IMPORTANCE OF ANTIMICROBIAL AGENTS FROM PLANTS IN PRESENT SCENARIO: A REVIEW
HTML Full TextIMPORTANCE OF ANTIMICROBIAL AGENTS FROM PLANTS IN PRESENT SCENARIO: A REVIEW
Chetan Savant * 1, Venkatesh 1, Basheer Ahmed Abdulaziz Mannasaheb 2 and Hanumanthachar Joshi 1
Sarada Vilas College of Pharmacy 1, Krishnamurthypuram, Mysore - 570004, Karnataka, India.
East West College of Pharmacy 2, Anjana Nagar, Bangalore - 560091, Karnataka, India.
ABSTRACT: Since, human existence on earth, plants are being used for the treatment of various diseases. The specific uses of plants are mentioned in the traditional system of medicines like Ayurveda, Siddha, Unani, etc. Now a day’s plants are getting more important as medicines because plants are having fewer side effects, cost-effective, long effective life span and safe, etc. The present review covers the brief history of antimicrobials and list of plants as antimicrobials, approved, and banned antimicrobial drugs. In the present scenario, the use of herbal drugs is increasing progressively worldwide. Hence, this review shows useful data for researchers to develop new anti-microbials from plant origin.
| Keywords: |
Approved anti-microbial drugs, Banned anti-microbial drugs, Anti-microbial plants, Bacterial resistance
INTRODUCTION: According to the WHO, over 80% of the world’s population relies on traditional forms of medicine, largely plant-based to meet primary health care needs. In India, the collection and processing of medicinal plants and plant products contribute a major part each year to the national economy, as a source of both full and part-time employment. Plants are one of the most important sources of medicines. The application of plants as medicines has dated back to the prehistoric period. In India the references to the curative properties of some herbs in the Rig-Veda seems to be the earliest records of use of plants in medicines. The medicinal plants are extensively utilized throughout the world in two distinct areas of health management; traditional system of medicine and modern system of medicine 1.
Clinical microbiologists have two reasons to be interested in the topic of antimicrobial plant extracts. First, these phytochemicals will find their way into the arsenal of antimicrobial drugs prescribed by physicians; several are already being tested in humans. It is reported that, on average, two or three antibiotics derived from micro-organisms are launched each year 2. After a downturn in that pace in recent decades, the pace is again quickening as scientists realize that the effective life span of any antibiotic is limited. Worldwide spending on finding new anti-infective agents (including vaccines) is expected to increase 60% from the spending levels in 1993.3
New sources, especially plant sources, are also being investigated. Second, the public is becoming increasingly aware of problems with the over prescription and misuse of traditional antibiotics. Also, many people are interested in having more autonomy over their medical care. A multitude of plant compounds (often of unreliable purity) is readily available over-the-counter from herbal suppliers and natural-food stores, and self-medication with these substances is commonplace.
Brief History: Approximately 3000 plants species are known to have medicinal properties in India. The Rigveda (3700 B.C.) mentions the use of medicinal plants. Our traditional systems of medicines, viz., Ayurveda, Yunani, Siddha, and Homeopathy, etc. use herbs for treatment 1. It is estimated that there are 250,000 to 500,000 species of plants on Earth 4. A relatively small percentage (1 to 10%) of these is used as foods by both humans and other animal species. It is possible that even more are used for medicinal purposes 5. Hippocrates (in the late fifth century B.C.) mentioned 300 to 400 medicinal plants 6. In the first century A.D., Dioscorides wrote De Materia Medica, a medicinal plant catalog which became the prototype for Modern Pharmacopeia’s. The Bible offers descriptions of approximately 30 healing plants. Frankincense and myrrh reported to have antiseptic properties; they were even employed as mouthwashes.
The fall of ancient civilizations forestalled Western advances in the understanding of medicinal plants, with much of the documentation of plant pharmaceuticals being destroyed or lost 7. During the Dark Ages, the Arab world continued to excavate their own older works and to build upon them. Asian cultures were also busy compiling their pharmacopeia. In the West, the Renaissance years saw a revival of ancient medicine, which was built largely on plant medicinal. North America’s history of plant medicinal use follows two strands-their use by indigenous cultures (Native Americans), dating from prehistory 8 and an “alternative” movement among Americans of European origin, beginning in the 19th century. Native American use of plant medicinal has been reviewed extensively in a series of articles by Moerman 5. He reported that while various Native American groups have used 1,625 species of plants as food, 2,564 have found use as drugs 9.
According to his calculations, this leaves approximately 18,000 species of plants which were used for neither food nor drugs. Speculations as to how and why a selected number of plant species came into use for either food or drugs. In 1861 Holmes wrote, “If the whole Materia Medica as now used could be sunk to the bottom of the sea, it would be all the better for mankind-and all the worse for the fishes.” In 1887 alternative practitioners compiled their catalogs, notably the Homeopathic Pharmacopoeia of the United States.
Mainstream medicine is increasingly receptive to the use of antimicrobial and other drugs derived from plants, as traditional antibiotics (products of microorganisms or their synthesized derivatives) become ineffective and as new, particularly viral, diseases remain intractable to this type of drug. Another driving factor for the renewed interest in plant antimicrobials in the past 20 years has been the rapid rate of (plant) species extinction. (10) There is a feeling among natural-products chemists and microbiologists alike that the multitude of potentially useful phytochemical structures which could be synthesized chemically is at risk of being lost irretrievably 11.
There is a scientific discipline known as ethnobotany (or ethnopharmacology), whose goal is to utilize the impressive array of knowledge assembled by indigenous peoples about the plant and animal products they have used to maintain health. Lastly, the ascendancy of the human immunodeficiency virus (HIV) has spurred intensive investigation into the plant derivatives which may be effective, especially for use in underdeveloped nations with little access to expensive Western medicines 12.
Development of Antibiotics: There are two broad routes to drug discovery: the natural product and the synthetic route.
Natural Route: The antibiotic discovery from natural source began with the discovery of penicillin from a mold by Alexander Fleming in 1928. Natural drug discovery involves the exploration of natural sources such as soil, bacteria, mold, and trees for new chemical entities which could be further developed and licensed for clinical use. Majority of the antibiotics used worldwide are of natural origins 13, 14, 15.
In this method, natural products or extracts from the herbs, bacteria and so forth are first tested for antibacterial activity, followed by purification and characterization of promising candidates 16. However, the ease with which pathogens acquire resistance to antibacterial compounds has resulted in a continuous search for a lasting solution.
Semi-Synthetic Route: The semi-synthetic route was ventured into due to antibiotic resistance to natural antibiotics and instability to acidic medium 17. Following the purification and identification of the pharmacophore of penicillin 18 and the understanding of the mechanism of resistance by lactamase enzymes 19, 20 the pharmaceutical companies began to modify the antibiotic molecules in such a way as to retain activity while resisting inactivation by microbial enzymes.
This method became possible when the structure of the beta-lactam ring which is the pharmacophore of penicillin was determined. After it was discovered that the beta-lactamase enzymes hydrolyze the β-lactam bond to render the pharmacophore inactive 20 attempts were made to introduce a moiety that would stabilize the pharmacophore against the attack of the enzymes. Aminopenicillins and methicillin are also examples of derivatives produced by the synthetic route. 18
The modification affected the properties of the compounds as it pertains to acid and alkaline stability and stability to degradation by bacterial enzymes. Aminopenicillin is stable to stomach acid and can be administered orally while methicillin is stable against the beta-lactamase enzyme. Thus methicillin (carboxypenicillin) and ticarcillin were fashioned from penicillin for the treatment of infections due to resistant Staphylococcus and pseudomonas respectively 13. The synthesis of other semi-synthetic antibiotics was also reported. Minocycline and doxycycline are both tetracycline analogs 19. Second and third generation cephalosporins were possible through semi-synthesis 21, 22.
Semi-synthesis was not, however, very effective in combating the dynamic resistance posed by the microbial world. Cross-resistance between different antibiotics of the same family was a major problem. It was thought that the ease with which bacteria develop resistance to natural compounds could be due to co-evolution between the organism and the antibacterial. The companies thought of introducing xenobiotics which are compounds that are not known to nature 23.
Synthetic Route: The sulphonamides were the first antibiotics that were synthesized. Prontosil, a component of a dye which was prepared for use in the textile industry was accidentally found to be active against microorganisms by a German chemist 24. The next product of pure synthesis did not arrive until 1962 when the quinolones were synthesized. It took about 40 years for another synthesized compound Linezolid (oxazolidinone) to be approved for clinical use 25.
The rapid development and promises of the synthetic route were thought to pull the pharmaceutical industry away from the natural drug discovery route. Combinatorial chemistry has played a large role in the search for drug leads, but it was not until 2005 when the first product of combinatorial chemistry as a model of drug discovery was approved for clinical use. The FDA approved sorafenib (Nexavar) for the treatment of advanced renal cancer, and it received its marketing license in Europe in 2006.26, 27
Antimicrobial Resistance:
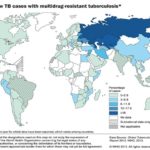
Resistance in Bacteria: WHO’s 2014 report on global surveillance of antimicrobial resistance is happening right now, across the world, and is putting at risk to treat common infections in the community and hospitals Fig. 1.
- The treatment with third-generation cephalosporins has been a failure for gonorrhea in many countries due to which there is increased complications like infertility, adverse pregnancy outcomes, and neonatal blindness.
- coli caused urinary tract infections to become resistance to fluoroquinolones is also very widespread.
- The severe infections caused by Staphylococcus aureus becomes resistance to first-line antibiotics in many populations.
- In most of the countries, life-threatening infections caused by common intestinal bacteria are resistant to carbapenem antibiotics.
Resistance in HIV: Resistance is a pressing concern for the treatment of HIV infection, after the rapid expansion in access to antiretroviral drugs in recent years; national surveys are underway to detect and monitor resistance.
- At the end of 2011, more than 8 million people were receiving antiretroviral therapy in low- and middle-income countries to treat HIV. Although it can be minimized through good programmed practices, some amount of resistance to the medications used to treat HIV is expected to emerge.
- Analysis of data from WHO surveys that target people who have been recently infected with HIV indicates increasing levels of resistance to the non-
- Nucleoside reverse transcriptase (NNRTI) class of drug used to treat HIV. This increase is particularly noticeable in Africa, where the prevalence of resistance to NNRTI reached 3.4% in 2009.
- There is no clear evidence of increasing levels of resistance to other classes of HIV drugs. Of 72 surveys of transmitted HIV drug resistance conducted between 2004 and 2010, 20 (28%) were classified as having moderate (between 5% and 15%) prevalence of resistance.
- Available data suggest that there is an association between higher levels of coverage of antiretroviral therapy and increased levels of HIV drug resistance28.
FIG. 1: PERCENTAGE OF NEW TB CASES WITH MULTI-DRUG RESISTANT TUBERCULOSIS
TABLE 1: THERE ARE NUMEROUS PLANTS WHICH ARE PROVED FOR THEIR ANTIMICROBIAL ACTIVITY 29
| S. no. | Plant name | Part used | Extract | Active against |
| 1 | Stephania glabra | Rhizome | Ethanol, | Staphylococcus mutans, Staphylococcus epidermidis, Escherichia coli, Klebsiella pneumonia |
| 2 | Woodfordia fruticosa | Stem and flowers | Petroleum ether, chloroform, diethyl ether & acetone | Escherichia coli, Bacillus subtili,
Staphylococcus aureus and Pseudomonas aeruginosa |
| 3 | Betula utilis | Bark | Petroleum ether, chloroform,
methanol, ethanol and water |
Escherichia coli, Klebsiella pneumonia, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella paratyphi, Salmonella typhi, Salmonella typhimurium, Shigella flexneri, Shigella sonnei, Staphylococcus aureus, Streptococcus faecalis, Shigella boydii, Citrobacter sp., Salmonella paratyphi B and Shigella boydii |
| 4 | Calotropis gigantean | Latex | Ethanol | Candida albicans, Saccharomyc escerevisiae, T. mentagrophytes, T. rubrum, Aspergillus fumigates, Aspergillus lavus, Aspergillus niger, Penicillium chrysogenum |
| 5 | Nelumbo nucifera | Flowers. (Both white and pink) | Hydroethanolic extract | Escherichia coli, Klebsiella pneumonia,
Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus |
| 6 | Hemidesmus indicus | Roots | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 7 | Eclipta alba | Fruits | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 8 | Coscinium fenestratum
|
Stems | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 9 | Cucurbita pepo | Seeds | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 10 | Tephrosia
purpura |
Roots | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 11 | Mentha piperita | Leaves | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 12 | Pongamia pinnata | Seeds | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 13 | Symplocos racemosa
|
Barks | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 14 | Euphorbia hirta | Roots | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 15 | Tinospora
cordyfolia |
Roots | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 16 | Thespesia populnea, | Roots | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 17 | Jasminum officinale | Flowers | Ethanol | Propionibacterium acnes and
Staphylococcus epidermidis |
| 18 | Albizia
lebbeck (L.), |
Leaf | Benzene, water, acetone | Escherichia coli (MDR),
Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 19 | Cleistanthus collinus (Roxb.)., | Leaf | Benzene, water, acetone | Escherichia coli (MDR),
Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans. |
| 20 | Emblica officinalis (Phyllanthus emblica L.), | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 21 | Eucalyptus deglupta (Eucalyptus tereticornis), | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 22 | Eupatorium odoratum (Chromolaena odorata ), | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 23 | Oxalis corniculata L., | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans. |
| 24 | Hevea brasiliensis and | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 25 | Lantana camara L | Leaf | Benzene, water, acetone | Escherichia coli (MDR), Staphylococcus aureus (MDR), Klebsiella pneumoniae, Bacillus cereus, Vibrio cholerae and Candida albicans |
| 26 | Acacia nilotica,
|
Leaf, root, bark | Methanol | Bacillus subtilis, Escherichia coli,
Pseudomonas fluorescens, Staphylococcus aureus and Xanthomonas axonopodis pv. Malvacearum |
| 27 | Sida cordifolia | Leaf, root, bark | Methanol | Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens, Staphylococcus aureus and Xanthomonas axonopodis pv. Malvacearum |
| 28 | Tinospora cordifolia | Leaf, root, bark | Methanol | Bacillus subtilis, Escherichia coli,Pseudomonas fluorescens, Staphylococcus aureus and Xanthomonas axonopodis pv. Malvacearum |
| 29 | Withania somnifer | Leaf, root, bark | Methanol | Bacillus subtilis, Escherichia coli, Pseudomonas fluorescens, Staphylococcus aureus, and Xanthomonas axonopodis PV. Malvacearum |
| 30 | Ziziphus mauritiana | Leaf, root, bark | Methanol | Bacillus subtilis, Escherichia coli,Pseudomonas fluorescens, Staphylococcus aureus and Xanthomonas axonopodis pv. Malvacearum |
| 31 | Adhatoda vasica, | Leaves | Aqueous extract | M. tuberculosis, M. fortuitum |
| 32 | Acalypha indica, | Leaves | Aqueous extract | M. tuberculosis, M. fortuitum |
| 33 | Allium cepa, | - | Aqueous extract | M. tuberculosis, M. fortuitum |
| 34 | Allium sativum | Bulb | Aqueous extract | M. tuberculosis, M. fortuitum |
| 35 | Aloe vera | gel | Aqueous extract | M. tuberculosis, M. fortuitum |
| 36 | Mikania glomerata | - | 70% methanol | Staphylococcus aureus strains |
| 37 | Syzygium aromaticum | - | 70% methanol | Staphylococcus aureus strains |
| 38 | Allium sativum | - | 70% methanol | Staphylococcus aureus strains |
| 39 | Cymbopogon citratus | - | 70% methanol | Staphylococcus aureus strains |
| 40 | Zingiber officinale | - | 70% methanol | Staphylococcus aureus strains |
| 41 | Baccharis trimera | - | 70% methanol | Staphylococcus aureus strains |
| 42 | Mentha piperita | - | 70% methanol | Staphylococcus aureus strains |
| 43 | Arnebia nobilis | Crude extract | Ethanol | P. aeruginosa, Staphylococcus aureus
positive, Escherichia coli, S. aureus negative and fungi Candida albicans. |
| 44 | Garcinia indica, | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans |
| 45 | Boehavia diffusa, | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans |
| 46 | Solanum albicaule | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans. |
| 47 | Vitex negundu | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans. |
| 48 | Bunium
persicum |
Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans. |
| 49 | Acacia concinna | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative andfungi Candida albicans. |
| 50 | Albizia lebbeck | Crude extract | Ethanol | Pseudomonas aeruginosa, Staphylococcus aureus
positive, Escherichia coli, Staphylococcus aureus negative and fungi Candida albicans. |
| 51 | Lantana indica roxb | Leaves | Ethyl acetate and methanol | Staphylococcus aureus, Bacillus
subtilis, Steptococcus pyrogens, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Aspergillus niger and Candida albicans |
| 52 | Jatropha curcas | Stem Bark | Crude ethanolic, methanolic and water extracts | Staphylococcus aureus, Pseudomonas
aeruginosa, Escherichia coli, Streptococcus faecalis, Staphylococcus epidermidis, Shigelladysenteriae, Micrococcus kristinae |
| 53 | Azadirachta indica Linn. | Leaves, seed, oil | Hexane, chloroform, and methanol | Escherichia, Klebsiella pneumonia, Proteus vulgaris, Micrococcus luteus, Bacillus subtilis, Enterococcus faecalis and Streptococcus faecalis |
| 54 | Ficus carica | Leaves | Methanol | Streptococcus mutans, Streptococcus sanguinis, Streptococcus sobrinus, Streptococcus ratti , Streptococcus criceti, Streptococcus anginosus
and Streptococcus gordonii, A. actinomycetemcomitans, Fusobacterium nucleatum, Prevotella intermedia and Porphyromonas gingivalis |
| 55 | Bidens
pilosa |
Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 56 | Bixa Orellana | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 57 | Cecropia peltata | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 58 | Cinchona
officinalis |
Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 59 | Gliricidia sepium | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 60 | Jacaranda mimosifolia | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 61 | Justicia secunda | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 62 | Piper fulcrum | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 63 | P. paniculata | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
| 64 | Spilanthes americana | Whole plant parts | Ethanol, water and hexane | Staphylococcus aureus, Streptococcus β hemolític, Bacillus cereus, P. aeruginosa, and Escherichia coli, and one yeast Candida albicans |
TABLE 2: LIST OF ANTIMICROBIAL DRUGS APPROVED SINCE 2000 BY FDA 30
| Year
Approved |
Drug
Name |
Class | Bacteria
Type |
Lead
source |
NP-Lead source
organism |
| 2000 | Linezolid | Oxazolidinone | G +ve | S | --------------- |
| 2001 | Telithromycin | Macrolide | G +ve/G -ve | NP derived | Actinomycete |
| 2002 | Biapenem | Carbapenem | G +ve/G -ve | NP derived | Actinomycete |
| 2002 | Ertapenem | Carbapenem | G +ve/G -ve | NP derived | Actinomycete |
| 2002 | Prulifloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2002 | Pazufloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2002 | Balofloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2003 | Daptomycin | Lipopeptide | G +ve | NP derived | Actinomycete |
| 2004 | Gemifloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2005 | Doripenem | Carbapenem | G +ve/G -ve | NP derived | Actinomycete |
| 2005 | Tigecycline | Tetracycline | G +ve/G -ve | NP derived | Actinomycete |
| 2007 | Retapamulin | Pleuromutilin | G +ve | NP derived | Fungus |
| 2007 | Garenoxacin | Quinolone | G +ve/G -ve | s | ------------- |
| 2008 | Ceftobiprole medocaril | Cephalosporin | G +ve/G -ve | NP derived derived | Fungus |
| 2008 | Sitafloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2009 | Tebipenem pivoxil | Carbapenem | G +ve/G -ve | NP derived | Actinomycete |
| 2009 | Telavancin | Glycopeptide | G +ve | NP derived | Actinomycete |
| 2009 | Antofloxacin | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2009 | Besifloxacine | Fluoroquinolone | G +ve/G -ve | S | ------------- |
| 2010 | Ceftaroline fosamil | Cephalosporin | G +ve/G -ve | NP derived | Fungus |
| 2011 | Fidaxomicin | Tiacumicin | G +ve | NP derived | Actinomycete |
| 2012 | Bedaquiline | Diarylquinoline | G +ve (TB) | S | ------------- |
Recently Approved Antimicrobial Drugs:
- Flublok (seasonal influenza vaccine); Protein Sciences; for the active immunization against influenza virus subtypes A and type B, Approved January 2013.
- Luzu (luliconazole) Cream 1%; Valeant Pharmaceuticals; for the treatment of interdigital tinea pedis, tinea cruris, and tinea corporis, November of 2013.
- Olysio (simeprevir); Janssen Therapeutics; for the treatment of hepatitis C, November of 2013.
- Sitavig (acyclovir) buccal tablets; BioAlliance Pharma; for the treatment of recurrent herpes labialis in adults, Approved April 2013.
- Sovaldi (sofosbuvir); Gilead Sciences; for the treatment of hepatitis C, December of 2013.
- VariZIG, Varicella Zoster Immune Globulin (Human); Cangene; for the post-exposure prophylaxis of varicella zoster (chickenpox), Approved January 2013.
- Vibativ (telavancin); Theravance; for the treatment of hospital-acquired and ventilator-associated bacterial pneumonia caused by aureus, Approved June 2014.
- Dalvance (dalbavancin); Durata Therapeutics; for the treatment of acute bacterial skin and skin structure infections, Approved May 2014.
- Impavido (miltefosine); Knight Therapeutics; for the treatment of visceral, cutaneous and mucosal leishmaniasis, Approved March 2014.
- Jublia (efinaconazole) 10% topical gel; Valeant Pharmaceuticals; for the treatment of onychomycosis of the toenails, Approved June 2014.
- Metronidazole 1.3% Vaginal Gel; Actavis, Inc.; for the treatment of bacterial vaginosis, Approved April 2014. 31
The Government of India to Ban Over-The-Counter Sale of 92 Antibiotics: Resistance to antibiotics is becoming a serious threat for India because of popular habit to pop pills at will. Even the World Health Organization (WHO) recently warned that the world is staring at a post-antibiotic era when common infections will no longer have a cure.
WHO director-general Dr. Margaret Chan had said, "The world is on the brink of losing these miracle cures." Even director of Centres for Disease Control Atlanta chief Dr. Thomas R Frieden, who was in India said that drug resistance due to irrational use of antibiotics, will increase in the future. Drug Controller General of India (DCGI) Dr. G N Singh has written to the Union health minister to notify a new schedule H1 in the Drugs and Cosmetics Rules. Once notified, following clearance from the law ministry, these drugs cannot be sold without a prescription. The drugs will also have to carry a prominent label in red color on the left corner with the following warning: "It is dangerous to take this prescription except by medical advice and not to be sold by retail without the prescription of the registered medical practitioner." He added, "These drugs will only be sold against a prescription that the chemist will have to retain.
The drugs to come under H1 includes Moxifloxacin, Meropenem, Imipenem, Ertapenem, Doripenem, Colistin, Linezolid, Cefpirome, Gentamicin, Amikacin, Penicillin, Oxacillin, Zolpidem, Cefalexin, Norfloxacin, Cefaclor, Cefdinir, Tigecycline, Tobramycin, Tramadol, and Vancomycin 32.
List of Banned Drugs in India:
- Furazolidone: Furazolidone is a nitrofuran antibacterial. It was marketed by Roberts Laboratories under the brand name Furoxone and by GlaxoSmithKline as Dependal- M.
- Nitrofurazone: Nitrofurazone is bactericidal for most pathogens that commonly cause surface skin infections, including aureus, S. Escherichia coli, Clostridium perfringens, Enterobacter aerogenes, and Proteus organisms. It was marketed with brand name Furacin Soluble Dressing; Furacin Topical Cream; Furacin Topical Solution.
- Quiniodochlor : it was antibacterial and antibiotic marketed with brand names Betnovate-C (20 gm), Enteroquinol, Dermican, Dexaquin, Quinoderm, Skycet Gel, Dexaquin33.
Importance of Plants as Antimicrobial: Hong-Xi Xu et al., 2001 34 in their research work showed, thirty-eight plant-derived flavonoids representing seven different structural groups were tested for activities against antibiotic-resistant bacteria using the disc-diffusion assay and broth dilution assay. Among the flavonoids examined, four flavonols (myricetin, datiscetin, kaempferol, and quercetin) and two-flavones (flavone and luteolin) exhibited inhibitory activity against methicillin-resistant Staphylococcus aureus (MRSA).
Myricetin was also found to inhibit the growth of multidrug - resistant B. cepacia, vancomycin - resistant enterococci (VRE) and other medically important organisms such as Klebsiella pneumoniae and Staphylococcus epidermidis. Myricetin was bactericidal to B. cepacia. The results of the radiolabel incorporation assay showed that myricetin inhibited protein synthesis by B. Cepacia.
Ortega-Ramirez LA et al., 35 and Seow YX et al., 36 proposed, medicinal plants traditionally used to treat health disorders and to prevent diseases, as a source of bioactive compounds having food additive properties. Medicinal plants are rich in terpenes and phenolic compounds that present antimicrobial and antioxidant properties and also the essential oils derived from plants exhibit biological activities, including antioxidant, anticancer, and antimicrobial activity, use of these substances as antimicrobials in food products, factors that affect their efficacy, synergism between components or with available food preservatives as well as the challenges and future directions of using essential oils and phytochemicals as natural food preservatives.
A study was done by Nautiyala et al., in which it was observed that 1 h treatment with medicinal smoke, released by burning wood and mixture of odoriferous and medicinal herbs, lead to 94% reduction of bacterial counts by 60 min. The absence of pathogenic bacteria (Corynebacterium urealyticum, Enterobacter aerogenes, Enterobacter aerogenes, Klebsiella mobilize, Kocuria rosea, Pseudomonas syringae pv. persicae, S. lentus) in the open room even after 30 days is indicative of the bactericidal potential of the medicinal smoke treatment. Medicinal smoke from natural herbal products has the potential for use as a smoke/inhalational form of drug delivery 37.
Plant-based antimicrobials represent a vast untapped source for medicines. Continued and further exploration of plant antimicrobials needs to occur. Plants based antimicrobials have enormous therapeutic potential. They are effective in the treatment of infectious diseases while simultaneously mitigating many of the side effects that are often associated with synthetic anti-microbials. They are effective, yet gentle. Many plants have tropisms to specific organs or systems in the body. Phytomedicines usually have multiple effects on the body. Their actions often act beyond the symptomatic treatment of disease. An example of this is Hydrastis canadensis. Hydrastis not only has antimicrobial activity but also increases blood supply to the spleen promoting the optimal activity of the spleen to release mediating compounds 38.
The Market of Herbal Product of Selected Countries in 2014 India: Herbal / traditional products continued to grow at a strong rate of 13% regarding value in 2013. This was due to consumers’ continued trust in these products as they have no side effects. Herbal/traditional products are expected to grow at by a value CAGR at constant 2013 pieces of 7% during the forecast period of 2013-2018. This is expected to be driven by herbal / traditional vitamins and dietary supplements, topical analgesics and dermatological 39.
USA: The herbal/traditional products category continues to be dominated by dietary supplements, with a 68% share of value sales in 2013. Following this was herbal/a traditional cough, cold and allergy remedies with a 17% value share and digestive remedies with 5%. Together these categories account for 90% of category value sales. Herbal/traditional products are expected to grow by 14% to reach US$5.3 billion by 2018. Some consumers, afraid of complex chemical and drug interactions, will continue to look for more natural remedies and products and therefore the category can be expected to maintain a modest momentum with a CAGR of 3% in value sales at constant 2013 prices over the forecast period. As in the previous year, pediatrics dietary supplements are expected to witness the fastest value growth over the forecast period 40.
China: Herbal/traditional products are expected to see a constant value CAGR of 7% in the forecast period. Such healthy growth momentum is anticipated to be driven by consumers’ steady demand for such products, with rising health-consciousness, in pursuit of more natural cures for health problems.
Herbal/traditional topical analgesics are likely to continue to see the strongest value growth, due to the demand to relieve pain with milder products. Due to the long presence of herbal/traditional products in the market, consumers have developed a preference for such products, mainly due to their fewer side-effects and mild product nature 41.
Australia: Herbal/traditional products grew by 8% in current value terms throughout 2013 to reach a market size of A $498 million. Consumer sentiment has continued to shift over the review period to become more accepting of herbal alternatives as the health benefits have become widely publicized. Although, some concerns remain surrounding the efficacy of herbal alternatives, Australian consumers’ shift towards healthier lifestyle choices has moved demand towards products perceived as more natural. This trend has been magnified by the rise of ethnic populations in Australia, with an increased emphasis on herbal and traditional products and remedies. Herbal and traditional products will grow at a constant value CAGR of 4% over the forecast period. Australian consumers’ desire to minimize artificial ingredients will see them lean towards herbal/traditional products they see as having adequate efficacy 42.
United Kingdom: Herbal/traditional products remained stagnant in current value terms in 2013. The implementation of recent EU regulations on herbal and medicinal products, coupled with the declining interest of consumers in herbal/traditional remedies, contributed to the poor performance of the category. According to the trade sources interviewed, no major regulatory developments are expected to be implemented during the forecast period which will affect the industry the way the European Traditional Herbal Medicinal Product Directive did. The category is expected to continue to become more concentrated and less dynamic as a result of the implementation of the EU regulation 43.
CONCLUSION: In the beginning of the 21st century, the widespread emergence of antimicrobial resistance has made the current antimicrobials ineffective. Various efforts have been made to combat this resistance so that newer targets can be identified and the next generation of effective antimicrobials be produced. There is an urgent need for complete understanding of the various aspects of drug resistance in microbes which can help in the choice of good targets, vital for the discovery of new antibacterial drugs obtained from plant origin.
Shortly, the next challenge will be to identify newer agents for the treatment of multidrug-resistant pathogens which are emerging at a rapid rate. As the synthetic antimicrobials soon become resistant to the pathogen, this made more emphasis on antimicrobials from plant origins which are having a long duration of effectiveness. Hence the present review concludes the importance of plant drugs as antimicrobials over the synthetic drugs.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Mazid M, Khan TA and Mohammad F: Medicinal Plants of Rural India: A Review of Use by Indian Folks. Indo Global Journal of Pharmaceutical Sciences 2012; 2(3): 286-304.
- Clark AM: Natural products as a resource for new drugs. Pharm Res 1996; 13: 1996
- Alper J: The effort to combat microbial resistance lags. ASM News 1998; 64: 440-441.
- Borris RP: Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol 1996; 51: 29-38.
- Moerman DE: An analysis of the food plants and drug plants of native North America. J Ethnopharmacol 1996; 52: 1-22.
- Schultes RE: The kingdom of plants. In W. A. R. Thomson (ed.), Medicines from the Earth. McGraw-Hill Book Co., New York, 1978: 208.
- Stockwell C: Nature’s pharmacy. Century Hutchinson Ltd., London, United Kingdom 1988.
- Weiner MA: Earth medicine-earth food: plant remedies, drugs and natural foods of the North American Indians. Macmillan, New York, 1980.
- Klink B: Alternative medicines: is natural better? Drug Top 1997; 141: 99-100.
- Lewis WH and Elvin-Lewis MP: Medicinal plants as sources of new therapeutics. Ann Mo Bot Gard 1995; 82: 16-24.
- Borris RP: Natural products research: perspectives from a major pharmaceutical company. J Ethnopharmacol 1996; 51: 29-38.
- Cowan MM: Plant products as antimicrobial Agents. Clinical Microbiology Reviews 1999; 12(4): 564-82.
- Singh SB and Barrett JF: Empirical antibacterial drug discovery-foundation in natural products. Biochemical Pharmacology 2006; 71(7): 1006-1015.
- Cragg GM, Newman DJ and Snader KM: Natural products in drug discovery and development. Journal of Natural Products 1997; 60(1): 52-60.
- Newman DJ, Cragg GM and Snader KM: Natural products as sources of new drugs over the period 1981-2002. Journal of Natural Products 2003; 66(7): 1022-1037
- Li JWH and Veederas JC: Drug Discovery and Natural Products: End of an Era or An Endless Frontier? Science 2009; 325: 161-165.
- Clardy J, Fischbach MA and Walsh CT: New antibiotics from natural bacterial products. Nat Biotech 2006; 24(12): 1541-1550.
- Rolinson GN and Geddes AM. The 50th anniversary of the discovery of 6-amino penicillanic acid (6-APA). International Journal of Antimicrobial Agents 2007; 29(1): 3-8.
- Kirby WMM”: Extraction of a highly potent penicillin in activator from penicillin-resistant Staphylococci. Science 1944; 99(2579): 452-453.
- Majiduddin FK, Materon IC and Palzkill TG: Molecular analysis of beta-lactamase structure and function. International Journal of Medical Microbiology 2002; 292(2): 127-37.
- Péhourcq F and Jarry C: Determination of third-generation cephalosporins by High - Performance Liquid Chromatography in connection with pharmacokinetic studies. Journal of Chromatography A 1998; 812(1-2): 159-78.
- Bryskier A, Procyk T and Labro MT: Cefodizime a new 2-aminothiazolyl cephalosporin: physicochemical properties, toxicology and structure-activity relationships. Journal of Antimicrobial Chemotherapy 1990; 26(SplC): 1-8.
- Cassell GH and Mekalanos J: Development of anti-microbial agents in the era of new and reemerging infectious diseases and increasing antibiotic resistance. JAMA: The Journal of the American Medical Association 2001; 285(5): 601-5.
- 2Otten H: Domagk and the development of the sulphonamides. Journal of Antimicrobial Chemotherapy. 1986; 17(6): 689-90.
- Walsh C: Antibiotics: actions, origin, resistance. ASM Press 2003.
- http://www.thefreelibrary.com/FDA+Approves+Nexavar(R)+for+Treatment+of+Patients + with+Advanced...-a0139 964558.
- Medical News Today 2007. Nexavar (Sorafenib) Launched in the UK for hepatocellular carcinoma. Medical News Today, http://www.medicalnewstoday.com/articles/ 87657.php (accessed 20/1/010).
- http://who.int/iris/bitstream/10665/112647/1/WHO_HSE_PED_AIP_2014.2_eng.pdf?ua=1
- Vashist H and Jindal A: Antimicrobial activities of medicinal plants: Review. International Journal of Research in Pharmaceutical and Biomedical Sciences, 2012; 3(1).
- Butler MS, Blaskovich MA and Cooper MA: Antibiotics in the clinical pipeline in 2013. The Journal of Antibiotics 2013; 66: 571-91.
- 3http://www.centerwatch.com/drug-information/fdaapprov ed-drugs/year/2014
- Sinha K: Government to ban over-the-counter sale of 92 antibiotics. The Economic Times TNN 4, 2012, 11.04AM IST.
- Full List of Banned Drugs in India. In Business Health on November 1, 2012, at 12:03.
- Xu HX and Lee SF: The activity of plant flavonoids against antibiotic-resistant bacteria. Phytotherapy Research 2001; 15(1): 39-43.
- Ortega-Ramirez LA, Rodriguez GI, Leyva JM, Cruz-Valenzuela MR, Silva-Espinoza BA and Gonzalez-Aguilar GA: Potential of medicinal plants as antimicrobial and antioxidant agents in food industry: a hypothesis. J Food Sci. 2014; 79(2): 129-37.
- Seow YX, Yeo CR, Chung HL and Yuk HG: Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr 2014; 54(5): 625-44.
- Nautiyal CS, Chauhan PS and Nene YL: Medicinal smoke reduces airborne bacteria. J Ethnopharmacol 2007; 114: 446‑51.
- Murray M: The healing power of herbs. Prima Publishing. Rocklin, CA. 1995: 162-71.
- http://www.euromonitor.com/herbal-traditional-products-in-india/report may 2014 page no-38
- http://www.euromonitor.com/herbal-traditional-products-in-the-us/report may 2014 page no-47
- http://www.euromonitor.com/herbal-traditional-products-in-china/report May 2014 page no-54.
- http://www.euromonitor.com/herbal-traditional-products-in-australia/report May 2014 page no 31.
- http://www.euromonitor.com/herbal-traditional-products-in-the-united-kingdom/report may 2014 page no-31.
How to cite this article:
Savant C, Venkatesh, Mannasaheb BAA and Joshi H: Importance of antimicrobial agents from plants in present scenario: A review. Int J Pharmacognosy 2014; 1(8): 472-84. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(8).472-84.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
472-484
700
3339
English
IJPSR
C. Savant*, Venkatesh, B. A. Mannasaheb and H. Joshi
Department of Pharmacology, Sarada Vilas College of Pharmacy, Krishnamurthypuram, Mysore, Karnataka, India.
chetan.savant@yahoo.com
28 June 2014
23 July 2014
29 July 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(8).472-84
01 August 2014