Chopra IC: Glossary of Indian medicinal plants, CSIR, New Delhi 1956; 81.
- Batna PA and Balaraman R: Faculty of Technology and Engineering; Phytomedicine 2005, 12(4): 264-270.
- New medical dictionary. 2nd ed. Oxford and IBH Publishing Co. Pvt. Ltd.: New Delhi 2005.
- Tripathi KD: Essentials of medical pharmacology. 6th ed. Jaypee Brothers Medical Publishers (P) Ltd.: New Delhi; 2008.
- Kokashi CJ, Kokashi RJ and Sharma M: Fluorescence of powdered vegetable drugs inultra-violet radiation, J. Am. Pharm. Assoc 1958; 47: 715-717.
- Farnsworth NR: Biological and phytochemical screening of plants, J. Pharm. Sci. 1966; 55: 225-276.
- Harbone JB: Phenolic Glycosides and their Natural Distribution in the Biochemistry of phenolic compounds, Academic Press, New York, London 1973; 152-162.
- Thomas-Barberan FA, Msonthi JD and Hostettmann K: Phytochemistry 1988, 27(3): 753-755.
- Pratt RT and Chase ER: Fluorescence powder vegetable drugs in particular to development system of identification, J Am Pharm Assoc 1949; 38: 324-331
- Huang SS, Chiu CS and Chen HJ: Antinociceptive activities and the mechanisms of anti-inflammation of asiatic acid in mice. Evidence-Based Complementary and Alternative Medicine pages 2011.
- Vashista PC: Taxonomy of Angiosperms. P.B.M. Press, New Delhi India 1974.
- Balunas MJ and Kinghorn, AD: Drug discovery from medicinal plants. Life Sci 2005; 78: 431-441.
- Trease GE and Evans WC: Pharmacognosy, Bailliere Tindal, East Bourne 1986; 12: 136-204.
- Seshadri VS: Cucurbits. In: Bose, T.K. and M.G. Some (eds.) Vegetable Crops in India. Naya Prakash, Calcutta, 1986: 91-164.
How to cite this article:
Anitha J and Miruthula S: Traditional medicinal uses, phytochemical profile and pharmacological activities of Luffa acutangula Linn. Int J Pharmacognosy 2014; 1(3): 174-83. doi: 10.13040/IJPSR.0975-8232.1(3).174-83.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
174-183
722
3625
English
IJP
J. Anitha * and S. Miruthula
Department of Biotechnology, Arunai Engineering College Thiruvannamalai, Tamil Nadu, India.
mvragav444@yahoo.com
02 December 2013
13 February 2014
28 February 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(3).174-83
01 March 2014