STUDY OF ANTI-DIABETIC ACTION OF NYMPHAEA NOUCHALI FLOWER EXTRACT IN ALLOXAN-INDUCED DIABETES IN RATS
HTML Full TextSTUDY OF ANTI-DIABETIC ACTION OF NYMPHAEA NOUCHALI FLOWER EXTRACT IN ALLOXAN-INDUCED DIABETES IN RATS
Ashfaque Alam * and Nishi Prakash Jain
RKDF College of Pharmacy, Bhopal, Madhya Pradesh, India.
ABSTRACT: The present work focused on preparing ethanolic extract of Nymphae nouchali flower and evaluating its anti-diabetic potential in rats. The extraction of flowers was carried out using ethanol and the extraction yield was found to be 18.6% w/w. The result of the total phenolic content of the extract examined using Folin-Ciocalteu method revealed total phenolic content of ethanolic extract of Nymphae nouchali was 41.26 ± 2.13 GAE mg/g. The antidiabetic action of the extract was evaluated in terms of the effect of extract on body weight and serum glucose level. It was found that alloxan administration reduced the body weight of animals significantly in comparison with the normal control animals. The results also revealed that the serum glucose levels were highly elevated by the administration of alloxan in all the groups by day 7. In the treatment groups III to V, the glucose levels dropped in comparison to group II and by day 21, the levels reached almost the level of control group in group III and V. This indicates that the extract exhibits significant glucose lowering potential.
Keywords: Antidiabetic, Nymphae nouchali, Serum glucose, Lipid, Biochemical
INTRODUCTION: Diabetes encompasses a range of metabolic problems and is a significant global health concern 1. The remarkable economic progress and swift urbanization in Asian nations, notably in India, have resulted in a transition of health issues from communicable to non-communicable diseases. Developing countries will account for more than 85 percent of the world's diabetic patients by the year 2030. The incidence of diabetes in India is projected to rise from 31 million in 2000 to 79.4 million in 2030 2. Plants and their derivatives have been utilized for millennia in the treatment of ailments 3.
Many animal investigations have shown that these herbs possess anti-hyperglycemic properties, confirming their purported action 4-10. Furthermore, clinical investigations have demonstrated that certain plants possess beneficial properties as anti-diabetic medications. However, the isolated chemical compounds derived from these plants do not share structural similarities with the anti-diabetic drugs now used in clinical practice, nor do they operate through similar modes of action. However, the ongoing pursuit of a new anti-diabetic medication supports the use of plants as a promising resource.
This can be accomplished by applying advanced scientific techniques and the latest understanding of the physiological changes associated with diabetes 11. Nymphae nouchali is a flowering plant that has been associated with constituents like gallic acid, kaempferol, quercetin and nymphayol 12. The presence of these constituents offers several pharmacological properties to the flowers of this plant 13, 14. Literature has revealed that roots and leaves of various species of Genus Nymphaea have been helpful in preventing experimentally induced diabetes in rodents, but the effect of N. nouochali flowers has not been scientifically explored in diabetes. Hence in this study we attempted to assess the antidiabetic potential of the extract of the Nymphae nouchali flowers in experimentally induced diabetes
MATERIAL AND METHODS:
Extraction of Phytoconstituents 15: The extraction technique involved the utilization of powdered flowers through the hot continuous extraction method utilizing a Soxhlet equipment. A total of 47 grams of powdered flower was uniformly distributed within the extractor of the device. Subsequently, 250 milliliters of ethanol were poured over the powder and allowed to accumulate in the flask that was attached to it. The extraction process involved heating the solvent to a temperature of 65°C for a duration of 9 hours until a solution devoid of color was obtained and collected in the siphon tube of the device. The extract underwent filtration using a Whatman filter and was subsequently concentrated using a rotating vacuum evaporator. The resinous extract was gathered and placed in a desiccator to eliminate any excess moisture. The dehydrated extract was kept in a desiccator for subsequent processing.
Preliminary Phytochemical Screening 16: The extract underwent qualitative phytochemical analysis to determine the specific plant secondary metabolites it contained. The screening was conducted to detect triterpenes/steroids, alkaloids, glycosides, flavonoids, saponins, tannins, and phenolic acids. Analytical responses to these tests were determined by either the color intensity or the production of a precipitate.
Total Phenolic Content 17: To ascertain the overall phenolic content, a mixture of 0.5 grams of dry powder and 5 milliliters of methanol was prepared and left undisturbed for the duration of one night. The suspension underwent filtration using a qualitative cellulose filter paper, and the resulting liquid was then diluted to a volume of 10 mL using methanol. The solution was stored at a temperature of 4°C in amber bottles and used as the primary solution for further studies. To determine the total phenolic content, 200 μL of the sample was combined with 1.4 mL of filtered water and 100 μL of Folin-Ciocalteu reagent. After a duration of 3 minutes, a volume of 300 microliters of a 20% aqueous solution of Na2CO3 was introduced to the mixture, followed by a settling period of 2 hours. The measurement of absorbance was conducted at a wavelength of 760 nm using a UV-Vis spectrophotometer. The calibration curve was obtained by treating standard solutions of gallic acid (20-100 ppm) in a similar manner. The control solution comprised 200 μL of water and appropriate reagents. It was produced and incubated under identical circumstances to the other samples. The results were quantified as milligrams of gallic acid equivalent (GAE) per 100 grams of the dry material.
Pharmacological Study:
Animal Used: Male rats weighing 180–230 g was used, and they were obtained from Bhopal-approved vendors. The rodents had unlimited access to water and a pellet meal (Lipton India Ltd., Mumbai, Ind.). Throughout the trials, all laboratory setups and animal care were conducted in accordance with CPCSEA rules.
Acute Toxicity Study: Both medications' short- and long-term harmful effects, as well as the extracts from them, were evaluated in accordance with OECD guideline no. 423. The 150–200 g albino rats used in the study were housed in a 12-hour day night cycle with unlimited access to water. The extract was dissolved in one percent Tween 80, which was made using purified water. The animals were given a 12 hour fast before the extract was given to them orally in dosages up to 2000 mg/kg, which was the maximum weight that was considered.
Any strange behavior, such as changes in skin and fur, eyes, hyperactivity, grooming, convulsions, sedation, hypothermia, salivation, tremor, coma, lethargy, body weight, and mortality, should be observed within the first four hours. Based on research and observations, therapeutic doses of one-tenth and one-fifth of the lethal dose were employed, with 200 and 400 mg/kg as cut-off values to test dose-dependent effect and nootropic activity 18.
Induction of Diabetes: After a 12-hour fast, the animals received intraperitoneally (i.p.) 55 mg/kg bodyweight of freshly produced alloxan in 0.1 mol/L cold citrate buffer (pH 4.5). To reverse the drug-induced hypoglycemia, the rats receiving alloxoan were given access to a 5% glucose solution to consume throughout the night. Rats exhibiting chronic glycosuria and hyperglycemia, defined as fasting blood glucose levels greater than 250 mg/dL on the third day following the alloxan injection, were classified as diabetic and utilized in subsequent studies 19.
Experimental Design: The rats were split up into five groups, each with six members. Group II represented alloxan (160 mg/kg b.w., i.p.)-induced diabetic rats serving as the diabetic control group; Group I served as the normal receiving water. Glibenclamide 5 mg/kg b.w./p.o. was administered to diabetic rats induced by alloxan (160 mg/kg b.w., i.p.); NNE 200 mg/kg b.w./p.o. was administered to diabetic rats induced by alloxan (160 mg/kg b.w., i.p.); and For 21 days, NNE 400 mg/kg b.w./p.o.20
Before extracts were administered, blood glucose levels were assessed after a fast. On the first, seventh, fourteenth, and twenty-first days of the therapy period, blood glucose levels were measured. After the rat's tail was chopped off, blood was extracted. Glucose oxidase peroxidase reactive strips and a glucometer were used to measure blood glucose levels.
Biochemical Study: Blood samples were taken, serum was extracted using a centrifuge, and the animal was sacrificed by beheading on the last day in order to examine the biochemical parameters. The Lowry technique 21 was used to estimate the amount of protein. The Folch method 22 was used to extract serum lipids, and the Zlatkis method 23 was used to estimate serum cholesterol. The Burstein method 24 was used to estimate HDL cholesterol and the Foster and Dunn method was used to quantify serum triglycerides. The TG/5 mg/dl formula was used to calculate the VLDL cholesterol. The Friedwal method 25 was used to estimate the serum LDL cholesterol. Using the Reitman and Frankel method (a colorimetric approach), SGOT and SGPT were measured 26. The diacetylmonoxime method 27 was used to measure serum urea, while Jaffe's method28 was used to detect plasma creatinine.
RESULTS AND DISCUSSION: The ethanol extraction yield of Nymphae nouchali flowers using hot continuous extraction method was determined to be 18.6% w/w. The extract possessed a resinous consistency and exhibited a black hue. The phytochemical examination indicates the presence of phenolics, tannins, proteins, and flavonoids in the ethanolic extract of the flowers.
Total Phenolic Content: The total phenolic content of Nymphaenouchali flowers was measured using an ethanolic extract. A standard curve for gallic acid was constructed using pure water. The Folin-Ciocalteu method was used to analyze the total phenolic content of the extract, yielding the result. The ethanolic extract of Petunia hybrida was determined to have a total phenolic content of 41.26±2.13 GAE mg/g.
Acute Toxicity Study: No signs, symptoms, or harmful consequences were observed in rodents for either plant, even at a greater dose of 2000 mg/kg body weight. Therefore, a dosage equivalent to one-tenth of the maximal dose was chosen as the effective dose. The threshold value of 200 and a fractional dose of 1/5, specifically 400 mg/kg, were selected to assess the effectiveness of memory enhancement.
Antidiabetic Activity of NNE: The extract's antidiabetic efficacy was assessed by examining its impact on body weight Table 1 and serum glucose level. In addition, other indicators such as cholesterol and serum urea were assessed.
TABLE 1: EFFECT OF NNENNE ON BODY WEIGHT
| Group | Body weight (g) | |||
| Day 0 | Day 7 | Day 14 | Day 21 | |
| I | 185.4±1.910 | 184.3±2.257 | 204.2±1.033 | 212.6±1.161 |
| II | 187.3±2.010 | 147.4±1.042 | 131.5±1.266 | 122.7±0.964 |
| III | 182.6±1.930 | 171.8±2.010 | 172.5±1.033 | 180.9±1.780 |
| IV | 181.6±3.165 | 161.4±2.026 | 162.9±0.933 | 167.5±1.033 |
| V | 184.3±2.186 | 167.4±1.865 | 169.1±1.166 | 173.2±1.303 |
The experimental induction of hyperglycemia using alloxan is linked to a distinct decrease in body weight. This decrease is caused by the breakdown or degradation of structural proteins, resulting in increased muscle wasting. The loss of tissue protein, specifically structural proteins, is known to contribute to the reduction in body weight. Alloxan administration considerably decreased the body weight of animals compared to the normal control animals (Group I). However, the treatment of glibenclamide or NNE resulted in an increase in body weight compared to Group II.
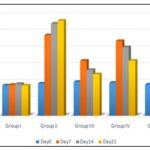
FIG. 1: COMPARISON OF BLOOD GLUCOSE OF TEST ANIMAL IN VARIOUS GROUPS
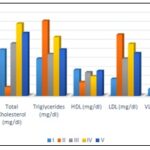
The findings demonstrated that the serum glucose levels were significantly increased by the injection of STZ in all groups by day 7. In treatment groups III to V, the glucose levels decreased compared to group II. By day 21, the levels in groups III and V practically reached the level of the control group. Fig. 1 demonstrates that the extract has a notable ability to reduce glucose levels. One possible mechanism for lowering blood glucose levels is a decrease in glucose transport or absorption from the gut. Additionally, there may be an increase in glucose utilization in peripheral tissues due to extra pancreatic action. This could be accompanied by an increase in the activity of enzymes involved in glycogen production or glycolysis in peripheral tissues. Another contributing factor may be a decrease in the secretion of counter-regulatory hormones such as glucagon and growth hormones. Hyperglycemia typically coexists with dyslipidemia. In normal conditions, insulin stimulates the action of lipoprotein lipase, an enzyme that breaks down triglycerides. However, in a diabetic state, lipoprotein lipase remains inactive due to a lack of insulin, leading to high levels of triglycerides in the blood. Additionally, insulin insufficiency is also linked to high levels of cholesterol due to metabolic irregularities. The results demonstrate that the administration of EEPH effectively controlled the levels of lipids, including cholesterol and triglycerides, indicating its notable influence on enhancing metabolic rate Fig. 2.
FIG. 2: COMPARISON OF LIPID LEVELS OF TEST ANIMAL IN VARIOUS GROUPS
TABLE 2: EFFECT ON NNE ON SGOT, SGPT, CREATININE AND UREA
| Group | Serum Urea (mg/dl) | Creatinine (mg/dl) | SGOT(U/L) | SGPT (U/L) |
| I | 5.38±0.167 | 0.54±0.018 | 45.78±0.21 | 21.80±0.21 |
| II | 4.24±0.143 | 1.59±0.037 | 112.43±0.46 | 46.29±0.45 |
| III | 5.48±0.182 | 0.57±0.015 | 42.77±0.85 | 24.65±0.54 |
| IV | 5.25±0.217 | 1.26±0.026 | 62.77±0.91 | 35.13±0.69 |
| V | 5.82±0.138 | 0.76±0.018 | 50.86±0.63 | 29.55±0.33 |
Both SGOT and SGPT enzyme levels increase in cases of liver injury, with a higher elevation observed in diabetic rats. The results indicate a decrease in the levels of these enzymes in the rats treated with EEPH. Elevated levels of serum creatinine are linked to kidney dysfunction in individuals with diabetes. The EEPH therapy successfully replenished the levels of creatinine, indicating a possible antidiabetic effect Table 2.
CONCLUSION: The aim of this study was to extract Nymphae nouchali flowers using ethanol as the solvent, identify the specific class of secondary metabolites in the extract, measure the total phenolics and flavonoids present in the extract, and assess the extract's anti-diabetic effects in rats/mice. The study's findings indicate that the plant's blooms contain phenolics and flavonoids, which have a positive impact on regulating lipid profiles and glucose levels in rats with alloxan-induced diabetes. The extract also regulated the animals' body weight and levels of protein, creatinine, SGOT, and SGPT. Therefore, it can be inferred from the study that Nymphae nouchali flowers have notable anti-diabetic properties and should be further investigated to identify the specific components responsible for their effects.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- King H, Aubert RE and Herman WH: Global burden of diabetes, 1995-2025 -Prevalence, numerical estimates and projections. Diabetes Care 1998; 21: 1414-1431.
- Wild S, Roglic G, Green A, Sicree R and King H: Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-1053.
- Prezeor M: Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022; 15: 65. Doi: 10.3390/ph15010065
- Angadi KK, Kandru A and Rahaman A: Antihyperglycaemic, antihyperlipidaemic and antioxidant assays (in-vivo) of Nymphaea pubescens leaf extract. International Journal of Pharmaceutical and Biological Sciences 2013; 4(2): 624-630.
- Tafesse TB, Hymete A, Mekonnen Y and Tadesse M: Antidiabetic activity and phytochemical screening of extracts of the leaves of ajugaremotabenth on alloxan-induced diabetic mice. BMC Complementary and Alternative Medicine 2017; 17: 243.
- Shaha MA, Khalil R, Ul-Haq Z and Panichayupakaranant P: Α-Glucosidase inhibitory effect of rhinacanthins-Rich extract from Rhinacanthus nasutus leaf and synergistic effect in combination with acarbose. Journal of Functional Foods 2017; 36: 325-331.
- Thomson M, Khaled K. Al-Qattan, Divya JS and Ali M: Anti-diabetic and anti-oxidant potential of aged garlic extract (age) in streptozotocin-induced diabetic rats. BMC Complementary and Alternative Medicine 2016; 16: 17.
- Wang J, Teng L, Liu Y, Hu W, Chen W, Hu X, Wang Y and Wang D: Studies on the antidiabetic and antinephritic activities of paecilomyceshepiali water extract in diet-streptozotocin-induced diabetic sprague dawley rats. Journal of Diabetes Research 2016.
- Moodley K, Joseph K, Naidoo Y, Islam S and Mackraj I: Antioxidant, antidiabetic and hypolipidemic effects of Tulbaghia violacea Harv. (Wild garlic) rhizome methanolic extract in a diabetic rat model. BMC Complementary and Alternative Medicine 2015; 15: 408.
- Reejo BD, Natarajan P and Thangathirupathi A: Evaluation of antidiabetic activity of samaneasaman (Jacq.) Merr. International Journal of Research in Pharmaceutical and Nano Sciences 2014; 3(4): 352- 356.
- Shukla R, Sharma SB and Puri D: Medicinal plants for treatment of diabetes. Indian J Clin Biochem 2000; 15: 169.
- Angadi KK, Kandru A and Rahaman A: Antihyperglycaemic, antihyperlipidaemic and antioxidant assays (in-vivo) of Nymphaea pubescens leaf extract. Int J Pharm Bio Sci 2013; 4(2): 624-630.
- Thongdonphum B, Vanichkul K, Bunchaleamchai A and Powthong P: In-vitro antimicrobial activity of Nymphaea pubescens (Pink Water Lily) leaf extracts. Plants 2023; 12: 3588. Doi: 10.3390/plants12203588
- Amreen R and Chaurey M: Evaluation of estrogenic potential of ethanolic and aqueous extract of Pitunia hybrida. Journal of Pharmacology and Biomedicine 2021; 5(3): 312-318.
- Abubakar AR and Haque M: Preparation of medicinal plants: basic extraction and fractionation procedures for experimental purposes. Journal of Pharmacy & Bioallied Sciences 2020; 12(1): 1-10.
- Aiyegoro OA and Okoh AI: Preliminary phytochemical screening and in-vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complementary and Alternative Medicine 2010; 10: 21. https://doi.org/10.1186/1472-6882-10-21
- Tiwari P, Joshi A and Dubey BK: Total phenolic content, flavonoid concentration, antimicrobial and insecticidal screening of aqueous extracts of Annona squamosa (seeds), Azadira chtaindica (leaves) and Lavandula angustifolia (flower). Journal of Pharmacology and Biomedicine. 2017; 1(1): 30-43
- OECD Guidelines 2001. “Guidance document on acute oral toxicity testing” Series on testing and assessment No. 23, Organization for Economic Co-operation and Development, OECD Environment, health and safety publications, Paris Available from: http://www. Oecd.org/ehs
- Yogesha Mohan, Grace Nirmala Jesuthankaraj and Narendhirakannan Ramasamy Thangavelu: Antidiabetic and Antioxidant Properties of Triticum aestivum in Streptozotocin-Induced Diabetic Rats. Advances in Pharmacological Sciences. 2013, Article ID 716073.
- Vishwanath Jannu, Sai Vishal D, Ranjith Babu V, Harisha B and Ravi Chandra Sekhara Reddy D: Antidiabetic activity of hydro-alcoholic extract of Cissampelos pareira linn. Leaves in streptozotocin induced diabetic rats. International Journal of Pharmacy & Technology.
- Lowry OH, Rosenbrough NJ, Farr AL and Randall RJ: Protein measurement with Folin-phenol reagent. Journal of Biological Chemistry 1951; 193: 265–275.
- Folch J, Lees M and Solane SGH: A simple method for isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry 1957; 26: 497–509.
- Zlatkis A, Zak B and Boyle AJ: A new method for the direct determination of serum cholesterol. Journal of Laboratory and Clinical Medicine 1953; 41: 486–492.
- Burstein M, Scholnichk HR and Morin R: Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. Journal of Lipid Research 1970; 11: 583–595.
- Friedwald WT, Levy RI and Fredrickson DS: Estimation of the concentration of LDL-cholesterol in plasma without the use of the preparative ultracentrifuge. Clinical Chemistry 1972; 18: 499-502.
- Reitman S and Frankel S: Colorimetric method for the determination of serum glutamic oxaloacetic acid and glutamic pyruvic transaminases. American Journal of Clinical Pathology 1957; 28: 56–63.
- Wybenga DR, Di Giorgio J and Pileggi VJ: Manual and automated methods for urea nitrogen measurement in whole serum. Clinical Chemistry 1971; 17: 891–895.
- Slot C: Plasma creatinine determination. Jaffe’s a new and specific reaction method. Scandavian Journal of Clinical Laboratory Investigation 1965; 17: 381–387.
How to cite this article:
Alam A and Jain NP: Study of anti-diabetic action of Nymphaea nouchali flower extract in alloxan-induced diabetes in rats. Int J Pharmacognosy 2024; 11(7): 322-27. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(7).322-27.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.