STUDY OF THE LATEST DISCOVERIES OF POTENTIAL ANTIOXIDANT COMPOUNDS TO MODULATE OXIDATIVE STRESS-MEDIATED BY FREE RADICALS LEADING TO NEUROTOXICITY IN PD
HTML Full TextSTUDY OF THE LATEST DISCOVERIES OF POTENTIAL ANTIOXIDANT COMPOUNDS TO MODULATE OXIDATIVE STRESS-MEDIATED BY FREE RADICALS LEADING TO NEUROTOXICITY IN PD
Seyyed Hossein Hassanpour * and Seyyedeh Zeinab Karami
Young Researchers and Elite Club, Yassoj Branch, Islamic Azad University, Yasooj, Iran.
ABSTRACT: Neurodegenerative movement disorder of the central nervous system (CNS) (Parkinson’s disease (PD)) is characterized by necrosis of dopaminergic neurons within the substan tianigra pars compacta are ain the midbrain. The PD cause is still unknown and seems to be multifactorial. Oxidative stress and mitochondrial impairment have been the main consequences, which offer prominent clues regarding the disease mechanisms. The effect of free radicals and oxidative stress on the cascade of events resulting in dopamine cell degeneration in PD has been demonstrated. In-built protective mechanisms, including enzymatic and non-enzymatic antioxidants within the CNS, effectively prevent neuronal cell loss because of free radicals. However, aging is associated with a decrease in the production of these antioxidants. Thus, antioxidant therapy alone or combined with available treatment methods offers an attractive method to treat or prevent neurodegeneration in PD. We summarized the recent discoveries of potential antioxidant agents to modulate free radical-mediated oxidative stress resulting in neurotoxicity in PD.
Keywords: Neuroprotection, PD, Radical, Neuro-degerative, Oxidative stress
INTRODUCTION: Neurodegenerative diseases are chronica and progressive diseases causing neuronal cell death. Alzheimer's disease (AD), Parkinson's disease (PD) Huntington's disease (HD) and cerebral ischemia are caused by oxidative stress. The Brain as a very active organ accounts for only 2% of the body weight and uses 20% of body oxygen and 25% of body glucose while resting. Reactive oxygen species (ROS)
generated in tissues are directly proportional to the tissue oxygen use leading to an increase in intellectual processes, such as planning, thinking and reasoning; therefore, brain is always under oxidation/anti-oxidation process, making it prone to oxidative damage. The brain uses many antioxidant mechanisms for combating ROS. Catalase (CAT) and glutathione peroxidase are found in the cytosol of Brain cells that can hydrolyze H2O2 and reduce organic hydroperoxides, respectively.
Neuronal mitochondria include upper oxide dismutase (SOD) to convert. O2- to H2O2, which is then metabolized by CAT, forming neurotoxic and inflammatory cytokine-inducing. ONOO- from. O2 - and NO. Brain cells are characterized by defense mechanisms to deal with ROS, but when ROS levels are unusually high or antioxidant defense is at a low level, cells bear oxidative damage resulting in neurodegenerative disorders. Exogenous H2O2 can generate ROS over the cellular defense system capacity resulting in apoptotic cell death. No effective drugs are available in the conventional system to deal with or check the onset or progression of neurodegenerative disorders. But, Ayurveda has long employed several herbs for treating and preventing neurodegenerative diseases. A few studies have been done on the neuroprotective activity of these plants. Thus, searching for new therapies associated with few side effects is increasing. Medhya rasayana as Ayurvedic drugs increase physical and mental health and immunity of the body.
Antioxidants have been shown with health-promoting effects protecting against different age-related diseases. They are endogenous or exogenous molecules, which prevent oxidative stress and its related effects on cellular systems. Oxidative imbalance during neurodegenerative processes can be triggered by brain aging, mitochondrial dysfunction, genetic predisposition, free radical generation, and environmental toxins. For overcoming free radical-mediated complications caused by disease processes and drug therapies, antioxidants have been considered as persuasive therapeutics to combat neuronal loss because they can neutralize free radicals. Therefore, we reviewed the recent study on the neuroprotective potential of antioxidants agents in PD experimental models.
MATERIALS AND METHODS:
Chemicals and Reagents:
Immunocyto-fluorescence: Used in western blotting, main antibodies were monoclonal anti- HSP70 (Clone BRM-22, Sigma-Aldrich) and anti-Grp 75 (Mortalin) (Abcam), and anti-α- tubulin (Clone AA13, Sigma-Aldrich). Anti-mouse IgG: HRP (Bangalore genei) and antimouse Alexa Fluor 568 (Invitrogen) were appliedas secondary antibodies. MTT, quercetin, and 1’-1” Diphenyl- 2’-picrylhydrazyl (DPPH) were prepared bySigma-Aldrich. The PCR reagents, such as Random Hexamer Primer, dNTP Mix, Reverse Transcriptase, 100bp ladder and Taq DNA Polymerase, were obtained from Thermo Fisher Scientific. Primers to synthesized DNA for α-HSP, tubulin, and Mortalin were obtained from Bio link, India. Other chemical agents and reagents were prepared in their purest from companies in India.
Preparing CP-MEx, CP-WEx, and CP-EEx: Dried C. pluricaulis was prepared from local Ayurvedic Merchants and identified by The Department of Botanical and Environmental Sciences, Guru Nanak Dev University, India. The plant was powdered, and dry rhizome powder (10 g) was suspended in methanol/ethanol/ distilled water (DW) (100 ml) and stirred for 48 h at 30 ± 5 ºC and then filtrated in sterile conditions, followed by concentrating using the vacuum rotary evaporator (Buchi, Switzerland) at 35 ºC and the pressures of 280, 170 and 60 mbar respectively for methanolic, ethanolic, water extracts. The concentrated extracts thus obtained are air-dried to obtain a powder. The powder was further diluted in respective solvent for obtaining a final concentration of 50 μg/ml each for CPMEx, CP-EEx, and CP-WEx.
Effect of Curcumin (Cur) on Rotenone Toxicity, the LC50: To test effect of CUR on rotenone toxicity, the LC50 (the concentration leading to 80% cell death) of rotenone was used. Pre-treatment cells with CUR at 0, 0.1, 05, 1, and 5 μM for 1 h, followed by exposing to LC80 of rotenone for 24 h was done Z-VAD as a pan-caspase inhibitor and positive control (100 μM) was applied. For measuring cell death, we used Trypan blue exclusion by counting the rate of dead (blue) and live cells in the cultures following rotenone exposure and/or CUR treatment. In Experiment I, incubation of cells with various levels of rotenone (2.5, 5, 50, 100, and 200 nM) was done for 24 h, and for detecting rotenone IC50 value, MTT assay was done. In Experiment II, pre-treatment of the cells was done using various levels of hesperidin (2.5, 5, 10, 20 and 40 μg) for 4 h, followed by incubation with rotenone (effective dosage) for 24 h. This hesperidin effective dose was applied for identifying possible neuroprotective effects on rotenone toxicity.
Cell Culture and Treatments: Human Neuroblastoma cell was prepared from NCCS, India and kept on Dulbecco’s Modified Eagle’s Medium (DMEM) treatedwith10% FCS (Life Technologies), streptomycin (100 U/ml), and gentamycin (100 μg/ml), at 37 ºC and humid environment including 5% CO2. The H2O2 level IC50 for neuroprotection assessments was determined by treatment of the cells with H2O2 (7.5 μM to 1000 μM diluted in medium) at 50% confluency for 24 h in serum-free medium. To obtain the cytotoxicity profile and nontoxic dose of C. pluricaulis extracts were tested at higher doses from 25 to 2000 μg/ml. The Human Neuroblastoma cells were treated with CP-MEx, CP-EEx, CP-WEx, and quercetin at 1.5 μg/ml to 50 μg/ml diluted in the medium for 24 h at 30-40% confluency, followed by subjecting to H2O2 IC50 concentration i.e 250 μM) for 24 h in serum-free medium. The control culture medium with no H2O2 and extract was replaced with a fresh medium.
Cell Viability Assay: To assess cell integrity and extract cytotoxicity, MTT was used to monitor the uptake of the vital mitochondrial dye, MTT via cell mitochondria.
Chemical Standardization of CP-MEX and Nature of Active Components: CP-MEx was phytochemically screened for alkaloids, amino acids, anthroquinones, tannins, flavonoids, saponins, phytosterols, triterpenoids steroids, and reducing sugars using of Harborne method. Then, thin-layer chromatography (TLC) was used with chloroform-methanol (19:1) as the solvent front. TLC plate was exposed to iodine vapours for observation.
Estimation of Activities of Antioxidant Enzymes and Levels of Antioxidants:
Catalase: CAT activity was measured based on the Aebi method. The decomposition rate of H2O2 by catalase was measured spectrophotometrically at 240 nm. The reaction mixture (1 ml) included 0.8 ml phosphate buffer (0.2 M, pH 7.0) containing 12 mM H2O2 as substrate, 100 µl of enzyme sample, and DW to make up the volume. A decrease in absorbance/minute at 240 nm was recorded against the H2O2-phosphate buffer as blank.
Superoxide Dismutase (SOD): SOD was estimated using the Kono method. The inhibitory effects of SOD are assessed on the reduction of nitro blue tetrazolium (NBT) dye using superoxide radicals produced through the autoxidation of hydroxylamine hydrochloride.
The NBT reduction was followed by a rise in absorbance at 540 nm. The reaction mixture included sodium carbonate buffer (50 mM; 1.3 ml), pH 10.0, NBT (96 µM; 500 µl) and triton X-100 (100 µl; 0.6%). The reaction was started by adding100 µl of hydroxylamine hydrochloride (20 mM; pH 6.0). Two minutes later, we added 50 µl of enzyme samples, and the rate of inhibition of NBT reduction was noted.
Reduced Glutathione (GSH) and Glutathione Peroxidase (Gpx): Total GSH was measured using the Sedlak and Lindsay method. Briefly, 100 µl of the samples were added to 500 µl of trichloroacetic acid (50% w/v) and 4.4 ml of 10 mM EDTA, followed by centrifuging at 3000 × g for 15 min. The supernatant was added to 5, 5-dithiobis (2-nitrobenzoic acid) (10 mM 50 µl), and absorbance was read at 540 nm. A standard curve was constructed with pure GSH. GPx activity was assessed indirectly through monitoring the NADPH oxidation. Then, 1 ml of the reaction mixture including 100 mM GSH, 15 nM H2O2 and 15nM NADPH in potassium phosphate buffer (50 mM, pH 7.5) was added to the sample (50 µl) and changes in absorbance was read at 340 nm. GPx activity was considered as 1 µmol NADPH oxidized per min at pH 7.5 at 25 ºC with purified GPx enzyme.
Lipid Peroxidation (LPX): LPx was assessed by the Beuge and Aust Method. Lipid peroxides are unstable, and their decomposition produces a complex series of materials, like reactive carbonyl compounds. Polyunsaturated fatty acid peroxides produce melondialdehyde (MDA) after decomposition, which can generate a 1:2 adduct with thiobarbituric acid (TBA), resulting in a product in red color with absorption of 532 nm. The sample (100 µl) was subjected to incubation with 100 µl of ascorbic acid (1.5 mM), FeSO4 (1 mM), and Tris- HCl Buffer (150 mM, pH 7.1) in a final volume of 1 ml using DDW (15 min /37 ºC). After the addition of trichloroacetic acid (10% w/v; 1 ml), the reaction was stopped, and then, 2 ml thiobarbituric acid (0.375% w/v) was added. The mixture was kept in boiling water-bath for 15 min, and the contents were subjected to cooling off and centrifuging, and the supernatant absorbance was read at 532 nm.
Immuno Cytochemistry: The control and treated cells were rinsed three times using ice-cold 0.1M PBS, followed by fixing with Paraformaldehyde (4%) for 30 min. Permeabilization was done using 0.32% PBST for 15 min. After washing Coverslips for three times with 0.1% PBST, it was blocked with 5% Normal Goat Serum obtained in 0.1% PBST for 1 h at room temperature. Incubation of the Cells was done using mouse anti-NF-200, anti-HSP70 and anti-Mortalin and diluted in 0.1% PBST at 4 ºC for 24 h in a humid chamber. Then, .1% PBST was used to wash Coverslips three times. Secondary antibody (anti-mouse Alexa Fluor 568, anti-rabbit Alexa Fluor 488 and anti-mouse Alexa Fluor 488) was used diluted (1:200) in 0.32% PBST for at room temperature 2h. After washing coverslips with 0.1% PBST thrice, the final washing was done using0.1M PBS. The coverslips were on the slides containing anti-fading mounting media (Sigma), and the fluorescent microscope Nikon E600 was used for observing. The Cool Snap CCD camera was used to capture images, and analysis of the pictures was done using Image J 1.44p, NIH, USA.
Reverse Transcription-PCR: Human Neuro-blastoma cell line cells from 25 cm2 culture flask were homogenized in TRI Reagent (Sigma). RNA extraction and reverse transcription were done based on the manufacturer’s instruction.
RESULT AND DISCUSSION:
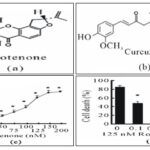
Antioxidant Compounds in Experimental Models of PD: CUR as the component of yellow curry spice Fig. 1A and B obtained from turmeric is used as a food preservative and also for therapeutic purposes in India.
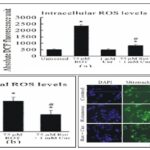
Turmeric was effective against MPTP-induced neurotoxicity in-vivo in a mice model of PD. Also, CUR was effective against rotenone-related toxicity in SH-SY5Y cells, and CUR administration (1 mM) could protect against rotenen-related cell death dose-dependently by reducing the intracellular ROS levels cytochrome release, mitochondrial depolarization, and activation of caspase-9 and caspase-3.
FIG. 1A: CURCUMIN (CUR) WAS PROTECTIVE AGAINST CELL DEATH BY ROTENONE IN-VITRO. (A) ROTENONE CHEMICAL STRUCTURE; (B) DOSE-RESPONSE CURVE INDICATING CELL DEATH CAUSED BY ROTENONE IN SH-SY5Y CELLS. TREATMENT OFSH-SY5Y CELLS WITH ROTENONE WAS DONE FOR 24 H IN 2% FBS OPTI-1 MEDIA. TRYPAN BLUE ASSAY WAS USED TO MEASURE CELL DEATH. DATAARE PRESENTEDAS MEANS ± SEM. CELL DEATH RATIO IN MULTIPLE GROUPS SHOWED A SIGNIFICANT INCREASE COMPARED WITH THE CONTROL GROUP WITHOUT ROTENONE EXPOSURE (*P < 0.05); (C) CURCHEMICAL STRUCTURE ; (D) PRE-TREATMENT OF SH-SY5Y CELLS WITH CURFOR 1 H, FOLLOWED BYEITHER LEFT UNTREATED (CONTROL) OR TREATED WITH 125 NM ROTENONE FOR 24 H. TRYPAN BLUE EX- CLUSION ASSAY WAS USED TO MEASURE CELL DEATH. CELLS TREATED WITH CURINDICATED A SIGNIFICANT DECREASE IN CELL DEATH RATE THAN CELLS EXPOSED TO ROTENONE BUT NO CURE TREATMENT *P < 0.05
FIG. 1B: CURCUMIN (CUR) DECREASED INTRACELLULAR AND MITOCHONDRIAL ROS CONCENTRATIONS. (A) QUANTIFICATION OF DCF FLUORESCENCE IN SH- SY5Y CELLS. CURCAUSED A SIGNIFICANT REDUCTION IN ROTENONE-RELATED INTRACELLULAR ROS ELEVATION; (B) AND (C) CAR CAUSED A SIGNIFICANT REDUCTION IN ROTENONE-RELATED ROWS IN MITOCHONDRIA. DIGITAL PHOTOMICROGRAPH UNDER FLUORESCENT ILLUMINATION INDICATING MITOCHONDRIAL SUPEROXIDE SIGNAL (C) AND THE MEAN OPTICAL DENSITY OF MITOSOX (B). THE ROS CONCENTRATION IS SHOWN BY THE MEAN OF CELLULAR MITOSOX OPTICAL DENSITY IN 10 RANDOMLY SELECTED FIELDS IN EACH CONDITION. VALUES ARE EXPRESSED AS MEAN ± SEM FOR THREE INDIVIDUAL EXPERIMENTS. (*P < 0.05, ROS CONCENTRATIONS AS SIGNIFICANTLY DIFFERENT THAN THE CELLS WITHOUT ROTENONE AND NO CURE TREATMENT. #P < 0.05, ROS CONCENTRATION WAS SIGNIFICANTLY DIFFERENT THAN THE CELLS WITH ROTENONE TREATMENT
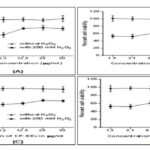
FIG. 2A: THE QUERCETIN EFFECTS ON IMR32 NEUROBLASTOMA CELL VIABILITY IN THE PRESENCE OR ABSENCE OF H2O2. (A) INCUBATINGQUERCETIN WITH IMR32 CELLS (24 H) COULD PROTECT AGAINST THE H2O2CYTOTOXICITY CONCENTRATION-DEPENDENTLY. THERE WAS NO CYTOTOXICITY IN THE CELLS FOLLOWING INCUBATING WITH QUERCETIN ALONE FOR 24 H AT 1.5 -50 ΜG/ML. (B) THE EFFECTS OF CP-MEX ON IMR32 CELL VIABILITY IN THE H2O2PRESENCE OR ABSENCE. INCUBATING THE EXTRACT WITH HEPG2 CELLS (24 H) COULD PROTECT AGAINST THE CYTOTOXIC EFFECT OF H2O2 CONCENTRATION-DEPENDENTLY. THERE WASNO CYTOTOXICITY IN THE CELLS FOLLOWING INCUBATING WITH THE EXTRACT ALONE FOR 24 H AT 1.5 -50 ΜG/ML. (C) THE CP-EEX EFFECTS ON IMR32 CELL VIABILITY IN THE PRESENCE OR ABSENCE OF H2O2. INCUBATING THE EXTRACT WITH HEPG2 CELLS (24 H) COULD PROTECT AGAINST THE H2O2CYTOTOXIC EFFECT CONCENTRATION-DEPENDENTLY. THERE WASNO CYTOTOXICITY IN THE CELLS FOLLOWING INCUBATING WITH THE EXTRACT ALONE FOR 24 H AT 1.5 -50 ΜG/ML. (D) C) THE CP-WEX EFFECTS ON IMR32 CELL VIABILITY IN THE PRESENCE OR ABSENCE OF H2O2. INCUBATING THE EXTRACT WITH HEPG2 CELLS (24 H)COULD PROTECT AGAINST THE H2O2CYTOTOXICITY CONCENTRATION-DEPENDENTLY. THERE WAS CYTOTOXICITY IN THE CELLS FOLLOWING INCUBATING WITH THE EXTRACT ALONE FOR 24 H AT 1.5 -50 ΜG/ML
Fig. 2A and B Convolvulus pluricaulis is commonly prescribed to improve learning and memory and treat mental health disorders. CP-MEX, CP-EEX and CP-WEX have been reported to protect the IMR32 neuroblastoma cell from inducing the toxic effect of H2O2 at a dose-dependent manner (Kshitija et al., 2012). The authors show that CP-MEX 25 µM yield better result compare to CP-EEX and CP-WEX
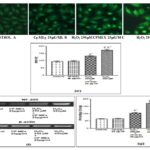
FIG. 2B: NF-200 LOCALIZATION IN IMR 32 NEUROBLASTOMA CELLS (A), CONTROL (B), CP-MEX-EXPOSED (C) CP-MEX+H2O2-EXPOSED (D) H2O2-EXPOSED. CELLS AFTER GROWTH ON COVERSLIPS (N = 5) FOR FOUR DAYS WERE SUBJECTED TO FIXATION AND STAININGFOR NF-200 IMMUNOREACTIVITY (ALEXA FLUOR 488). (E) RELATIVE INTENSITY ASSESSMENT OF NF-200 IMMUNOFLUORESCENCE CONDUCTED BY IMAGEJ 1.44P. (F) REPRESENTATIVE REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION (RT-PCR) INDICATING THE EXPRESSION OF NF-200 AND Β-ACTIN IN THE CONTROL, CPMEX EXPOSED, CP-MEX+H2O2EXPOSED, H2O2EXPOSED IMR 32 NEUROBLASTOMA CELLS. (G) RELATIVE OPTICAL DENSITY ASSESSMENT OF THE NF-200 MEAN EXPRESSION LEVEL IN RT-PCR FOR EACH GROUP PRESENTED AS Β-ACTIN PERCENTAGE. A P < .05 WAS CONSIDERED SIGNIFICANT. A’, SIGNIFICANT CHANGES IN H2O2-EXPOSED CULTURES THAN THE CONTROL CULTURES; A”, SIGNIFICANT CHANGES IN CP-MEX + H2O2EXPOSED CULTURES THAN THE CP-MEX EXPOSED CULTURES; A’”, SIGNIFICANT CHANGES IN H2O2EXPOSED CULTURES THAN THE CP-MEX + H2O2 TREATED CULTURES.
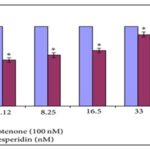
Hesperidin Fig. 1, as flavonone major flavanone availablein citrus and other plants are isolated from peels of Citrus aurantium (bitter orange) 1. It has been reported that this plant exerts several Pharmacological activities, like antioxidant, anti-inflammatory, anti-hyper cholesterolemic, and anti-carcinogenic 2. Antioxidant activity of Hesperidin has been tested and reported to protect SK-N-SH human neuroblastoma cells against cytotoxicity induced by rotenone at the dose of 20 µg 3
FIG. 3: HESPERIDIN EFFECT ON ROTENONE-RELATED DECREASE IN CELL PROLIFERATION OFSK-N-SH NEUROBLASTOMA CELLS. HESPERIDIN ALONE (BLUE COLUMN) (2.5, 5, 10, 20, AND 40 ΜG) HAD NO EFFECTON CELL PROLIFERATION. PRE-TREATMENT WITH HESPERIDIN (2.5, 5, 10 AND 20 ΜG) CAUSED A DOSE-DEPENDENT INCREASE IN CELL PROLIFERATION AGAINST ROTENONE TOXIC EFFECT
Quercetin: Fig. 1B, a major flavonoid has beneficial effects against neural damage in-vitro and in-vivo. Neuroprotective effect of quercetin on PC12 cells in zebrafish was assessed. Quercetin (25, 50 and 100 μM) could prevent 6-OHDA-related PC12 cell apoptosis. In zebrafish, pre-treatment with quercetin (6 and 12 μM) caused a significant attenuation in6-OHDA-stimulated DA ergic neuron loss resulting in its development as a promising candidate for treating PD.
Also, it is effective on hypoxia and ischemia-related neuroprotection by suppressing oxidative stress, improving behavioral function, reducing infarct volume, brain swelling, and cellular injury in-vivo and in-vitro because of its antioxidant functions.
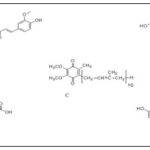
Coenzyme: Q10 (CoQ10; Fig. 1C as an important component of the electron transport chain is associated with ATP production. The therapeutic effect of CoQ10 and decreased CoQ10 was assessed in the MPTP model of Parkinsonism mice. CoQ10 at 1600 mg/kg/day caused significant protection against DA loss after MPTP treatment (10 mg/kg, i.p., each 2 h × 3 doses), and also a significant increase was observed in plasma levels of CoQ10. In a chronic MPTP model (40 mg/kg per day for one month), CoQ10 at 1600 mg/kg/day was found with excellent therapeutic effects through a significant inhibition of striatal DA depletion, dopaminergic neuron loss in the SNpc and forming SNCA aggregate in the dopaminergic neurons of mice.
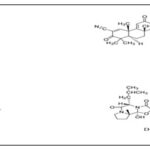
FIG. 4: THE MOLECULAR STRUCTURES RELATED TOCURCUMIN (A); QUERCETIN (B); COENZYME Q10 (C); CREATINE (D) AND RESVERATROL E
Treatment with 1% CoQ10 and 2% creatine Fig. 1D was assessed for one week in an MPTP mouse model of PD. This treatment (40 mg/kg body weight once a day for 28 days byosmotic pumps) caused additive neuroprotective impacts against striatal dopamine depletion and tyrosine hydroxylase (TH) neuron loss in the SNpc, decreased LPx and pathologic SNCA accumulation in SNpc neurons and DAergic neuron losss.
Resveratrol: Fig. 1E as an antioxidant have extensive pharmacological effects, like anti-tumor, anti-mutation, anti-inflammatory, and blood fat regulatory effects. Resveratrol administrated orally or as a resveratrol liposome (20 mg/kg daily) for 14 days could protect DAergic neurons in PD rats. The total ROS LEVELS showed a marked decrease, and the total antioxidant effect of nigral tissues increased markedly. Radical scavenging capacity and antioxidant effects of resveratrol are possibly due to its potent neuroprotection in PD. Also, resveratrol at up to 5 g induced no severe adverse effects in healthy controls in clinical studies due to its safe application for neuroprotection.
Luteolin: Fig 2A and 5 as a polyphenolic compound is available in foods, such as artichoke leaves, peanut shells, celery, parsley, peppers, rosemary, olive oil, lemons, sage, peppermint, and thyme. It (5, 10, and 20 μM) could significantly attenuate an elevation in ROS production and prevent a decrease in mitochondria, GSH and CAT activities in ROS-insulted primary neurons. The neuroprotection of luteolin in ROS-insulted primary neurons can occur by the rebalance of pro-oxidant-antioxidant status.
Brassinosteroids: (BRs) Fig 2 as highly oxygenated steroids are isolated from different vegetables, such as Vicia faba seeds and pollen.
Two BRs (natural) and five analogs (synthetic) were produced, and their neuroprotective effect was assessed against MPP+-related neuronal PC12 cells. The selected BRs and analogs could protect neuronal PC12 cells in response to MPP+ toxicity and exert neuroprotective impacts because of their anti-oxidative effects. Also, the steroid B-ring and lateral chain have anti-oxidative effects, which need to be assessed on animal models of PD in-vivo.
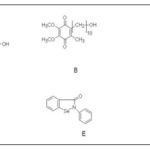
FIG. 5: THE MOLECULAR STRUCTURES OF LUTEOLIN (A); IDEBENONE (B); 3Α- ACETOXYEUDESMA-1,4 (15), 11(13)-TRIEN-12,6A-OLIDE (C); S-ALLYLCYSTEINE (D); EBSELEN (E) AND DIPHENYL DISELENIDE (F)
Idebenone Fig. 2B and Fig. 5, extended lifespan and improved motor function of HtrA2 knockout mice. Oral treatment with idebenone at 500 mg/kg body weight/day could extend lifespan and delay the deterioration of the motor phenotype.
Idebenone acts through the down-regulation of the integrated stress reaction. It can ameliorate disease signs in HtrA2 knockout mice, which indicates that antioxidants are able to delay neuronal degeneration of the striata in these subjects.
This finding indicates the idebenone ability to treat neurodegenerative diseases, such as PD.
3α-Acetoxyeudesma-1,4(15), 11(13)-Trien-12,6a-Olide: (AETO, Fig. 2C and Fig 5, is available in the leaves of Laurus nobilis L. It (0.4, 2, and 10 μM) reduces the active form of caspase-3 and the concentrations of p53 associated with high levels of Bcl-2 dose-dependently. According to the Flow cytometry and Western blot results, AETO markedly inhibited DA-induced apoptosis and suppressed intracellular tyrosinase activity and ROS, SNCA, and quino protein production. Thus, AETO inhibited DA-related apoptosis associated with the inhibition of intracellular tyrosinase activity as well as forming α-syn, quino protein, and ROS, in SH-SY5Y cells.
S-allylcysteine: (SAC, Fig. 2D and Fig. 5) has sulfur obtained from garlic with different biological effects. The most common organosulfur compound found in aged garlic extracts was assessed for its protective effects against MPP+-induced oxidative stress in the C57BL/6J mice striatum. Pre-treatment with SAC at 125 mg/kg i.p. once a day for 17 days and also the administration of MPP + at 0.72 mg/kg i.c.v., caused a significant attenuation in MPP + -related loss of DA levels in striatum (32%). SAC markedly blocked (100% of protection) LPx, and a decrease in of superoxide radical generation was indicated by up-regulating Cu-Zn-SOD activities in MPP+-induced mice. According to Behavioral studies, SAC improved MPP+-related impairment of locomotion (35%). Thus, SAC attenuated MPP +-induced neurotoxicity in the striatal area of mice through its strong antioxidant effects against oxidative stress caused by MPP +.
Organoselenides: ebselen and diphenyl diselenide Fig 2E & F and Fig. 5 were evaluated for neuroprotective effects on differentiated human neuroblastoma SH-SY5Y cells after challenging with 6-OHDA was assessed. Screening of several organoselenides to investigate their antioxidant potential at 3 μM indicated a neuroprotective effect in these cells. These selected organoselenium molecules can be developed as possible pharmacological and therapeutic agents for treating PD.
Deprenyl: Fig 3A and Fig 6 as a selective MAO-B inhibitor can cause a reduction in the progression of symptoms in PD cases. Deprenyl at 10, 20, 50, and 100 μM could up-regulate NQO1 activity and expression, attenuate an elevation in quino protein concentrations in MPP+-treated PC12 cell lines, and protect against oxidative damage through triggering the Nrf2/ARE pathway. Also, deprenyl effects on NQO1 up-regulation showed a great attenuation in Nrf2 siRNA transfected cells. Nrf2/ARE signaling activation with deprenyl in PC12 cell lines is not dependent on MAO-B inhibition.
SCM 198: or 4-guanidino-n-butyl syringate Fig. 3B and Fig. 6 as a chemical compound has cardioprotective impacts in myocardial infarction models and also neuroprotective impacts on rat middle cerebral artery. Pre-treating with SCM198 at 0.1, 1, and 10 mM could markedly increase SOD activity, ameliorate intracellular ROS production, prevent the membrane potential dissipation of mitochondria, decrease apoptotic cell death, down-regulate Bax and up-regulate Bcl-2 mRNA and protein concentrations than 6-OHDA damaged cells. Administration of SCM198 (18 or 60 mg/kg/day) intragastrically for four weeks markedly ameliorated apomorphine-related contralateral rotations of 6-OHDA-lesioned rats. The underlying SCM198 mechanisms to deliver strong euro protective impacts against 6-OHDA-related toxicity in vivo and in vitro can be done through the inhibition of oxidative stress and apoptosis.
Phenothiazine: Fig. 3C and Fig. 6 as an organic compound is available in different anti-psychotic and antihistaminic agents. Phenothiazine at 500 nM exerted strong neuroprotective activities at the cellular level leading to better performance in behavioral tests. Therefore, chain-breaking agents, like phenothiazine are therapeutic compounds for PD because they rescue DAergic toxic effect in-vivo at nanomolar levels according to strong antioxidant effects.
Although the evaluated doses in-vivo and in-vitro in PD models seem far below the toxic levels, side effects, like extrapyramidal symptoms, such as akathisia and tardive dyskinesia, neuroleptic malignant syndrome, hyper-prolactinaemia, and also substantial weight gain should be considered.
The Dl-3n-Butylphthalide: (NBP, Fig. 3D and Fig. 6 evaluated the therapeutic potential by treating clinically affected PD patient. NBP at 0.1, 1.0 and 10 μM reduced MPP + cytotoxicity through the suppression of the permeability transition in mitochondria, decreasing oxidative stress and elevating cellular GSH content in MPP+-received PC12 cells.
Also, NBP reduced SNCA accumulation as the more important component of Lewy bodies. SUN N8075 is a novel antioxidant Fig. 3E and Fig. 6 that is used for stroke patients. It has a potent neuroprotective effect on rodent transient middle cerebral artery occlusion. The underlying neuroprotective mechanism may be through protecting against oxidative stress. The same scientist assessed the neuroprotective activities of SUNN8075 in-vitro on H2O2 related ROS production and 6-OHDA-relatedcell death in human neuroblastoma SH-SY5Y cell lines. Its putative neuroprotective impacts on MPTP-related neurotoxicity were assessed in a mouse model of PD. Treatment with SUNN8075 at micromolar levels significantly reduced the H2O2-induced generation of ROS and was protective against 6-OHDA-related cell death. Intra-peritoneal SUNN8075 injections at 30 mg/kg, two-time sata 5 h interval inhibited LPx in the forebrain of mice in-vivo. Also, SUN N8075 at 10 and 30 mg/kg i.p., (two times) was protective against the MPTP-induced reduction in TH-positive cells within the substantia nigra. Thus, the SUN N8075 protective effects in experimental PD models can be through its antioxidant effects Fig. 3.
FIG. 6: THE MOLECULAR STRUCTURES OF DEPRENYL (A); SCM198 (B); PHENOTHIAZINE (C); DL-3N-BUTYLPHTHALIDE (D), AND SUN N8075 (E)
N-acetyl-l-cysteine: (NAC, Fig. 4A and Fig. 7 as a nutritional supplement and pharmaceutical drug is applied as a mucolytic agent and also for the treatment of paracetamol overdose. Drinking water supplemented with NAC (40 mM) prevents SNCA toxicity. Oxidative stress increases accumulating toxic forms of SNCA DA-dependently. Treatment of transgenic mice aged six weeks to one-year over-expressing wild-type human SNCA with drank water supplemented with NAC indicated that NAC enhanced SN levels of GSH between 5 and 7 weeks of treatment.
The loss of DAergic terminals after one year linked to SNCA over-expression showed a significant attenuation by NAC supplementation. Also, NAC markedly decreased the concentrations of human SNCA in the PDGFb-SNCA transgenic mice brains than the controls. Increased oxidative stress because of early GSH deficiency in the SN can result in increased toxic effect of SNCA in DAergic SN neurons, indicating that techniques for increasing GSH or blocking oxidative stress using NAC can protect against the SNCA toxic effect seen in PD.
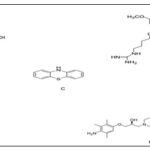
Oleanolic Acidasa triterpenoid has long been applied in Asian medicine because of its anti-inflammatory effects. The synthetic triterpenoid, CDDO-methyl amide (2-cyano-N-methyl-3,12-dioxooleana-1, 9(11)-dien-28 amide; CDDO-MA) Fig 4B and Fig 7 has been shown at least 200,000 times more strong compared to its naturally occurring distant parent.
Oleanolic acid induces NQO-1. CDDO-MA (800 mg/kg of diet) has high neuroprotective impacts on MPTP and 3-nitropropionic acid neurotoxicity. The neuroprotective effects are because of its antioxidant activities, inducing pathways regulated by the Nrf2/ antioxidant response element (ARE) pathway, like GSH synthesis Fig. 4.
FIG. 7: THE MOLECULAR STRUCTURES OF N-ACETYL-L-CYSTEINE (A); CDDO-METHYL AMIDE (B); NP7 (C); BROMOCRIPTINE D
NP7: Fig. 4C and Fig 7 as a novel marine-derived antioxidant has many chemical molecular structures compared to classic phytochemicals, derived from lead optimization system from Streptomyces spp. NP7 protective effects on cell death caused by oxidative stress have been shown in neuronal as well as glial midbrain cultures inparkin null mice (PK-KO). NP7 at 5-10 μM could prevent H2O2-relatedapoptosis and midbrain neuronal and glial cultures necrosis in wild-type and PK-KO mice. NP7 inhibited activation of microglia and the H2O2-related dropout of DA neurons. NP7 can be an appropriate neuro protecting compound against oxidative stress in PD.
Bromocriptine: Fig 4D, Fig. 7 as a DA agonist is applied in PD clinics since 1974 for delaying and minimizing deleterious motor fluctuations following long-term l-dopa therapy. It is a free radical scavenger scavenging hydroxyl and superoxide radicals in-vitro and is an antioxidant inhibiting free radical formation. The cytoprotective mechanism of bromocriptine in response to oxidative damage in PC12 cells treated withH2O2 was investigated. Bromocriptine at 5 μM could up-regulate the activity and expression of NQO1 and attenuate an elevation in the protein-bound quinone in these cells. It also could protect PC12 cell lines against oxidative damage and enhance the nuclear translocation and expression of Nrf2. The Nrf2-inducedcytoprotective and anti-oxidative impacts of bromocriptine are associated with DA receptor activation. Synthetic compounds, like selenium, melatonin, rosmarinic acid, R-alpha-lipoic acid, metal loporphryins compounds, eugenol is oborneol, and metal ion chelators possess neuroprotective impacts in PD models due to their anti-oxidative properties.
CONCLUSIONS: Although there are many drug classes, like l-dopa, monoamine oxidase inhibitors, catechol-O-methyl transferase inhibitors DA agonists, and anti-cholinergic compound for the PD symptomatic treatment, its treatment remains elusive. The accurate nature of the mechanism leading to neurodegeneration in PD is poorly understood, but oxidative stress is an important risk factor for initiating and/or promoting the degeneration of DA neurons. Thus, antioxidant therapy can reduce or prevent the progression of PD. Antioxidant compounds can protect neuronal cells by scavenging free radicals or activating the antioxidant mechanisms. Several in vivo and in vitro animal studies recently declared assessed oxidative stress and ROS-related mechanisms, like metal chelating, radical scavenging, and/or regulating antioxidant enzymes. Nonetheless, oxidative stress is not the only deleterious factor causing the death of DAergic neurons' death. Different mechanisms associated with modulatory impacts on gene expression and signal transduction pathways are linked to the neuroprotection of progressive PD. The discussed compounds can act by regulation of such pathways and anti-oxidative mechanisms that can be synergistic to cause beneficial effects in PD. Also, combination therapy using antioxidants and available drugs is possibly helpful and increases the effectiveness of standard therapy to treat PD. Different types of free radicals are generated and antioxidants are different regarding their capacity to quench such free radicals; thus, supplementation with different antioxidants and using a cocktail of agents, which can target one of the aspects of the degenerative mechanism at the correct time and using proper dose, may lead to better results for achieving promising clinical effects. Nonetheless, investigate the critical factors, such as the optimum doses required, the type of the needed biologically active forms, and the crossing of such compound into the blood-brain barrier for potential therapeutic effects. A comprehensive understanding of the ROS specificity molecular mechanisms in PD and larger epidemiologic investigations and randomized clinical trials on humans and animals should be considered to confirm these results and benefit from treating PD.
Funding/Support: This study was supported by the authors of this article.
ACKNOWLEDGEMENT: The authors would like to thank the martyr Dr. Mustafa Chamran.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest regarding this study.
REFERENCES:
- Chen D, Zhang T and Lee TH: Cellular mechanisms of melatonin: insight from neurodegenerative diseases. Biomolecules 2020; 10(8): 1158.
- Maragkoudaki X, Naylor M, Papacleovoulou G, Stolarczyk E, Rees D, Pombo JM and Williamson C: Supplementation with a prebiotic (polydextrose) in obese mouse pregnancy improves maternal glucose homeostasis and protects against offspring obesity. International Journal of Obesity 2020; 1-12.
- Catanzaro E, Bishayee A and Fimognari C: On a beam of light: photoprotective activities of the marine carotenoids astaxanthin and fucoxanthin in suppression of inflammation and cancer. Marin Drugs 2020; 18(11): 544.
- You Z, Zhang Z, Blagg BS and Dobrowsky RT: KU-596 decreases mitochondrial superoxide and improves bioenergetics following downregulation of manganese superoxide dismutase in diabetic sensory neurons. Experimental Neurology 2019; 313: 88-97.
- Durdik M, Kosik P, Markova E, Somsedikova A, Gajdosechova B, Nikitina E and Belyaev I: Microwaves from mobile phone induce reactive oxygen species but not DNA damage, preleukemic fusion genes and apoptosis in hematopoietic stem/progenitor cells. Scientific Reports 2019; 9(1): 1-12.
- Zhou DR, Eid R, Miller KA, Boucher E, Mandato CA and Greenwood MT: Intracellular second messengers mediate stress inducible hormesis and programmed cell death: a review. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2019; 1866(5): 773-92.
- Teixeira MI, Lopes CM, Amaral MH and Costa PC: Current insights on lipid nanocarrier-assisted drug delivery in the treatment of neurodegenerative diseases. European J of Pharmaceutics and Biopharma 2020; 149: 192-17.
- Anand U, Jacobo-Herrera N, Altemimi A and Lakhssassi N: A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolit 2020; 9(11): 258.
- D'Angelo S: Current evidence on the effect of dietary polyphenols intake on brain health. Current Nutrition & Food Science 2020; 16(8): 1170-82.
- Bhatt KL and Khader A: Conceptual and clinical approach of immunology in ayurveda. Journal of Ayurveda and Integrated Medical Sciences 2020; 5(4): 164-68.
- Ribaudo G, Bortoli M, Pavan C, Zagotto G and Orian L: Antioxidant potential of psychotropic drugs: From clinical evidence to in vitro and in vivo assessment and toward a new challenge for in silico molecular design. Antioxidants 2020; 9(8): 714.
- Prasad S and Srivastava SK: Oxidative stress and cancer: chemopreventive and therapeutic role of triphala. Antioxidants 2020; 9(1): 72.
- Agnihotri A and Aruoma OI: Alzheimer’s disease and Parkinson’s disease: a nutritional toxicology perspective of the impact of oxidative stress, mitochondrial dysfunction, nutrigenomics and environmental chemicals. Journal of the American College of Nutrition 2020; 39(1): 16-27.
- Sali VK and Vasanthi HR: Protective effect of rutin isolated from Spermococe hispida against cobalt chloride-induced hypoxic injury in H9c2 cells by inhibiting oxidative stress and inducing apoptosis. Phytomedicine 2019; 51: 196-04.
- Dhuna K, Dhuna V, Bhatia G, Singh J and Kamboj SS: Neuroprotective effect of convolvulus pluricaulis methanol extract on hydrogen peroxide induced oxidative stress in human imr32 neuroblastoma cell line. Biotechnology Journal International 2012; 192-10.
- Dhuna K, Dhuna V, Bhatia G, Singh J and Kamboj SS: Neuroprotective effect of convolvulus pluricaulis methanol extract on hydrogen peroxide induced oxidative stress in human imr32 neuroblastoma cell line. Biotechnology Journal International 2012; 192-10.
- Umar NM, Parumasivam T, Aminu N and Toh SM: Phytochemical and pharmacological properties of Curcuma aromatica Salisb (wild turmeric). Journal of Applied Pharmaceutical Science 2015; 10(10): 180-94.
- Pranda MA, Murugesan BJ, Knoll AJ, Oehrlein GS and Stroka KM: Sensitivity of tumor versus normal cell migration and morphology to cold atmospheric plasma‐treated media in varying culture conditions. Plasma Processes and Polymers 2020; 17(2): 1900103.
- Verdoodt F, Dehlendorff C, Jäättelä M, Strauss R, Pottegård A, Hallas J and Kjaer SK: Antihistamines and ovarian cancer survival: nationwide cohort study and in-vitro cell viability assay. JNCI: Journal of the National Cancer Institute 2020; 112(9): 964-67.
- Dhuna K, Dhuna V, Bhatia G, Singh J and Kamboj SS: Cytoprotective effect of methanolic extract of Nardostachys jatamansi against hydrogen peroxide induced oxidative damage in C6 glioma cells. Acta Biochimica Polonica 2013; 60(1).
- Saadi S, Hussein RH and Mohammed S: Estimation some antioxidants enzymes in stress patients in babylon city. Indian J of Forensic Med & Toxico 2020; 14(3): 2463-66.
- Irawan B, Labeda I, Lusikooy RE, Sampetoding S, Kusuma MI, Uwuratuw JA and Faruk M: Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: A cross-sectional study. Annals of Medicine and Surgery 2020; 58: 194-99.
- Irawan B, Labeda I, Lusikooy RE, Sampetoding S, Kusuma MI, Uwuratuw JA and Faruk M: Association of superoxide dismutase enzyme with staging and grade of differentiation colorectal cancer: A cross-sectional study. Annals of Medicine and Surgery 2020; 58: 194-99.
- Shamsher N and Prabhu C: Quit puff: a simple method using lipid peroxidative changes in saliva to assess the risk of oral precancerous lesions and oral squamous cell carcinoma in chronic smokers. Indian Journal of Medical and Paediatric Oncology 2020; 41(5): 670.
- Lin Z, Wang Z, Zhou X, Zhang M, Gao D, Zhang L and Miao J: Discovery of new fluorescent thiazole–pyrazoline derivatives as autophagy inducers by inhibiting mTOR activity in A549 human lung cancer cells. Cell death & Disease 2020; 11(7): 1-12.
- Zhang T, Tian C, Wu J, Zhang Y, Wang J, Kong Q and Zhao W: MicroRNA‐182 exacerbates blood‐brain barrier (BBB) disruption by down regulating the mTOR/FOXO1 pathway in cerebral ischemia. The FASEB Journal 2020; 34(10): 13762-75.
- Sharifi-Rad J, El Rayess Y, Abi-Rizk A, Sadaka C, Zgheib R, Zam W and Salehi B: Curcumin and heath: a devoted review to bioactive effects and safety profiles for food, biotechnological and medicinal applications. Frontiers in Pharmacology 2020; 11: 1021.
- Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar, A and Oancea R: Nutraceuticals in neurological disorders. Intern Journal of Molecular Sciences 2020; 21(12): 4424.
- Zhao C, Wang F, Lian Y, Xiao H and Zheng J: Biosynthesis of citrus flavonoids and their health effects. Critical Reviews in Food Science and Nutrition 2020; 60(4): 566-83.
- Slanina T, Petrovičová L and Massányi P: Role of natural substances and vitamin supplementation in tinnitus prevention and treatment. Journal of Microbiology Biotechnology and Food Sciences 2020; 9(4): 987-94.
- Khan A, Jahan S, Imtiyaz Z, Alshahrani S, Antar Makeen H, Mohammed Alshehri B and Rehman MU: Neuroprotection: targeting multiple pathways by naturally occurring phytochemicals. Biomedicines 2020; 8(8): 284.
- Wu D, Zhang S, Sun N, Zhu B and Lin S: Neuroprotective function of a novel hexapeptide qmddq from shrimp via activation of the pka/creb/bndf signaling pathway and its structure–activity relationship. Journal of Agricultural and Food Chemistry 2020; 68(24): 6759-69.
- Abdulwanis Mohamed Z, Mohamed Eliaser E, Mazzon E, Rollin P, Cheng Lian Ee G and Abdull Razis AF: Neuroprotective potential of secondary metabolites from melicope lunu-ankenda (rutaceae). Molecules 2019; 24(17): 3109.
- Bazmandegan G, Shamsizadeh A, FathiNajafi M, Assadollahi Z, Allahtavakoli M, Kamiab Z and Boroushaki MT: Iranian brown propolis possesses neuroprotective effect against ischemic neuronal damage in mice. J of Herb med Pharmacology 2019; 9(2): 121-29.
- Park HW, Park CG, Park M, Lee SH, Park HR, Lim J and Choy YB: Intrastriatal administration of coenzyme Q10 enhances neuroprotection in a Parkinson’s disease rat model. Scientific Reports 2020; 10(1): 1-12.
- Jeong JS, Piao Y, Kang S, Son M, Kang YC, Du XF and Hong SP: Triple herbal extract DA-9805 exerts a neuroprotective effect via amelioration of mitochondrial damage in experimental models of Parkinson’s disease. Scientific Reports 2020; 8(1): 1-13.
- D'Angelo S, Scafuro M and Meccariello R: BPA and nutraceuticals, simultaneous effects on endocrine functions. Endocrine, Metabolic & Immune Disorders-Drug Targets Formerly Current Drug Targets-Immune Endocrine & Metabolic Disorders 2020; 19(5): 594-04.
- Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P and Gupta SC: Health benefits of resveratrol: Evidence from clinical studies. Medicinal Research Reviews 2019; 39(5): 1851-91.
- Ikram M, Park TJ, Ali T and Kim MO: Antioxidant and neuroprotective effects of caffeine against alzheimer’s and parkinson’s disease: insight into the role of nrf-2 and a2ar signaling. Antioxidants 2019; 9(9): 902.
- Venema JH, Giuffrida F. R. A. N. C. E. S. C. O, Paponov I. V. A. N, Albacete A. L. F. O. N. S. O, Perez-Alfocea F R. A. N. C. I. S. C. O, Dodd I. C and Colla G: Rootstock-scion signalling: key factors mediating scion performance. Vegetable grafting: principles and practices. Wallingford UK CABI 94-31.
- Cichon N, Saluk-Bijak J, Gorniak L, Przyslo L and Bijak M: Flavonoids as a natural enhancer of neuroplasticity-an overview of the mechanism of neurorestorative action. Antioxidants 2020; 9(11): 1035.
- Blanco LP, Pedersen HL, Wang X, Lightfoot YL, Seto N, Carmona‐Rivera C and Kaplan MJ: Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme q10 analog idebenone. Arthritis & Rheum 2020; 72(3): 454-64.
- Ahmadian-Moghadam H, Sadat-Shirazi MS and Zarrindast MR: Therapeutic potential of stem cells for treatment of neurodegenerative diseases. Biotechnology Letters 2020; 42(7): 1073-01.
- Koo U, Nam KW, Ham A, Lyu D, Kim B, Lee SJ and Shin J: Neuroprotective effects of 3α-acetoxyeudesma-1, 4 (15), 11 (13)-trien-12, 6α-olide against dopamine-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurochemical research, 36(11), 1991-2001.
- Zhong, J., Xie, J., Xiao, J., Li, D., Xu, B., Wang, X.,& Wang, H. (2019). Inhibition of PDE4 by FCPR16 induces AMPK-dependent autophagy and confers neuroprotection in SH-SY5Y cells and neurons exposed to MPP+-induced oxidative insult. Free Rad Bio and Med 2019; 135: 87-101.
- Yoshitomi R, Nakayama K, Yamashita S, Kumazoe M, Lin TA, Mei CY and Tachibana H: Plasma homocysteine concentration is associated with the expression level of folate receptor 3. Scientific Reports 2020; 10(1): 1-8.
- Radenković N, Kostić M, Đorđević N, Doličanin Z, Soldatović T, Živanović M and Divac VM: Synthesis of new pt (ii) complex bearing organoselenium ligands and evaluation of cytotoxic activity of some structurally related pd (ii) complexes. Macedonian Journal of Chemistry and Chemical Engineering 2020; 39(1): 59-64.
- Lai TT, Yang CM and Yang CH: Astaxanthin protects retinal photoreceptor cells against high glucose-induced oxidative stress by induction of antioxidant enzymes via the PI3K/Akt/Nrf2 pathway. Antioxidants 2019; 9(8): 729.
- Sanders O and Rajagopal L: phosphodiesterase inhibitors for alzheimer’s disease: a systematic review of clinical trials and epidemiology with a mechanistic rationale. J of Alzheimer's Disease Reports 2020; 4(1): 185-15.
- Koppula S, Kumar H, More SV, Kim BW, Kim IS and Choi DK: Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. IJMS 2012; 13(8): 10608-29.
- Ning K, Wang MJ, Lin G, Zhang YL, Li MY, Yang B and Zhu L: NOS-nitric oxide system contributes to a novel antiatherogenic effect of leonurine via inflammation inhibition and plaque stabilization. Journal of Pharma and Experimental Therapeutics 2020; 373(3): 463-75.
- Benfeito S, Oliveira C, Fernandes C, Cagide F, Teixeira J., Amorim R and Remião F: Fine-tuning the neuroprotective and blood-brain barrier permeability profile of multi-target agents designed to prevent progressive mitochondrial dysfunction. European J of Med Chem 2020; 167: 525-45.
- Xie J, Liang R, Wang Y, Huang J, Cao X and Niu B: Progress in Target Drug Molecules for Alzheimer's Disease. Current Topics in Medicinal Chemis 20(1), 4-36.
- Bangwal R, Bisht S, Saklani S Garg S and Dhayani M: Psychotic disorders, definition, sign and symptoms, antipsychotic drugs, mechanism of action, pharmacokinetics & pharmacodynamics with side effects & adverse drug reactions: updated systematic review article. J of Drug Deliv and Therap 2020; 10(1): 163-72.
- Bieri G, Brahic M, Bousset L, Couthouis J, Kramer NJ, Ma R and Shamloo M: LRRK2 modifies α-syn pathology and spread in mouse models and human neurons. Acta Neuropathologica 2019; 137(6): 961-80.
- Koppula S, Kumar H, More SV, Kim BW, Kim IS and Choi DK: Recent advances on the neuroprotective potential of antioxidants in experimental models of Parkinson’s disease. IJMR 2012; 13(8): 10608-29.
- Jantas D, Chwastek J, Grygier B and Lasoń W: Neuroprotective effects of necrostatin-1 against oxidative stress–induced cell damage: an involvement of cathepsin d inhibition. Neurotoxicity Research 2020; 37(3): 525-42.
- Noda Y, Motoyama S, Nakamura S, Shimazawa M and Hara H: Neuropeptide VGF-derived peptide LQEQ-19 has neuroprotective effects in an in-vitro model of amyotrophic lateral sclerosis. Neurochemical Research 2020; 44(4): 897-04.
- Fazary AE, Awwad NS, Ibrahium HA, Shati AA, Alfaifi, MY and Ju YH: Protonation equilibria of n-acetylcysteine. ACS Omega 2020; 5(31): 19598-05.
- Wong YC, Luk K, Purtell K, Burke Nanni S, Stoessl AJ, Trudeau LE and Volpicelli‐Daley LA: Neuronal vulnerability in Parkinson disease: Should the focus be on axons and synaptic terminals. Movement Disorders 2022; 34(10): 1406-22.
- Srivastava AK, Choudhury SR and Karmakar S: Melatonin/polydopamine nanostructures for collective neuroprotection-based Parkinson's disease therapy. Biomaterials Science 2020; 8(5): 1345-63.
- Henchcliffe C and Beal MF: Potential therapies for mitochondrial dysfunction. In Mitochondrial Dysfunction in Neurodegenerative Disorders 2020; 215-30.
- Surinlert P, Kongthong N, Watthanard M, Sae-lao T, Sookbangnop P, Pholpramool C and Tipbunjong C: Styrene oxide caused cell cycle arrest and abolished myogenic differentiation of c2c12 myoblasts. Journal of Toxicology 2020; 2020.
- Son J and Lee SY: therapeutic potential of ursonic acid: comparison with ursolic acid. Biomol 2020; 10(11): 1505.
- Luo YH, Cheng HJ, Tsai, FY, Tsou, TC, Lin SY and Lin P: Primary amine modified gold nanodots regulate macrophage function and antioxidant response: potential therapeutics targeting of nrf2. IJN 2019; 15: 8411.
- Mena MA, Casarejos MJ, Solano R, Rodríguez-Navarro JA, Gómez A, Rodal, I and De Yebenes JG: NP7 protects from cell death induced by oxidative stress in neuronal and glial midbrain cultures from parkin null mice. FEBS letters 2009; 583(1): 168-74.
- Ahmed SS, Pallavi K, Aiswarya PS, Ramakrishnan V and Husain RA: Neuroprotective compounds from marine microorganisms. Encyclopedia of Marine Biotechnology 2020; 3: 1559-79.
- Tábi T, Vécsei L, Youdim, MB, Riederer and Szökő É: Selegiline: a molecule with innovative potential. Journal of Neural Transmission 2020; 127(5): 831-42.
- Castro-González LM, Alvarez-Idaboy JR and Galano, A: Computationally designed sesamol derivatives proposed as potent antioxidants. ACS Omega 2020; 5(16): 9566-75.
- Vasconcelos AR, Dos Santos NB, Scavone C and Munhoz CD: Nrf2/ARE pathway modulation by dietary energy regulation in neurological disorders. Frontiers in Pharmacology 2010; 10: 33.
- Liu H, Yu Q, Fang C, Chen S, Tang X, Ajuwon, KM and Fang R: Effect of selenium source and level on performance, egg quality, egg selenium content, and serum biochemical parameters in laying hens. F 2020; 9(1): 68.
- Lissoni P, Messina G, Borsotti G, Tosatto A, Frigerio S, Tassoni S and Fede DG: Modulation of immune and anti-tumor effects of cancer immunotherapy with anti-pd-1 monoclonal antibodies by the pineal hormone melatonin. Preliminary Clinical Results J Im Allerg 2021; 1(1): 1-6.
- Lv R, Du L, Zhou F, Yuan X, Liu X and Zhang L: Rosmarinic acid alleviates inflammation, apoptosis, and oxidative stress through regulating mir-155-5p in a mice model of parkinson’s disease. ACS Chemical Neuroscience 2020; 11(20): 3259-66.
- Liu Y, Zhu W, Ni D, Zhou Z, Gu, JH, Zhang W and Liu F: Alpha lipoic acid antagonizes cytotoxicity of cobalt nanoparticles by inhibiting ferroptosis-like cell death. Journal of Nanobiotechnology 2019; 18(1): 1-14.
- Fekrirad Z, Gattali B and Kashef N: Quorum sensing-regulated functions of Serratia marcescens are reduced by eugenol. Iranian Journal of Microbiology 12(5): 451-59.
- Rosato A, Sblano S, Salvagno L, Carocci A, Clodoveo M L, Corbo F and Fracchiolla G: Anti-biofilm inhibitory synergistic effects of combinations of essential oils and antibiotics. Antibiotics 2010; 9(10): 637.
- Tosato M and Di Marco V: Metal chelation therapy and Parkinson’s disease: A critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 2019; 9(7): 269.
How to cite this article:
Hassanpour SH and Karami SZ: Study of the latest discoveries of potential antioxidant compounds to modulate oxidative stress mediated by free radicals leading to neurotoxicity in pd. Int J pharmacognosy 2021; 8(11): 462-75. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(11). 462-75.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
02
462-475
1143 KB
747
English
IJP
Seyyed Hossein Hassanpour * and Seyyedeh Zeinab Karami
Young Researchers and Elite Club, Yassoj Branch, Islamic Azad University, Yasooj, Iran.
Dr.hossein1366@yahoo.com
13 September 2021
11 November 2021
20 November 2021
10.13040/IJPSR.0975-8232.IJP.8(11).462-75
30 November 2021