SUNSCREEN FORMULATION BASED ON CAMELLIA SINENSIS LEAF EXTRACT
HTML Full TextSUNSCREEN FORMULATION BASED ON CAMELLIA SINENSIS LEAF EXTRACT
Mohammed Adil Selka *, Souheila Guendouz, Fethallah Ouahrani, Akram Yassine Mohammedi Mohammed Yacineachouri, Amel Chenafa and Nazim Bellifa
Pharmacy Department, Faculty of Medicine, Toximed Laboratory, University of Tlemcen, Algeria.
ABSTRACT: Topical emulsions are widely used in dermo-cosmetology. Several topical formulations, such as sunscreen and anti-wrinkle creams, are prepared based on plant extracts for different dermatological affections. The main objective of this study was to formulate an emulsion-type sunscreen by incorporating tea leaf extract as an active ingredient. A spectrophotometric assay of the main phenolic groups and the screening of the antioxidant power using DPPH assay were carried out. Different O/W emulsions were prepared using tea leaf extract as an active ingredient, and their physical stability was assessed based on various physicochemical characteristics, including color, creaming, liquefaction, centrifugation, and pH. The results showed the potential of tea leaf extract and the possibility to be used as an active ingredient in new natural sunscreens due to its antioxidant activity. The tea leaf extract-based emulsion was stable and gave satisfactory results regarding the stability tests carried out during the preparation.
Keywords: Camellia sinensis, Sunscreen, Polyphenol, Tannin
INTRODUCTION: The skin is continually exposed to environmental stresses, including ultraviolet (UV) radiation. UVB is an inherent sunlight component that penetrates the epidermis and reaches the upper dermis, leading to high oxidative stress levels, inflammatory response activation, and DNA damage accumulation. The Earth's UVB radiation increasing due to stratospheric ozone destruction is a major environmental threat to the skin, enhancing the risk of damage with long-term consequences, such as photoaging and photocarcinogenesis 1. Plant extracts and natural compounds have been historically used in traditional medicine for skin care as creams and ointments, but most of these compound's effects remain to be investigated.
With the growing concern of the population about life quality and beauty issues, the cosmetic market for anti-aging and photoprotective products based on natural compounds is expanding, and there is a growing need for further research development in this field 2. Tea is one of the most popular beverages in the world. White, green, and black teas are produced from Camellia sinensis leaves, and they differ by their production method therefore, they are different by their bioactive compounds availability. Green tea is obtained by pan-frying or steaming the leaves, a process that inactivates the endogenous polyphenol oxidase 3.
Green tea chemical compounds are mostly polyphenols, caffeine, and amino acids. Tea also contains flavonoids. Most of these components have been shown to provide antioxidant, anti-inflammatory, and immunomodulatory effects, which offer additional protection against UV radiations exposure 2. The objective of this study was to formulate an emulsion sunscreen with tea leaf extract as an active ingredient.
MATERIAL AND METHODS:
Plant Material: Tea leaves were bought from an arborist in the region of Adrar in southern Algeria
Sample Identification:
Macroscopic Identification: Macroscopic observation was performed with the naked eye; an organoleptic examination was also carried out.
Microscopic Identification: Green tea leaves were pulverized into a fine powder, a sample was spread on a microscope slide and mounted with two drops of acidified chloral hydrate solution, and a cover slip was put over. A light microscope did the observation at different magnifications. Each element was then identified and photographed.
Thin Layer Chromatography: 5 ml of EtOH-water (v/v 40/60) was added to 1 g of pulverized tea leaves; the mixture was macerated at 40 °C with constant stirring for 15 min and then filtered. Caffeine standard (10 mg) and theobromine standard (5mg) were dissolved in 10 mL of EtOH-water (v/v 40/60).
Tea leaves extracts and standard solutions was spotted on the plates with microcapillary tubes (10-μL) methanol, methylene chloride (5:95 V/V) was used as a mobile phase, the development distance was 10 cm, at the end of the migration the plate was dried. TLC Chromatogram was observed under UV light at 254 nm (A) and visible light at 366 nm, Another revelation was used by spraying an equal volume mixture of hydrochloric acid and EtOH 96%, then another prepared solution by dissolving 1 g of iodine and 1 g of potassium iodide in 100 mL of EtOH 96%. The observation was made in daylight 4.
Phenolic Compound Extraction: 200 g of dried, ground, and powdered plant material was macerated with 1000 Ml of ethanol-water solution (56-44) v/v. After 72 hours, 170 ml was collected and stored in an amber container at a low temperature (4∘C). The remaining extract was concentrated at low temperature under reduced pressure using a rotary evaporator until a volume of 30 ml was obtained, which extract was added to the 170 ml extraction solution producing 200 ml of Camellia sinensis hydroalcoholic extract, so the final solution obtained was considered as 1 : 1 (weight/volume) 5.
Determination of the Total Phenolic Content: The total polyphenol content of tea leaves extract was determined by the Folin-Ciocalteu method. 400 microliters of methanolic extract were mixed with 1.6 ml of 7,5% sodium carbonate solution (Na2CO3) and with 2 ml of freshly prepared Folin-Ciocalteu reagent (diluted (1:10). The mixture was vortexed and incubated at room temperature for 90 min, the absorbance was measured at 765 nm. The standard calibration curve was plotted using gallic acid. The results were expressed as mg Gallic acid equivalents/ g dry matter. All operations were performed in triplicate 6.
Determination of the Total Flavonoid Content: The total flavonoid content was estimated using aluminum chloride colorimetric assay. 1 mL of diluted extract was mixed was mixed with 3mL of distilled water and 300 µl of 7% sodium nitrite (NaNO2) solution. This was incubated for 5 min. Later 300 µl of 10% aluminum chloride (AlCl3) and 1 mL of 1 M sodium hydroxide were added and left at room temperature for 15 min. The absorbance of the mixtures was measured at 510 nm (and total flavonoid contents were calculated as catechine equivalents from a calibration curve of catechin. The calibration curve was prepared similarly using 0.01562-1 mg/mL of catechin solutions in methanol. All operations were performed in triplicate 7.
Determination of Condensed Tannins Content: Condensed tannins content was estimated using the vanillin HCl method. 50 μl of diluted methanolic extract was mixed with 1500 μl of vanillin/methanol solution (4%, w/v) and 750 μl of 37% hydrochloric acid (HCl). After 20 minutes of incubation at room temperature, the absorbance of the mixture was measured at 500 nm. A calibration curve was obtained using catechin solution at different concentrations. The result was expressed as mg catechin equivalents/ g dry plant material. All operations were performed in triplicate 8.
Evaluation of the Antioxidant Activity: Free radical scavenging activity of tea leaves extract was measured by 1, 1- diphenyl-2-picrylhydrazyl (DPPH). 1.950 ml of freshly prepared DPPH- methanolic solution (0.025 g/l) was added to 50 μl of the extract at different concentrations (5, 10, 15, 20, 25, 30 μg/ml). The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance was measured at 517 nm (Optizen 3220, South Korea).
A negative control was prepared by mixing 50 μl of methanol with 1.95 ml of DPPH-methanolic solution. Radical scavenging activity was expressed as the inhibition concentration (IC50), i.e., the concentration of extract necessary to decrease the initial concentration of DPPH● by 50% (IC50) under the specified experimental condition 9.
In-vitro SPF Determination: The in-vitro sun protection factor (SPF) was determined in this paper according to the method of Mansur et al. 10. 100 mg of crude extract were dissolved in ethanol 60% at a concentration of 1µg/ml.
The absorption spectra of the extract in were obtained in the range of 290 to 450 nm using 1 cm quartz cell and ethanol as a blank. The absorption data were obtained in the range of 290 to 320, every 5 nm and three determinations were made at each point, followed by the application of Mansur equation 11.
Where CF = 10 (correction factor), EE(λ) = erythemogenic effect of radiation of wavelength λ, I(λ)= intensity of solar light of wavelength λ, Abs (λ) = spectrophotometric value for absorbance of wavelength l by a solution of the preparation.
Cream Formulation: Sunscreen can reduce the effects of UV exposure and prevent complications due to its direct action or by inhibiting the resulting metabolites. Hydrophilic creams are among patients' most accepted topical forms because of their tolerability, penetrability, ease of use, and washability with water compared to ointments. Tea leaves extract was used as an active ingredient, and the concentration of 2% w/w was chosen based on literature reports 12.
Different Formulas have been prepared by Changing:
- The oil type (paraffin oil and almond oil)
- Surfactants percentages
- Xanthan gum percentages
The proportions of the two surfactants were adjusted using the Griffin method to obtain HLBm of 6.5, 7, 8 and 8.5.
HLBm = [xHLBs + (100-x) HLBt ] / 100
X: span 80 percent, HLBs span 80 HLB, HLBt: tween 80 HLB, HLBm: HLB of the mixture. All the preparations were carried out according to the phase inversion method:
The aqueous phase was prepared by mixing tea leaf extract tween 80, glycerol, propylene glycol, and distilled water. The oil phase was prepared by mixing oil, span 80, and xanthan gum.
All the content of the oil phase and the water phase was heated up to 60°C separately. Afterward, the oil phase was added slowly to the aqueous phase with continuous stirring to form a crude emulsion. The resulting materials were cooled for 5 minutes using an ice bath.
The composition of the different formulas is given in Table 1.
TABLE 1: FORMULAS COMPOSITION
| Formula code | F1 | F2 | F3 | F4 | F5 | F6 |
| Almondoil | / | 5% | 5% | 15% | 15% | 15% |
| paraffinoil | 5% | / | / | 5% | 5% | 5% |
| xanthangum | 0.3% | 0 ,1% | 0.3% | 0.5 % | 0.3% | 0.3% |
| Span 80 | 1.2 | 1.2 | 3% | 1% | 2 | 3% |
| Tween 80 | 6.8 | 6.8 | 2% | 3% | 3 | 2% |
| glycerol, | 5% | 7 | 8% | 10% | 9 | 5% |
| propylene glycol | 2% | 5 | 8% | 10% | 9 | / |
| distilled water | 77,7% | 72.9% | 71,7% | 53.5% | 56.7% | 67.7% |
| Tea leaves extract | 2% | 2% | 2% | 2% | 2% | 2% |
| HLBm | 8,5 | 6,5 | 7 | 7 | 8 | 8,5 |
Cream Physical Parameters Evaluation: All preparations were checked macroscopically: a naked-eye observation of the main characteristics: texture, color, and homogeneity.
For the most stable formula, we determined:
Emulsion Type: using the dye method where two dyes were used, including methylene blue as a water-soluble dye and Soudan III as a lipophilic one.
pH Measurement: emulsions pH was measured using a calibrated pH meter after dilution (1:10) in neutral distilled water 13, 14.
Centrifugation Stability: centrifugation test was done by adding 2 g of the colored emulsion into centrifugation tubes to be centrifuged at 25°C and speed of 1000, 2000and 4000 rpm for 30 min 13.
RESULTS:
Macroscopic Observation: Tea leaves are green-gray, rolled, often folded, twisted on themselves, complete, or scattered.
Microscopic Observation: Microscopic observation of the powder showed the presence of an upper epidermis without stomata, formed by polyhedral cells, frequently associated with palisading parenchyma.
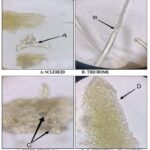
The lower epidermis is formed by polyhedral cells with an anomocytic stomata surrounded by 3 to 4 cells and many trichome unicellular flexuous and thick-walled. Many sclereids were found; the type was Astro sclereid with branched, pointed, and irregular walls. The microscope observation also revealed some druse calcium oxalate crystals Fig. 1.
FIG. 1: MICROSCOPIC OBSERVATION OF TEA LEAVES POWDER (400 ×)
SPOT A: TEAL LEAVES EXTRACT / SPOT B: CAFFEIN STANDARD/SPOT C: THEOBROMINE STANDARD
FIG. 2: TLC CHROMATOGRAM OF THE TEA LEAF EXTRACT
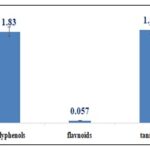
Total Phenolic, Flavonoid and Condensed Tannin Contents: Total polyphenols, flavonoids, and condensed tannins contents are summarized in Fig. 3.
FIG. 3: TOTAL PHENOLIC, FLAVONOID, AND CONDENSED TANNIN CONTENTS
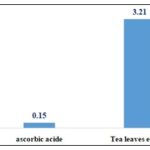
Antioxidant Activity: Fig. 4 shows IC50 of ascorbic acid and tea leaf extract.
FIG. 4: TEA LEAVES EXTRACT AND EMULSIONS DPPH RADICAL SCAVENGING ACTIVITY
In-vitro Sun Protection Factor (SPF): In-vitro sun protection factor (SPF) value for tea leaf extract at a 1mg/mL concentration was about 30.
Cream Formulation: Table 2 summarizes the physical appearance and homogeneity of the different formulations.
TABLE 2: PHYSICAL APPEARANCE AND HOMOGENEITY OF THE DIFFERENT FORMULATIONS
| Formulation | Physical appearance | Homogeneity |
| F1 | Very fluid | Very quick phase separation |
| F2 | fluid | Very quick phase separation |
| F3 | Thick | Very quick phase separation |
| F4 | Thick | Phase separation afterhours |
| F5 | Thick | Phase separation afterhours |
| F6 | Thick | Slight and delayed separation |
| F7 | Thick | No phase separation |
All formulations had a lightly tinted color. F6 formula (the most stable one) tests gave the following results:
- A rapid spread of methylene blue in the cream unlike Sudan red where there is no spread as shown in 5.
- pH value of 5.34.
- No separation phases after centrifugation at 1000 and 2000 rpm for 30 min but a very slight separation after 4000 rpm for 30 min as shown in 6.
FIG. 5: DYE SOLUBILITY METHOD
FIG. 6: EMULSION ASPECT AFTER CENTRIFUGATION TEST
DISCUSSION: Microscopic observation of plant powders is essential in drug control in Pharmacognosy. The microscopic study results of tea leaf powder agree with the French Pharmacopeia 11th edition, which cites the presence of flexuous unicellular tectorial hairs and large sclerites. The pharmacopeia also mentions fragments of parenchyma containing calcium oxalate star-shaped druses like those found in this study 4. Thin layer chromatography chromatogram is identical to that reported by the French Pharmacopoeia 11th edition; the caffeine band is highlighted compared to that of theobromine due to the high level of caffein in tea leaves. These results associated with microscopic analysis indicate tea sample conformity used in this study according to French Pharmacopoeia 11th edition tea monograph 4. Antioxidant activity studies of green tea are very numerous, this study was made on the one hand to confirm this antioxidant activity and on the other hand to take advantage of these properties for the formulation of a total screen based on a plant extract free of any toxicity. Several studies have reported that phenolic compounds are the main quality markers of green tea, mainly polyphenols. Kopjar et al. (2015) conducted a comparative experiment including several varieties of tea using the method described by the international standardization organization, which uses the Folin-Ciocalteu reagent, one of the oldest methods to determine polyphenols content; they reported that green tea leaves contained about 2 µg/mg. This value is very close to the value found in this study 1.83 µg/mg 15. Unachukwu et al. (2010) quantified phenolic content of green tea, black tea, and chamomile using a spectrophotometric method, green tea was found to have the highest level of polyphenols with a value of 1.17 µg/mg 16. Anesini et al (2008) studyin Argentina had as objective, phenolic content determination in both green and black teas, they confirmed that green tea is richer in polyphenols than black tea, based on this result, they were able to note that during the manufacturing process of black tea especially during fermentation, polyphenols are oxidized which will lead to a lower quality product 17. Aluminum chloride test described by Jaiswal et al (2012) has always been used for tea flavonoid quantification. Jaiswal et al found an amount of 0.36 µg/mg flavonoids, a value significantly higher than that found in this work 50. Tannin content was determined by The HCl–vanillin method, and the content was calculated from the calibration curve for catechin. Using the same procedure, Nakamura et al (2003) obtained a value of around 0.69 µg/mg which is lower than that found in this study (1.87 µg/mg) 18. Generally, higher concentrations of catechins have been observed in green tea compared to black tea; this is essentially explained by the fermentation process 17.
The values found in this work agree with some studies and differ with others, it should be noticed that phenolic compounds, flavonoids, and condensed tannins contents vary according to several factors, such as geographical distribution, processing methods, preparation mode, phenolic compounds structure, extraction mode, solvent polarity and temperature 19. The antioxidant activity of teal leaf extract was determined using the DPPH radical scavenging capacity test, taking ascorbic acid as a positive control. In this study, tea leaves have a lower antioxidant potential than ascorbic acid, with an IC50 value equal to 3.21 mg /l compared to an IC50 =0.15mg/l for ascorbic acid. These results are consistent with the work of Chang et al. (2020) who obtained an IC50 of 3.6 mg/l from black tea leaves infusion 20. This powerful antioxidant activity is due to black tea, flavonols, which are more potent antioxidants than those found in apples and oranges.
In this study, antioxidant activity of tea leaf extract, therefore, supports what was previously discussed about total polyphenol assay results based on the correlation between polyphenols and their antioxidant effects. Total polyphenol content and antioxidant activity are two parameters that indicate tea quality in terms of its biological properties, and both tests should be applied for quality control of manufactured and imported teas.
Pastoriza et al. (2011) made an Analysis comparing the antioxidant activity of Camellia sinensis using two different tests: one with DPPH on methanolic extract and the other by direct application of free radicals on sprayed leaves. The results showed that the highest antioxidant activity was obtained by directly applying free radicals so that we can consider a possible phenolic compound degradation during their extraction 21.
The preparations based on only sweet almond oil showed phase separation after a few minutes of their preparation; this is why t is necessary to add a mineral oil. Paraffin oil was chosen because of its viscosity characteristics, which improve cream stability and increase its viscosity. For HLBm 6.5 and 7 formulations, phase separation was still present but at different degrees. However, the separation was delayed for the HLBm 8 emulsion compared to the HLB 6.5 and 7 preparations. For the HLBm 8.5 preparations, no phase separation was noticed. This cream presented a tinted color, a neutral odor, and a viscosity adapted to skin application. Therefore, stability tests were performed on this formulation. The pH was 5.34, which is suitable for skin use. The dye tests confirmed that our cream is based on oil/water emulsion. Centrifugation stability tests showed no separation at 1000 and 2000 rpm. However, a slight separation was produced at 4000 rpm after 5 min.
CONCLUSION: It is well known that green tea has different beneficial effects on health; this work has extended the current knowledge by highlighting the potential of this plant in cosmetics. The results allowed us to understand tea's antioxidant potential by emphasizing its anti-free radical action and by determining bioactive compound contents involved in this activity.
Active metabolite contents obtained in this study have reinforced the objective of formulating a cream with a photoprotective action; clinical studies have pointed out tea's harmlessness skin metabolism, showing that it does not present any allergenic or irritating effect after topical use. All this data encouraged green tea extract integration as an active ingredient in a dermo-cosmetic formula. Emulsion stability when it was formulated showed that tea leaf extract did not compromise its integrity. These preliminary results should motivate researchers to focus on tea skin application, which is still limited and occasional; this would give this plant a real place in cosmetic use. It is obvious that this plant, still under-studied in cosmetology, will attract research attention shortly in this field.
ACKNOWLEDGEMENTS: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Prasanth MI: A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019; 11(2).
- Zink A and Traidl-Hoffmann C: Green tea in dermatology. Myths and Facts 2015; 13(8): 768-775.
- Becker LC: Safety assessment of Camellia sinensis derived ingredients as used in cosmetics. Int J Toxicol 2019; 38(3): 48-70.
- French Pharmacopeia 10th edition. Green tea, Camelia sinensis folium 2010.
- de Oliveira-Júnior RG: Development and Evaluation of Photoprotective O/W Emulsions Containing Hydroalcoholic Extract of Neoglaziovia variegata (Bromeliaceae). Scientific World Journal 2017; 2017: 5019458.
- Selka A: Formulation and Control of a Topical Emulsion, Containing Algerian Vitis vinifera. L Leaves Extract 5(1): 36-49.
- Ying C and Wan D: Quantitative determination of total and individual flavonoids in stems and leaves of Buddleja davidii and Buddleja albiflora. Pharmacogn Mag 2012; 8(32): 273-9.
- Julkunen-Tiitto RJJOA: and chemistry F., Phenolic constituents in the leaves of northern willows. Methods for the Analysis of Certain Phenolics 1985; 33(2): 213-217.
- Sánchez-Moreno CJFST. International, Methods used to evaluate the free radical scavenging activity in foods and biological systems 2002; 8(3): 121-137.
- Mansur JDS: Determinaçäo do fator de proteçäo solar por espectrofotometria 1986; 121-4.
- Smaoui S: Development and stability studies of sunscreen cream formulations containing three photoprotective filters. Arabian Journal of Chemistry 2017; 10: 1216-1222.
- Hong YH: Physiological effects of formulation containing tannase-converted green tea extract on skin care: physical stability, collagenase, elastase, and tyrosinase activities. Integr Med Res 2014; 3(1): 25-33.
- Khan BA: Development, characterization and antioxidant activity of polysorbate based O/W emulsion containing polyphenols derived from Hippophae rhamnoides and Cassia fistula 2013; 49: 763-773.
- Brossard D: Pharmacie galénique: Bonnes pratiques de fabrication des médicaments. Elsevier Health Scien 2016.
- Kopjar M: Phenol content and antioxidant activity of green. Yellow and Black Tea Leaves 2015; 2(1): 1-6.
- Shannon E, Jaiswal AK and Abu-Ghannam NJFR: Polyphenolic content and antioxidant capacity of white, green, black, and herbal teas. A Kine Stu 2018; 2(1): 1-11.
- Anesini C, Ferraro GE and Filip R: Total polyphenol content and antioxidant capacity of commercially available tea (C. sinensis) in Argentina. JAFC 2008; 56(19): 9225-9.
- Nakamura Y, Tsuji S and Tonogai YJJOHS: Analysis of proanthocyanidins in grape seed extracts. Health Foods and Grape Seed Oils 2003; 49(1): 45-54.
- Tadesse A: Quantification of total polyphenols, catechin, caffeine, L-theanine, determination of antioxidant activity and effect on antileishmanial drugs of ethiopian tea leaves extracts. Pharmacognosy Res 2015; 7(1): 7-14.
- Chang MY: Effects of infusion and storage on antioxidant activity and total phenolic content of black tea 2020; 10(8): 2685.
- Pastoriza S: A physiologic approach to test the global antioxidant response of foods. The GAR method. Food Chemistry 2011; 129(4): 1926-1932.
How to cite this article:
Selka MA, Guendouz S, Ouahrani F, Yacineachouri AYMM, Chenafa A and Bellifa N: Sunscreen formulation based on Camellia sinensis leaf extract. Int J Pharmacognosy 2023; 10(5): 276-83. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(5).276-83.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
276-283
966 KB
869
English
IJP
Mohammed Adil Selka *, Souheila Guendouz, Fethallah Ouahrani, Akram Yassine Mohammedi Mohammed Yacineachouri, Amel Chenafa and Nazim Bellifa
Pharmacy Department, Faculty of Medicine, Toximed Laboratory, University of Tlemcen, Algeria.
ad.selka@gmail.com
29 March 2023
27 May 2023
30 May 2023
10.13040/IJPSR.0975-8232.IJP.10(5).276-83
31 May 2023