STUDY OF ETHANOLIC EXTRACT OF JASMINUM SAMBAC USE AS AN ACID-BASE INDICATOR
HTML Full TextSTUDY OF ETHANOLIC EXTRACT OF JASMINUM SAMBAC USE AS AN ACID-BASE INDICATOR
Rutuja Bhoir, Ujwala Dube, Ayub Choudhary and Aniket Dalvi
Ideal College of Pharmacy & Research, University of Mumbai , Maharashtra, India.
ABSTRACT: The demand for today’s chemistry is finding an efficient alternative to synthetic indicators. Acid-base titration was conducted using natural indicators in the present research work. The natural indicator is prepared from Jasminum sambac, the most commonly occurring plant. The ethanolic flower extract was used as a natural indicator. For acid-base titration, Hydrochloric acid (HCl) as strong acid and Acetic acid (CH3COOH) used as a weak acid, and Sodium hydroxide (NaOH) were chosen as a strong base. Successful results were achieved when it was examined by using standard synthetic indicators. Titration shows colour change at the point of equivalence. Thus, for acid-base titration at any dilution, natural indicators from flowers can be used. Titration with natural indicator from ethanolic flower extract of Jasminum sambac has not yet been performed by any research team. It is very simple and economical to use ethanolic flower extract of Jasminum sambac as an indicator as it provides the same outcome accuracy as that provided by the synthetic indicator.
Keywords: Acid-base indicator, Anthocyanins, Titration, Phenolphthalein indicator
INTRODUCTION: Jasminum sambac flowering plant belonging to family Oleaceae. It is widely cultivated throughout the Malaysian region. In Tamil Nadu, Chazipur, Sikanderpur, Andhra Pradesh, Kannad and Karnataka 4. Jasminum sambac commonly known as lily jasmine is a sub-erect shrub, white colour very fragrant flowers commercially cultivated for flowers throughout the tropical and sub-tropical region of the world. Jasminum sambac bears single, double flowers with elongated or rounded petals. The different varieties showed variations in habit, internode length, size, the shape of a leaf, calyx, number of whorls of petals 4.
FIG. 1: JASMINUM SAMBAC FLOWERS
A sub-erect shrub with having pubescent branchlets. Twining shrub growing up to 0.5 to 3 m leaves opposite, variable in shape, usually oval or elliptic and sometimes scalloped, pointed, smooth, downy on the veins 5. The blooming of flowers takes place throughout the year 6. Flowers Containing chemical constituents such as 3-hexenol, 2- vinylpyridine indole, myrcene, linalool, geranyl linalool, alpha terpenol, beta terpenol, linalyl acetate, nerolidol, phytol, isophytol, farnesol, eugenol, benzyl alcohol, methyl benzoate, benzyl acetate,benzyl cyanide, cis-3-hexenyl-benzoate, benzoate, methylpalmitate and methyl linoleate. Glycosidic aroma precursor-like benzyl 6-O-β-D xylopyranosyl-β-D-glucopyranoside (β-primeveroside), 3(2-phenylethyl 6-O-α-Lrhamno-pyranosiyl-β-D-glucopyranoside (β-rutinoside) are identified from the flowers. Benzyl acetate, benzyl alcohol and cisjasmone give the fruity aromatic jasmine qualities 4.
The major pigments that cause colour of flowers are carotenoids, flavonoids (anthocyanins), and betalains. Other pigments that can rarely produce flower colour are chlorophyll, phenylphenalenones, and quinochacones. Different types of anthocyanins are responsible for different colors of flowers such as pink, red, orange, scarlet, purple, blue, etc. The anthocyanins are derived from 2 Greek words antos: flower and kyanos: blue 8. Although anthocyanins are petals' major coloring component, they are also present in other plant tissues such as roots, tubers, stems 7. Anthocyanins produce various types of colours in flowers. The type and shade of colour of the flowers depend majorly on the chemistry and concentration of anthocyanins, several other factors such as anthocyanidin equilibrium forms, the extent of anthocyanin glycosidation and acylation, the nature and concentration of copigmentation, metal complexes, influence of external factors like pH, salts, etc. are also responsible for shade and colour of the flower 7.
Special Features of Jasminum sambac: The antimicrobial activity carried out by using ethanolic and methanolic extracts J. sambac exhibited the highest activity against pathogens. Hence it is used as antibiotic 9, 11. They also have Anti-inflammatory, analgesic, and anti-pyretic activities carried out with standardized root extract of Jasminum sambac and have a significant role in the treatment of inflammation and associated problems 10. Because anthocyanins, flavonoids present in J. sambac flowers and are pH-sensitive, it was postulated that the flower extract may be used as an indication for various acid-base titrations. As a result, the flavonoids were extracted, separated and identified for use as an acid-base indicator in a variety of acid-base titrations 2.
MATERIALS AND METHODS:
Apparatus: filter paper, funnel burette, stand, conical flask beaker, test tube, test-tube stand, tripod stand, aluminium foil, conical flask, beaker, etc.
Chemicals: Hydrochloric acid (HCl), Glacial Acetic acid (CH3COOH), Sodium hydroxide (NaOH), Ammonia (NH3), Ethanol (C2H5OH) Phenolphthalein, Distilled water, etc. 1
Flowers: Jasminum sambac.
Method:
Extraction: The flowers of Jasminum sambac, were brought from the local market. All the flowers were authenticated by a Botanist of ‘Ramniranjan Jhunjhunwala College, Ghatkopar, Mumbai’. The flowers were properly washed to remove any dirt. After washing, the flowers were spread on filter paper and were dried in the shade. After complete drying, the petals were taken in a clean mortar and were triturated. Once trituration is complete, the triturated mass is transferred in a conical flask, and 30ml of ethanol is added. The mixture is mixed by slowly shaking the conical flask. After this, the mouth of the conical flask is covered properly with aluminium foil. After the above process, the mixture is kept for 24 hours for maceration. After 24 hours, the mixture was filtered with normal filter paper. The filtrate collected was immediately transferred into the clean conical flask, and its mouth was covered.
Preliminary Test of Floral Extract: It is just done to check that whether the extract changes the colour in different acidic and basic mediums. Five clean test tubes were taken and arranged on a test tube stand. In each test tube, 2ml distilled water, 1N HCl, 1N CH3COOH, 1N NaOH and ammonia solution were added in each. After this 2-3 drops of floral extract were added in each test tube and colour change was observed.
Titration of Standard: Clean burette was arranged on stand. It was filled with 1N NaOH. 10ml of 1N HCL was taken in a conical flask and 2 to 3 drops of phenolphthalein indicator solution was added to it.
This was titrated with 1 N NaOH till the endpoint (pink). It was repeated 5 times and burette reading was recorded. Similarly, 1N CH3COOH was titrated with 1N NaOH.
Titration with Floral Extract: Clean burette was arranged on the stand. It was filled with 1N NaOH. 10ml of 1N HCL was taken in conical flask and 5-6 drops of floral extract was added to it. This was titrated with 1 N NaOH till the endpoint. It was repeated five times, and burette rereading was recorded.
OBSERVATION:
Titration of Standard:
TABLE 1: 1N HCL WAS TITRATED WITH 1N NaOH BY USING PHENOLPHTHALEIN AS INDICATOR
| S. no. | Initial burette reading (ml) | Final burette reading (ml) |
| 1 | 0 | 10.8 |
| 2 | 0 | 11 |
| 3 | 0 | 11 |
| 4 | 0 | 11.2 |
| 5 | 0 | 11 |
TABLE 2: 1N CH3COOH WAS TITRATED WITH 1N NaOH BY USING PHENOLPHTHALEIN AS INDICATOR
| S. no. | Initial burette reading (ml) | Final burette reading (ml) |
| 1 | 0 | 11 |
| 2 | 0 | 10.9 |
| 3 | 0 | 10.9 |
| 4 | 0 | 11 |
| 5 | 0 | 10.8 |
TABLE 3: PRELIMINARY TEST
| Solution | Observation |
| H2O | Yellowish |
| HCl | Slightly turbid |
| CH3COOH | Colourless |
| NaOH | Dark yellow |
| NH3 | Yellow |
FIG. 2: PRELIMINARY TEST OF FLORAL EXTRACT OF JASMINUM SAMBAC
Titration with Floral Extract of Jasminum sambac:
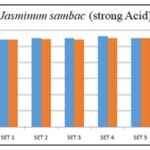
TABLE 4: HCL WAS TITRATED WITH 1N NaOH (STRONG ACID v/s STRONG BASE)
| S. no. | Initial burette reading (ml) | Final burette reading (ml) |
| 1 | 0 | 10.8 |
| 2 | 0 | 10.9 |
| 3 | 0 | 10.8 |
| 4 | 0 | 11 |
| 5 | 0 | 11 |
FIG. 3: TITRATION RESULT OF HCL v/s NaOH USING FLORAL EXTRACT OF JASMINUM SAMBAC AS INDICATOR
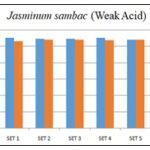
TABLE 5: 1N CH3COOH WAS TITRATED WITH 1N NaOH (WEAK ACID v/s STRONG BASE)
| S. no. | Initial burette reading (ml) | Final burette reading (ml) |
| 1 | 0 | 10.6 |
| 2 | 0 | 10.8 |
| 3 | 0 | 10.8 |
| 4 | 0 | 10.7 |
| 5 | 0 | 10.8 |
FIG. 4: TITRATION RESULT OF CH3COOH v/s NaOH USING FLORAL EXTRACT OF JASMINUM SAMBAC AS INDICATOR
RESULT:
FIG. 5: 1N HCL WAS TITRATED WITH 1N NaOH (STRONG ACID v/s STRONG BASE)
FIG. 6: 1N CH3COOH WAS TITRATED WITH 1N NaOH (WEAK ACID v/s STRONG BASE)
Comment: In both cases, i.e., with strong acid v/s strong base and weak acid v/s strong base, burette reading by using floral extract of Jasminum sambac as an indicator matches standard (phenolphthalein) indicator.
CONCLUSION: Commonly used indicators for acid-base titration are synthetic. This experiment was performed to find out eco-friendly and natural indicators from floral extracts of Jasminum sambac. These flower’s extracts are used to perform titration with Strong acid-Strong base and weak acid-Strong base. We have get sharp and clear colour change from flower extract. Flowers’s extract shows sharp color change with acid and base, making the floral extract suitable as acid-base indicators. As these flower's extracts are very simple, cost-effective, and excellent performances with sharp color change at the endpoints of the titration, it will be possible to replace the synthetic indicators used in labs with natural floral indicators.
ACKNOWLEDGEMENT: We are thankful to the ‘Ideal College of Pharmacy and Research’ for providing us the platform to carry out our research. We also express our guide Dr. Ujwala Dube (HOD of Pharmacognosy, Ideal College of Pharmacy and Research) under whose guidance in project is carried out. We also thank the lab assistant, Mrs. Priyanka mam, who helped us make all the apparatus and chemicals available to us. We also thank our friends who were directly or indirectly involved in the project.
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Abbas SK: Study of acid-base indicator property of flowers of Ipomoea biloba. International Current Pharmaceutical Journal 2012; 1(12): 420-22.
- Shishir MN, Laxman JR, Vinayak PN, Jacky DR, and Pradip KP: Use of Canna indica flower extract as a natural indicator in acid base titration. Journal of Pharmacy Research 2008; 1(1).
- Garba MD and Abubakar S: Flower extract as an improvised indicator in acid – base titration. Chemsearch Journal 2012; 3(1): 17-18.
- Sabharwal S, Sudan S and Ranjan V: Jasminum sambac linn (motia): a review. International Journal of Pharmaceutical Research and Bio-Science 2013; 2(5): 108-30.
- Encyclopedia of India medicinal plants, Rational Western Therapy, Ayurvedic Other Usage, Botany by C. P. Khare.
- Indian material medica, Indian medicinal plants by Dr. K.M Nadkarni
- Flavonoids- Chemistry, Biochemistry and Applications- edited by Oyvind M. Andersen, Kenneth R. Markham
- Anthocyanins biosynthesis, function and applications- edited by Kevin Gould, Kevin Davies, Chris Winefiled.
- Joy P and Raja DP: Anti-Bacterial activity studies of Jasminum grandiflorum and Jasminum sambac. Ethnobotanical Leaflets 2008; 12: 481-83.
- Sengar N, Joshi A, Prasad SK, Hemalatha S, Abdoul-Latif F, Edou P, Eba F, Mohamed N, Ali A, Djama S, Obame LC, Bassolé I and Dicko M: Anti-inflammatory, analgesic and anti-pyretic activities of standardized root extract of Jasminum sambac. Journal of Ethnopharmacology 2015; 160: 140-48.
- Abdoul-Latif F, Edou P, Eba F, Mohamed N, Ali A, Djama S, Obame LC, Bassolé I and Dicko M: Antimicrobial and antioxidant activities of essential oil and methanol extract of Jasminum sambac from Djibouti. African Journal of Plant Science 4(3): 038-043.
How to cite this article:
Bhoir R, Dube U, Choudhary A and Dalvi A: Study of ethanolic extract of Jasminum sambac use as an acid-base indicator. Int J Pharmacognosy 2021; 8(7): 311-14. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(7).311-14.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
303-306
738 KB
926
English
IJP
Rutuja Bhoir, Ujwala Dube, Ayub Choudhary and Aniket Dalvi
Ideal College of Pharmacy & Research, University of Mumbai , Maharashtra, India.
rutujab30499@gmail.com
11 June 2021
25 July 2021
27 July 2021
10.13040/IJPSR.0975-8232.IJP.8(8).311-14
31 July 2021