THE PROMISING THERAPEUTIC EFFECTS OF RESVERATROL: A REVIEW OF HUMAN CLINICAL STUDIES
HTML Full TextTHE PROMISING THERAPEUTIC EFFECTS OF RESVERATROL: A REVIEW OF HUMAN CLINICAL STUDIES
Dania Alkabbani * and Enas Al-Khafaji
Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Jordan Amman, Jordan.
ABSTRACT: Resveratrol (3, 5, 4′-trihydroxy stilbene) is a type of natural phenol, produced by several plants in response to injury or under attack by pathogens. These phytoalexin compounds are thought to have antioxidant, anticancer, and anti-inflammatory properties. Although resveratrol is commonly used as a dietary supplement, there is no conclusive evidence of whether or not resveratrol could be a viable treatment in humans. Despite the richness of in-vitro and in-vivo research, which confirms its potential therapeutic effects, there is insufficient clinical evidence on its beneficial results in humans. In this review, we have focused on the mechanism of action of resveratrol and on clinical trials that have evaluated the efficacy of reseveratrol in cancer, cardiovascular diseases and neurodegenerative disorders.
| Keywords: |
Resveratrol, Cancer, Cardiovascular diseases, Neurodegenerative disorders, Clinical trials, Therapeutic effects
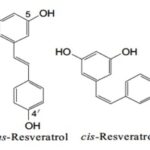
INTRODUCTION: There is considerable interest in the therapeutic potential of natural products because of their low toxicity and minor side effects. Plant-derived polyphenolic compounds have recently attracted a lot of research interest throughout history, due to their antioxidant properties and potential pharmacological effects 1. One of the most widely studied polyphenols is Resveratrol. Resveratrol (3, 4′, 5- trihydroxy stilbene) is a small phenolic compound that is produced naturally by several plants when they become attacked by bacterial or fungal pathogens (phytoalexin) 2, 3. Resveratrol is a member of the stilbene family (two benzene rings linked via isopropyl moiety separated by a double bond), which exists as cis and trans stereoisomers (Z and E) with the transform exhibiting the principal form found 4, 5 Fig. 1.
Resveratrol is found in various food and food products such as grapes, peanuts, wine, grape juice, berries, and various other plants. It was first isolated in 1940 from the roots of white hellebore (Veratrumgrandiflorum O. Loes) 6 Fig. 2 and later in 1963, from the roots of Polygonumcuspidatum, a plant used widely in traditional Chinese and Japanese medicine 7 Fig. 3. Resveratrol attracted little interest until 1992 when it was proposed to explain some of the cardioprotective effects of red wine 8. The presence of resveratrol in red wine at high concentrations (0.1–14.3 mg/L) suggested explaining the interesting “French Paradox” which suggested an unexpectedly low rate of heart disease among Southern French people who consume a lot of red wine, despite their diets being high in saturated fat 9, 10.
Since then, several reports have been conducted to evaluate the effect of resveratrol in the prevention and treatment of various diseases and medical illnesses 11, 12, 13, 14, 15, 16. From a pharmacokinetic perspective, results indicate that in spite of rapid absorption circulating resveratrol is rapidly undergo intestinal and hepatic metabolism via glucuronic acid and sulfates 17. The produced conjugates accumulate in plasma and urine, thus resulting in poor bioavailability of the compound. Also, the high lipophilicity of resveratrol leads to low aqueous solubility, which may potentiate its poor oral bioavailability 18. Accordingly, resve-ratrol has proven to be more effective when applied topically rather than administered orally 19. Resveratrol is considered to be a relatively non-toxic and well-tolerable agent. It is reported that doses up to 450 mg/day are safe dose for a 60 kg person. Among its few dose-independent adverse effects are nephrotoxicity and gastrointestinal problems 20. However, higher doses of resveratrol are reported to be toxic. The toxicity is thought to be due to the effect of resveratrol on several hepatic enzymes, where CYP3A4, CYP2C9, and CYP2D6 are inhibited while CYP1A2 is induced. Also, high doses of resveratrol may interact with many other drugs 21. Thus, orally administered high doses could be risky for the patients taking several medications. In this review, we have focused on the mechanism of action of resveratrol and on clinical trials that have evaluated the efficacy of reseveratrol in cancer, cardiovascular diseases, and neurodegenerative disorders.
FIG. 1: CHEMICAL STRUCTURE OF RESVERATROL
FIG. 2: VERATRUM GRANDIFLORUM O. LOES
FIG. 3: POLYGONUM CUSPIDATUM
1. Mechanism of Action of Resveratrol: Resveratrol has received a great deal of attention as a probable treatment of several human diseases. The mechanism by which resveratrol exerts its favorable effects across different disease models is not yet clear, but it seems that it can act on a number of molecules in the body.
One of the suggested mechanisms that resveratrol acts as a potent antioxidant by inhibiting reactive oxygen species (ROS) generations. This is achieved mainly by activating 5' AMP-activated protein kinase (AMPK). This makes resveratrol as a potent antioxidant capable of preventing oxidative stress.
Therefore, it can explain its actions such as anticancer 22, 23, and cardioprotective agent 24. Resveratrol is also found to act as an inhibitor for vascular cell adhesion molecules (VCAM) expression and to influence the activity of vascular smooth muscle cells which are involved in the development of atherosclerosis and high blood pressure (hypertension), respectively 25.
Also, resveratrol exhibit an ant platelet effect through inhibiting early signaling events in Washed platelets in-vitro but has little effect on platelet aggregation in whole blood. Thus, although resveratrol may function as a protective agent of coronary heart disease, its effects are not solely attributed to its effects on platelets in circulation 26. Resveratrol also suppresses COX-2 enzymes, which are responsible for the conversion of arachidonic acid into prostaglandins. The inhibition of this pathway reduces inflammation and suggests the possibility of resveratrol as a treatment for inflammatory conditions 27. Resveratrol is also an activator of SIRT1, a member of the sirtuin family of proteins 28. SIRT1 deacetylates proteins, including transcription factors. This regulated pathway is therefore expected to benefit several conditions such as abnormal metabolic control, cell cycle defects, and inflammation 29, 30. Resveratrol affects the nuclear factor κB (NF-κB) signaling pathway, which regulates inflammation, the immune response to infection, and cellular response to stimuli 31. In addition, it has been shown to significantly inhibit the IGF-1R/ Akt/Wnt pathways and activate p53, and therefore can influence its anti-cancer activities 32.
2. Medical uses of Resveratrol:
2.1. Resveratrol and Anti-Cancer Activity: Cancer remains a chronic health problem with a high mortality rate to date. In 1997, Jang et al. published a research article reported the ability of topical f resveratrol in reducing the number of skin tumors up to 98% in mice 33. These findings triggered research on resveratrol on cancer treatment worldwide. Several in-vitro research studies on the effect of resveratrol on cancer treatment have been reported.
These studies showed that in-vitro resveratrol interacts with multiple molecular targets, and has positive effects on the cells of breast 34, skin 35, gastric 36, colon 37, esophageal 38, prostate 39 and pancreatic cancer 40 and leukemia 41. Clinical studies, on the other side, must determine if the same effects can be seen in human patients.
However, few results of human clinical trials for the effect of resveratrol on cancer have been reported 42. Reason behind this limited number of clinical trial is the pharmacokinetics of resveratrol, where it was concluded that even high doses of resveratrol might be insufficient to achieve resve-ratrol concentrations required for the systemic prevention of cancer 43. This poor systemic bioavailability limits clinical trial for resveratrol on humans for the treatment of cancers 44.
The strongest evidence of anti-cancer action of resveratrol exists for tumors it can come into direct contact with, such as skin tumors 45. For other cancers, the evidence is uncertain, even if massive doses of resveratrol are used.
2.1.1. Prostate Cancer: The few clinical trials that have been conducted show that resveratrol has several targets within the cell, and its efficacy is dependent on the type and stage of cancer, dosage levels, and treatment periods. Among the clinical trials conducted, a phase 1 clinical study was conducted on 14 subjects to describe the effect of pulverized muscadine grape skin (MPX) extract, which contains resveratrol in appreciable concentration on prostate-specific antigen (PSA) doubling time. Every 500 mg of MPX has 4.4μg of trans-resveratrol. Length of the trial was 2-35 months depending on the patient’s condition. Results showed a delay in prostate-specific antigen (PSA) doubling time by 5.3 months; however, these results were not statistically significant (P = 0.17) 46. The results for prostate cancer was confirmed by another randomized placebo-controlled clinical study, which was conducted using two doses of resveratrol (150 mg or 1,000 mg resveratrol daily) for 4 months. This study concluded that resveratrol could not treat prostate cancer as it had no effect on the prostate volume or PSA levels 47.
Another double-blind, randomized, placebo-controlled trial was conducted to examine the effects of the specific phytotherapeutic intervention (containing turmeric, resveratrol, green tea, and broccoli sprouts) on 22 men with recurrent prostate cancer. Patients were randomized to either the active treatment arm or placebo for 12 weeks. Results found that there is no statistical difference between groups on PSA doubling time in this study 48. From these studies, it seems unlikely that resveratrol will prove to be an effective treatment for prostate cancer, but more clinical trials need to be performed to confirm this.
2.1.2. Colorectal Cancer: In a clinical trial conducted in patients with colorectal cancer, the results seem promising. Twenty patients with histo-logically confirmed colorectal cancer consumed eight daily doses of resveratrol at 0.5 or 1.0g 8 days before surgical resection. Cell proliferation, as reflected by Ki-67 (proliferation marker) staining, was compared in preintervention and post-intervention tissue samples. Results revealed a reduction of 5% in the rate of cellular proliferation in colorectal cancer tissue 49. Also, another phase 1 randomized, double-blind pilot study conducted on 9 patients with colorectal cancer showed activation of apoptosis and an increase in cleaved Caspase-3 level (apoptotic marker), which suggest the beneficial effect of resveratrol on colon cancer 50.
2.1.3. Breast Cancer: Alternatively, in breast cancer, a randomized double-blinded clinical trial was conducted over 12 weeks on 39 adult women at increased breast cancer risk. These women were randomized in a double-blind fashion to placebo, 5, or 50 mg trans-resveratrol twice daily for 12 wk.
Results found that the resveratrol affected the epigenetic pattern of RASSF-1α, a gene associated with breast cancer, and this effect correlated with the levels of circulating resveratrol 51. These results suggest that resveratrol may act as a chemo preventive agent for breast cancer by influencing the epigenetics of breast cancer-associated genes, a finding that needs to be confirmed in future clinical trials. Another pilot study was conducted on postmenopausal women with high body mass index to determine the clinical effect of resveratrol on estrogen hormones, which play a main role in breast cancer. Results showed that a daily 1 gm dose of resveratrol has favorable effects on estrogen metabolism thus may have a good effect on breast cancer reduction 52.
2.1.4. Skin Cancer: In a study conducted by Farris et al. (2014), a topically applied blend containing 1% resveratrol, 0.5% baicalin, and 1% vitamin E was applied to the skin of patients with mild to moderately photodamaged skin for 12 weeks.
Findings showed a statistically significant improvement in fine lines and wrinkles, skin firmness, skin elasticity, skin laxity, hyper-pigmentation, radiance, and skin roughness after 12 weeks compared to baseline 53. A summary of these clinical studies on a human for each cancer type is presented in Table 1.
TABLE 1: SUMMARY OF RESVERATROL CLINICAL EFFECTS ON SEVERAL TYPES OF CANCER
| S. no. | Year of the Study | Type of Cancer | Study Design | No. of Patients | Effect
|
| 1 | 2015 | Prostate cancer | Phase 1clinical trial | 14 patients | None 46 |
| 2 | 2015 | Prostate cancer | Randomized placebo-controlled clinical study | 66 patients | None 47 |
| 3 | 2017 | Prostate cancer | A double-blind, randomized, placebo-controlled trial | 22 men | None 48 |
| 4 | 2010 | Colorectal cancer | One group interventional study | 20 patients | Beneficial 49 |
| 5 | 2011 | Colorectal cancer | Phase 1 randomized, double-blind pilot study | 9 patients | Beneficial 50 |
| 6 | 2012 | Breast cancer | Randomized double-blinded clinical trial | 39 women | Beneficial 51 |
| 7 | 2014 | Breast cancer | One group interventional study on postmenopausal women | NA | Beneficial 52 |
| 8 | 2014 | Skin cancer | One group interventional study | NA | Beneficial 53 |
2.2. Resveratrol and Cardiovascular Diseases: The World Health Organization (WHO) reports that cardiovascular disease represents the main cause of death worldwide 54. It has long been known that moderate drinking of red wine reduces the risk of heart disease 55.
The consumption of red wine provided an explanation for the ‘French Paradox’ that is used to describe the observation that the French enjoy the relatively low risk of cardiovascular disease despite a diet that is high in saturated fat 56. Studies suggest that resveratrol is a main constituent in red wine, and it is suggested to play an important role in this phenomenon.
Several clinical trials have evaluated the effect of resveratrol in the management of cardiovascular diseases, one of these trials is a double-blind and placebo-controlled trial that was conducted on 40 post-infarction Caucasian patients, which were randomized into two groups.
One group received 10 mg resveratrol capsule daily for 3 months. Results of this study found that treatment with resveratrol in patients with stable coronary heart diseases improved left ventricular systolic and diastolic function. Also, the same study proved that resveratrol treatment also inhibited platelet aggregation and decreased LDL levels 57.
A randomized, double-blinded, active-controlled, parallel clinical trial was conducted on 87 subjects with stable angina pectoris. Subjects were divided into three groups: group 1 received oral supplementation with calcium fructoborate, group 2 received oral supplementation of resveratrol and group 3 received the combination of both. Treatment was continued for 60 days.
Results showed a significant decrease of high-sensitivity C-reactive protein in all groups at the 30 days and 60 days visits. The N-terminal prohormone of brain natriuretic peptide was significantly lowered for all groups; however, their combination was the most effective. Lipid markers showed slight changes from baseline in all groups. These results indicate that the resveratrol has beneficial effects in patients with angina 58.
A double-blind, randomized, placebo-controlled clinical trial comprised of 44 healthy subjects received either blend of phytochemicals (400 mg trans-resveratrol, 400 mg grape skin extract and 100 mg quercetin) or a cellulose placebo for 30 days. Results showed a decreased expression of endothelial cell ICAM, VCAM and IL-8, which may be an important mechanism contributing to resveratrol’s beneficial effects on cardiovascular function 59. A randomized placebo-controlled clinical trial performed on 18 patients investigated the effect of resveratrol on reducing diastolic blood pressure in conjunction with other phytochemicals substances (330 mg grape seed and skin, 100 mg green tea, 60 mg resveratrol, 60 mg blend of quercetin, ginkgo biloba, and bilberry). In this study, resveratrol was found to be effective in reducing diastolic blood pressure 60. Another randomized, double-blind crossover study was conducted by Timmers et al., 2011 on 11 healthy, obese men.
Subjects were divided into two groups: placebo and 150 mg/day resveratrol groups. Resveratrol was received for 30 days. Results showed that resveratrol significantly decreased intrahepatic lipid content, circulating glucose, triglycerides, alanine-aminotransferase, and inflammation markers. Also, systolic blood pressure dropped after resveratrol treatment 61. Another prospective, open-label, randomized, controlled trial was conducted on 26 patients with type 2 diabetes mellitus in India.
Patients were randomized into control and intervention (resveratrol) groups. The results reveal that supplementation of resveratrol for 3 months significantly improves the systolic blood pressure (Mean ± SD, 139.71 ± 16.10 vs 127.92 ± 15.37; P < .05) and total cholesterol (Mean ± SD, 4.70 ± 0.90 vs. 4.33 ± 0.76; P < .05) in type 2 diabetic patients 62. Table 2 provides a summary of these clinical trials studying the effect of resveratrol on cardiovascular diseases. Finally, resveratrol has demonstrated positive effects in studies of various cardiovascular conditions, however further research in human is necessary to verify its effectiveness.
TABLE 2: SUMMARY OF RESVERATROL CLINICAL EFFECTS ON CARDIOVASCULAR DISEASES
| Study no. | Year of the Study | Type of Cardiovascular Disease | Study Design | No. of Patients | Effect
|
| 1 | 2016 | Coronary heart disease | Randomized double-blind placebo-controlled | 40 post-infarction patients | Beneficial 57 |
| 2 | 2013 | Angina pectoris | Randomized double-blind active-controlled | 87 patients | Beneficial 58 |
| 3 | 2016 | Hypertension | randomized, placebo-controlled trial | 18 patients | Beneficial 60 |
| 4 | 2011 | Hypertension and hyperlipidemia | a randomized, double-blind placebo-controlled | 11 patients | Beneficial 61 |
| 5 | 2012 | Hypertension and hyperlipidemia | prospective, open-label, randomized placebo-controlled | 26 patients | Beneficial 62 |
| 6 | 2014 | Atherosclerosis | a randomized, double-blind placebo-controlled | 44 healthy subjects | Beneficial 59 |
2.3. Resveratrol and Neurodegenerative Diseases: Neurological disorders such as Alzheimer’s disease occur via oxidative and inflammatory damage to the central nervous system. The exact mechanism of Alzheimer’s disease development is unknown. Several bio-markers have been identified which help to characterize disease onset and progression and may serve as therapeutic targets. For instance, amyloid-β plaque accumulation caused by amyloid-beta precursor protein and increased inflammation and oxidative damage has been shown to be associated with Alzheimer’s disease 63. The potential therapeutic activity of resveratrol in Alzheimer’s disease was reported by few clinical trials, which have shown that resveratrol is safe to use in patients with mild to moderate Alzheimer’s disease, and it alters several Alzheimer’s disease biomarkers. A randomized, placebo-controlled, double-blind, 52-week phase 2 trial of resveratrol in individuals with mild to moderate Alzheimer disease was conducted on 119 patients.
Participants were randomized to placebo or resveratrol 500 mg orally once daily. The result showed that resveratrol stabilizes the progressive decline in cerebrospinal fluid Aβ40 and plasma Aβ40 levels as dementia advances. Also, it stabilizes CSF Aβ42 levels at week 5264. Another randomized, placebo-controlled, double-blind, phase 2 trial found that treatment of mild-moderate Alzheimer’s disease (AD) subjects (N = 119) for 52 weeks with resveratrol (up to 1 g by mouth twice daily) decreases levels of matrix metalloproteinase-9 (MMP-9) that degrades components of the extracellular matrix, an activity that is associated with Alzheimer’s disease.
The decrease in MMP-9 may indicate that resveratrol reduces permeability and the ability of pro-inflammatory agents from reaching the brain. Furthermore, patients receiving resveratrol had a decline of cerebrospinal fluid beta-amyloid (Aβ) 42 and Aβ40 levels, indicating lower accumulation of Aβs in the brain 65.
Another 10 subjects with a mild decline in cognition were included in a double-blinded placebo-controlled pilot study. Participants were randomized into an active grape formulation arm or a placebo arm which consumed a formulation free of polyphenols for six months. The placebo arm had declines in regions of the brain known to be significantly affected in the early stages of Alzheimer's disease, while the active formulation group was spared such decline. This suggests a protective effect of resveratrol contained in grapes against early pathologic metabolic decline in Alzheimer's disease 66.
Also, eighty post-menopausal women aged 45-85 years were randomized to take trans-resveratrol or placebo for 14 weeks to evaluate the effects on cognitive performance. These results indicate that regular consumption of a modest dose of resveratrol can enhance both cerebrovascular function and cognition in post-menopausal women, potentially offering a promising therapeutic treatment for menopause-related cognitive decline 67. A randomized, double-blind, placebo-controlled, crossover study was conducted to evaluate the effects of oral resveratrol on cognitive performance and localized cerebral blood flow variables in 22 healthy human adults.
Resveratrol administration resulted in dose-dependent increases in cerebral blood flow during task performance, as indexed by total concen-trations of hemoglobin. In contrast, the cognitive function was not affected by 68. Table 3 provides a summary of these clinical trials investigating the effect of resveratrol on neurodegenerative diseases.
TABLE 3: SUMMARY OF RESVERATROLS CLINICAL EFFECTS ON NEURODEGENERATIVE DISEASES
| Study no. | Year of the study | Type of neurodegenerative diseases | Study Design | No. of patients | Effect
|
| 1 | 2015 | Alzheimer’s disease | Randomized, placebo-controlled, double-blind, phase 2 trial | 119 patients | Beneficial 64
|
| 2 | 2017 | Alzheimer’s disease | Randomized placebo controlled | 119 patients | Beneficial 65 |
| 3 | 2017 | Alzheimer’s disease | Double-blinded placebo controlled pilot study. | 10 patients | Beneficial 66 |
| 4 | 2017 | cognitive decline | Randomized double-blinded placebo controlled study | 80 post-menopausal women patients | Beneficial 67 |
| 5 | 2010 | cognitive decline | randomized, double-blind, placebo-controlled, crossover study | 22 healthy subjects | Beneficial 68 |
CONCLUSION: The clinical trials presented in this review have demonstrated resveratrol’s therapeutic potency in the prevention and treatment of some diseases. In most of the clinical trials, the major problem was resveratrol’s poor bio-availability, which is due to extensive metabolism in the liver. We found that for cardiovascular diseases and neurodegenerative disorders, the majority of clinical data showed that resveratrol had beneficial effects in patients. But resveratrol had an unclear effect on certain types of cancers. It seems that resveratrol may have specificity for certain types of cancers, so it is difficult to conclude given the small number of clinical trials that have been conducted. Overall, more clinical data are necessary in order to better understanding of resveratrol’s therapeutic potential. In addition, future Pharmaceutical efforts should focus on developing a resveratrol derivative with better bioavailability.
ACKNOWLEDGEMENT: We would like to thank Dr. Mohammad Hudaib and Dr. Mayada Shihadeh at the University of Jordan for their support and guidance.
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Sies H: Polyphenols and health: update and perspectives. Archives of biochemistry and biophysic 2010; 501(1): 2-5.
- Dixon RA: Natural products and plant disease resistance. Nature 2001; 411(6839): 843-47.
- Timperio AM, D’Alessandro A, Fagioni M, Magro P and Zolla L: Production of the phytoalexins trans-resveratrol and delta-viniferin in two economy-relevant grape cultivars upon infection with Botrytis cinerea in field conditions. Plant Physiology and Bioche 2012; 50: 65-71.
- Abu-Amero KK, Kondkar AA and Chalam KV: Resveratrol and ophthalmic diseases. Nutrients 2016; 8(4): 200.
- Wang S, Liang X, Yang Q, Fu X, Zhu M, Rodgers B, Jiang Q, Dodson MV and Du M: Resveratrol enhances brown adipocyte formation and function by activating AMP‐activated protein kinase (AMPK) α1 in mice fed high‐fat diet. Molecular nutrition & food research 2017; 61(4): 1600746.
- Takaoka M: Of the phenolic substrate of hellebore (Veratrum grandiflorum fil.). J Fac Sci Hokkaido Imper Univ 1940; 3: 1-16.
- Nonomura S, Kanagawa H and Makimoto A: Chemical constituents of polygonaceous plants. i. studies on the components of ko-j o-kon.(polygonumcuspidatumsieb. et zucc.). Yakugakuzasshi Journal of the Pharmaceutical Society of Japan 1963; 83: 988-90.
- Siemann E and Creasy L: Concentration of the phytoalexin resveratrol in wine. American Journal of Enology and Viticulture 1992; 43(1): 49-52.
- Lippi G, Franchini M and Guidi GC: Red wine and cardiovascular health: the “French Paradox” revisited. International Journal of Wine Research 2010; 2(1): 1-7.
- Renaud Sd and de Lorgeril M: Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet 1992; 339(8808): 1523-26.
- Bonnefont-Rousselot D: Resveratrol and cardiovascular diseases. Nutrients 2016; 8(5): 250.
- Borriello A: Resveratrol in cancer prevention and treatment: focusing on molecular targets and mechanism of action. Multidisciplinary Digital Publishing Institute Proceedings 2017; 1(10): 976.
- Heebøll S, El‐Houri RB, Hellberg YEK, Haldrup D, Pedersen SB, Jessen N, Christensen LP and Grønbæk H: Effect of resveratrol on experimental non‐alcoholic fatty liver disease depends on severity of pathology and timing of treatment. Journal of gastroenterology and hepatology 2016; 31(3): 668-75.
- Martin I: Resveratrol for Alzheimer’s disease. Science translational medicine 2017; 9(375): 6055.
- Öztürk E, Arslan AKK, Yerer MB and Bishayee A: Resveratrol and diabetes: A critical review of clinical studies. Biomedicine & Pharmacothera 2017; 95: 230-34.
- Shahidi M, Vaziri F, Haerian A, Farzanegan A, Jafari S, Sharifi R and Shirazi FS: Proliferative and anti-inflammatory effects of resveratrol and silymarin on human gingival fibroblasts: a view to the future. Journal of Dentistry Tehran Iran 2017; 14(4): 203.
- Walle T, Hsieh F, DeLegge MH, Oatis JE and Walle UK: High absorption but very low bioavailability of oral resveratrol in humans. Drug metabolism and disposition 2004; 32(12): 1377-82.
- De Vries K, Strydom M and Steenkamp V: Bioavailability of resveratrol: Possibilities for enhancement. Journal of Herbal Medicine 2018; 11: 71-77.
- Amri A, Chaumeil J, Sfar S and Charrueau C: Admini-stration of resveratrol: what formulation solutions to bioavailability limitations. Journal of controlled release 2012; 158(2): 182-93.
- Shaito A, Posadino AM, Younes N, Hasan H, Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH and Nasrallah GK: Potential Adverse Effects of Resveratrol: A Literature Review. International Journal of Molecular Sciences 2020; 21(6): 2084.
- Detampel P, Beck M, Krähenbühl S and Huwyler J: Drug interaction potential of resveratrol. Drug metabolism reviews 2012; 44(3): 253-65.
- Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S and Aggarwal BB: Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer. International Journal of Cancer 2010; 127(2): 257-68.
- Khan MA, Chen H-c, Wan X-x, Tania M, Xu A-h, Chen F-z and Zhang D-z: Regulatory effects of resveratrol on antioxidant enzymes: a mechanism of growth inhibition and apoptosis induction in cancer cells. Molecules and Cells 2013; 35(3): 219-25.
- Xia N, Daiber A, Förstermann U and Li H: Antioxidant effects of resveratrol in the cardiovascular system. British Journal of Pharmacology 2017; 174(12): 1633-46.
- Wiciński M, Malinowski B, Węclewicz MM, Grześk E and Grześk G: Anti-atherogenic properties of resveratrol: 4-week resveratrol administration associated with serum concentrations of SIRT1, adiponectin, S100A8/A9 and VSMCs contractility in a rat model. Experimental and Therapeutic Medicine 2017; 13(5): 2071-78.
- Kirk RI, Deitch JA, Wu JM and Lerea KM: Resveratrol decreases early signaling events in washed platelets but has little effect on platelet aggregation in whole blood. Blood Cells Molecules and Diseases 2000; 26(2): 144-50.
- Yang C-M, Chen Y-W, Chi P-L, Lin C-C and Hsiao L-D: Resveratrol inhibits BK-induced COX-2 transcription by suppressing acetylation of AP-1 and NF-κB in human rheumatoid arthritis synovial fibroblasts. Biochemical Pharmacology 2017; 132: 77-91.
- Borra MT, Smith BC and Denu JM: Mechanism of human SIRT1 activation by resveratrol. Journal of Biological Chemistry 2005; 280(17): 17187-95.
- Liu S, Yang H, Hu B and Zhang M: Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol‑treated osteoarthritis chondrocytes via the Wnt/β‑catenin signaling pathways. Experimental and Therapeutic Medicine 2017; 14(5): 5057-62.
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P and Elliott P: Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006; 127(6): 1109-22.
- Kundu JK, Shin YK, Kim SH and Surh YJ: Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-κB in mouse skin by blocking IκB kinase activity. Carcinogenesis 2006; 27(7): 1465-74.
- Vanamala J, Reddivari L, Radhakrishnan S and Tarver C: Resveratrol suppresses IGF-1 induced human colon cancer cell proliferation and elevates apoptosis via suppression of IGF-1R/Wnt and activation of p53 signaling pathways. BMC Cancer 2010; 10(1): 238.
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD and Mehta RG: Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997; 275(5297): 218-20.
- Kim YN, Choe SR, Cho KH, Cho DY, Kang J, Park CG and Lee HY: Resveratrol suppresses breast cancer cell invasion by inactivating a RhoA/YAP signaling axis. Experimental & Molecular Medicine 2017; 49(2): 296.
- Nivelle L, Hubert J, Courot E, Borie N, Renault J-H, Nuzillard J-M, Harakat D, Clément C, Martiny L and Delmas D: Cytotoxicity of Labruscol, a New Resveratrol Dimer Produced by Grapevine Cell Suspensions, on Human Skin Melanoma Cancer Cell Line HT-144. Molecules 2017; 22(11): 1940.
- Xu J, Liu D, Niu H, Zhu G, Xu Y, Ye D, Li J and Zhang Q: Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Aktsignaling pathway in gastric cancer. Journal of Experimental & Clinical Cancer Research 2017; 36(1): 19.
- Saunier E, Antonio S, Regazzetti A, Auzeil N, Laprévote O, Shay JW, Coumoul X, Barouki R, Benelli C and Huc L: Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Scientific Reports 2017; 7(1): 6945.
- Zhou HB, Yan Y, Sun YN and Zhu JR: Resveratrol induces apoptosis in human esophageal carcinoma cells. World journal of gastroenterology 2003; 9(3): 408.
- Singh SK, Banerjee S, Acosta EP, Lillard JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017; 8(10): 17216.
- Vendrely V, Peuchant E, Buscail E, Moranvillier I, Rousseau B, Bedel A, Brillac A, de Verneuil H, Moreau-Gaudry F and Dabernat S: Resveratrol and capsaicin used together as food complements reduce tumor growth and rescue full efficiency of low dose gemcitabine in a pancreatic cancer model. Cance Letters 2017; 390: 91-102.
- Frazzi R and Guardi M: Cellular and Molecular Targets of Resveratrol on Lymphoma and Leukemia Cells. Molecules 2017; 22(6): 885.
- Berman AY, Motechin RA, Wiesenfeld MY and Holz MK: The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precision Oncology 2017; 1(1): 35.
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M and Gescher AJ: Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiology and Prevention Biomarkers 2007; 16(6): 1246-52.
- Niles RM, Cook CP, Meadows GG, Fu YM, McLaughlin JL and Rankin GO: Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenografttumor growth. The Journal of Nutrition 2006; 136(10): 2542-46.
- Ndiaye M, Philippe C, Mukhtar H and Ahmad N: The grape antioxidant resveratrol for skin disorders: promise, prospects, and challenges. Archives of biochemistry and biophysics 2011; 508(2): 164-170.
- Paller CJ, Rudek MA, Zhou XC, Wagner WD, Hudson TS, Anders N, Hammers HJ, Dowling D, King S and Antonarakis ES: A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: Safety, tolerability and dose determination. The Prostate 2015; 75(14): 1518-25.
- Kjær TN, Ornstrup MJ, Poulsen MM, Jørgensen JOL, Hougaard DM, Cohen AS, Neghabat S, Richelsen B and Pedersen SB: Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4‐month randomised trial in middle‐aged men. The Prostate 2015; 75(12): 1255-63.
- Van Die MD, Williams SG, Emery J, Bone KM, Taylor JM, Lusk E and Pirotta MV: A Placebo-Controlled Double-Blinded Randomized Pilot Study of Combination Phytotherapy in Biochemically Recurrent Prostate Cancer. Prostate 2017; 77(7): 765-75.
- Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M and Crowell JA: Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Research 2010; 70(19): 7392-99.
- Howells LM, Berry DP, Elliott PJ, Jacobson EW, Hoffmann E, Hegarty B, Brown K, Steward W and Gescher AJ: Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prevention Research 2011; 4(9): 1419-25.
- Zhu W, Qin W, Zhang K, Rottinghaus GE, Chen YC, Kliethermes B and Sauter ER: Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutrition and Cancer 2012; 64(3): 393-400.
- Chow HH, Garland LL, Heckman-Stoddard BM, Hsu CH, Butler VD, Cordova CA, Chew WM and Cornelison TL: A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. Journal of Translational Medicine 2014; 12: 223.
- Farris P, Yatskayer M, Chen N, Krol Y and Oresajo C: Evaluation of efficacy and tolerance of a nighttime topical antioxidant containing resveratrol, baicalin, and vitamin e for treatment of mild to moderately photodamaged skin. Journal of Drugs in Dermatol JDD 2014; 13(12): 1467-72.
- McAloon CJ, Boylan LM, Hamborg T, Stallard N, Osman F, Lim PB and Hayat SA: The changing face of cardiovascular disease 2000–2012: An analysis of the world health organisation global health estimates data. International Journal of Cardiology 2016; 224: 256-64.
- Tomé-Carneiro J, Larrosa M, González-Sarrías A, A Tomas-Barberan F, Teresa Garcia-Conesa M and Carlos Espin J: Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Current Pharmaceutical Design 2013; 19(34): 6064-93.
- Lippi G, Franchini M and Guidi GC: Red wine and cardiovascular health: the “French Paradox” revisited. International Journal of Wine Research 2010; 2: 1-7.
- Magyar K, Halmosi R, Palfi A, Feher G, Czopf L, Fulop A, Battyany I, Sumegi B, Toth K and Szabados E: Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clinical Hemorheology and Microcirculation 2012; 50(3): 179-87.
- Militaru C, Donoiu I, Craciun A, Scorei ID, Bulearca AM and Scorei RI: Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: effects on lipid profiles, inflammation markers, and quality of life. Nutrition (Burbank, Los Angeles County, Calif) 2013; 29(1): 178-83.
- Agarwal B, Campen MJ, Channell MM, Wherry SJ, Varamini B, Davis JG, Baur JA and Smoliga JM: Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. International Journal of Cardiology 2013; 166(1): 246-48.
- Biesinger S, Michaels H, Quadros A, Qian Y, Rabovsky A, Badger R and Jalili T: A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. European Journal of Clinical Nutrition 2016; 70(1): 10-16.
- Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten S, Moonen-Kornips E, Hesselink MKC, Kunz I, Schrauwen-Hinderling VB, Blaak E, Auwerx J and Schrauwen P: Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metabolism 2011; 14(5): 612-22.
- Bhatt JK, Thomas S and Nanjan MJ: Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutrition Research (New York, NY) 2012; 32(7): 537-41.
- Huang Y and Mucke L: Alzheimer mechanisms and therapeutic strategies. Cell 2012; 148(6): 1204-22.
- Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA, Raman R and Aisen PS: A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015; 85(16): 1383-91.
- Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS and Turner RS: Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation 2017; 14(1): 1.
- Lee J, Torosyan N and Silverman DH: Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo controlled pilot study. Experimental gerontology 2017; 87: 121-28.
- Evans HM, Howe PR and Wong RH: Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; a 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017; 9(1).
- Kennedy DO, Wightman EL, Reay JL, Lietz G, Okello EJ, Wilde A and Haskell CF: Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. The American Journal of Clinical Nutrition 2010; 91(6): 1590-97.
How to cite this article:
Alkabbani D and Al-Khafaji E: The promising therapeutic effects of resveratrol: a review of human clinical studies. Int J Pharmacognosy 2020; 7(11): 257-65. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(11).257-65.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
257-265
790
1119
English
IJP
D. Alkabbani * and E. Al-Khafaji
Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Jordan Amman, Jordan.
kabbani.dania@yahoo.com
05 May 2020
23 September 2020
29 September 2020
10.13040/IJPSR.0975-8232.IJP.7(11).257-65
01 November 2020