STANDARDIZATION OF AERIAL PARTS AND PHYTOCHEMICAL INVESTIGATION OF PLANT PHYLLANTHUS MADERASPATENSIS L. (MADRAS NELLI)
HTML Full TextSTANDARDIZATION OF AERIAL PARTS AND PHYTOCHEMICAL INVESTIGATION OF PLANT PHYLLANTHUS MADERASPATENSIS L. (MADRAS NELLI)
Ramesh Raj Padhaya 1, Sajan Maharjan *2, Amrit Khanal 3, Thakur Pokhrel 4 and Gopala Krishna Burdipad5
National Ayurveda Research and Training Centre 1, Kirtipur, Kathmandu, Nepal.
Central Institute of Science and Technology 2, New Baneshwor, Kathmandu, Nepal.
Ohm Hospital and Research Centre 3, Chabahil, Kathmandu, Nepal.
NIST College 4, Lainchaur, Kathmandu, Nepal.
R.R College of Pharmacy 5, Chikabanavara, Bangalore - 560090, Karnataka, India.
ABSTRACT: The study was designed to investigate the pharmacognostic characters and phytochemical profile of crude drugs obtained from powdered aerial parts of Phyllanthus maderaspatensis L. (Madras nelli). The macroscopic, microscopic, phytochemical screening, and HPTLC studies of powdered aerial parts of the plant were carried out. Powder microscopy of aerial part powder of Madras nelli showed an isocytic type of stomata and with the straight cell wall. Treatment of aerial parts powder of Madras nelli showed a positive test for starch, tannins, and lignins, whereas negative for oil globules and crystals. Loss on drying was 5%, Total Ash value, and Acid – insoluble Ash value was found to be 10% and 2%, respectively. Aqueous and Alcohol extractive values were found to be 11.80% and 9.80%, respectively. The phytochemical screening test revealed for the presence of carbohydrates and in ethanol and water extracts, phytosterols in ether extract, flavonoids, and tannins in ethanol extract and saponins in ethanol and water extract. HPTLC study of the alcoholic extract revealed five phytoconstituents at Rf -0.49, 0.60, 0.70, 0.72, 0.77.
| Keywords: |
Phyllanthus maderaspatensis L. Pharmacognosy, Phytochemical screening, HPTLC
INTRODUCTION: Herbal care or traditional system of medicine are used throughout the world, and from century herbs have been the original source for most of the drugs. Medicinal plants contain so many chemical compounds that are the major source of therapeutic agents to cure human diseases. Recent discovery and advancement in medicinal and aromatic plants have to lead to the enhancement of health care of mankind. Among them, Phyllanthus maderaspatensis L. is also one of the important traditional medicine commonly called Madras nelli.
The plant sap and leaf decoction are credited with emetic and purgative activities. In Tanzania, the whole plant is pounded, and the solution is applied to scabies. A root decoction is taken to cure constipation, diarrhea, lack of appetite, intestinal pain, menstrual problems, gastrointestinal disorders, testicular swelling, chest complaints, and snakebites.
Plant sap is used as nose drops to treat toothache. Ground leaves are rubbed on the skin with lemon juice as a treatment for rheumatism. In Nigeria, the plant is used as an aphrodisiac. In Somalia, Phyllanthus maderaspatensis is considered poisonous. In India, Phyllanthus maderaspatensis is medicinally used to treat headaches, bronchitis, earache, and ophthalmic. Powder from dried plant material mixed with milk is drunk to treat jaundice. In Kenya, smoke from the burning plants is used to kill caterpillars in maize 1. As Madras nelli is reported for the presence of flavonoids and also reported for treating a wide range of ailments, the plant is selected for its standardization of aerial parts and phytochemical investigation.
METHODOLOGY:
Collection of Plant Material: The plant Madras Nelli was collected from the Chittur district of Andhra Pradesh. It was dried under shade and made coarse powder.
Identification: The plant material collected was identified and authenticated by Assistant Prof. (Dr.) K. Madhava Chetty, Department of Botany, Shree Venkateswara University Tirupati Chittur district, Andhra Pradesh.
Pharmracognostical Studies: Different parameters viz; macroscopy, microscopy, and proximate values were investigated. The macroscopical features and microscopical features were investigated as per standard protocols.
Macroscopical Features: 2, 3 Under macroscopical features, features like color, taste, size, and other morphological features were examined.
Microscopical Features: 4, 5
Powder Characters: For the study of powder characters, the aerial parts were collected and washed thoroughly with water to remove any unwanted matter. The cleaned parts were further dried in the shade. After complete drying, it was powdered and passed through sieve no. 60. A small quantity of powder was treated with different reagents like chloral hydrate, phloroglucinol, and conc. HCl, iodine solution, etc. for the detection of the constituents like lignin, starch, and calcium oxalate crystals.
Powder Microscopic: For microscopical studies, 3g of powder was taken. The powder was treated by warming with a few drops of chloral hydrate, stained with phloroglucinol: conc. HCl (1:1). The powder was then mounted in glycerine for microscopical observations. The photographs of the images were captured using the normal camera by observing different sides of the material under a compound microscope.
Proximate Values: 3, 6 The physical constants like ash and extractive values help in establishing the pharmacopoeial standards of the drug.
Determination of Loss on Drying: About 5 g of the drug was weighed in a petri plate, kept in hot air oven at 105 °C and dried for a period until constant weight was obtained. The weight loss on drying was noted and difference in weight gives the loss on drying of the powdered drug. The total loss on drying of powder was noted.
Determination of Ash Value: About 3 g of the powdered drug was weighed and placed in a silica crucible, which was previously ignited and weighed. The powdered drug was spread uniformly in a fine layer at the bottom of the tarred silica crucible. The crucible was kept inside the muffle furnace and the temperature increased to make crucible dull red hot until free from carbon. The crucible was cooled, kept in a desiccator and weighed. The same procedure was repeated to arrive at constant weight. The percentage of total ash obtained was calculated with reference to the air dried drug. The total ash value of powdered sample noted.
Determination of Extractive Values:
Alcohol Soluble Extractive Value: 4.0 g of powdered air dried material was accurately weighed, in a glass-stopper conical flask, macerated with 100 ml of the ethanol for 6 h, shaking frequently and then allowed standing for 18 h. Filtered rapidly taking care not to lose any solvent, transferred 25 ml of the filtrate to a tarred flat-bottomed dish and evaporated to dryness on a water bath. Dried at 105 °C for 6 h, cooled in a desiccator for 30 min and weighed immediately. Finally, the content of extractable matter was calculated in w/w of air-dried material.
Water-soluble Extractive Value: Placed about 4.0 g of powdered air-dried material, accurately weighed, in a glass-stopper conical flask and macerated with 100 ml of the ethanol for 6 h, shaking frequently and then allowed standing for 18 h.
Filtered rapidly taking care not to lose any solvent, transferred 25 ml of the filtrate to a tarred flat-bottomed dish and evaporated to dryness on a water bath and dried at 105 °C for 6 h, cooled in a desiccator for 30 min and weighed immediately. Finally, the content of extractable matter was calculated in w/w of air-dried material.
Phytochemical Studies: Phytochemical screening was carried out by the methods referred from the textbook authored Pulok Mukherjee and Kokate. Chromatographic studies were carried out by referring textbook by E. Stall and Wagner et al.
Preliminary Phytochemical Screening: 5, 7
Preparation of extract: The previously powdered drug was used for preparing extract. Different extracts are prepared by extracting the plant material with different solvents with increasing polarity.
Successive Solvent Extraction: About 50 g of the air-dried powdered plant material was extracted successively with petroleum ether 40-60 °C, chloroform, and alcohol in a Soxhlet apparatus. Each time before extracting with the next solvent, the marc was air-dried below 50 °C. The extracts were filtered, the solvent was evaporated at room temperature, and the accurate weight of the extracts was taken. The extractive value (%) was calculated with reference to air-dried drug.
Preparation of Alcoholic and Aqueous Extract: About 300 g of the powdered drug is taken into a maceration chamber and made wet with 95% ethanol; the solvent level is maintained above the bed of powdered drug material. Maceration is carried out for 14 days with intermediate shaking. Another 300 gm of the powdered drug is macerated with chloroform, water following the above procedure. Both the extracts obtained were filtered carefully, and the solvent was evaporated at room temperature. The extractive value (%) was calculated with reference to air-dried drug.
Detection of Chemical Constituents: Different chemical tests were performed for detecting various chemical constituents.
Detection of Carbohydrates: Small quantity of acetone, alcohol, and aqueous extracts were dissolved separately in distilled water and filtered. The filtrate was subjected to various tests to detect the presence of different carbohydrates.
Molisch’s Test: The filtrates were treated with a solution of alcoholic a-Naphthol (Molisch’s reagent). 0.2 ml of conc. sulphuric acid was added slowly through the sides of the test column. The development of purple to violet ring at the junction of the liquids indicates the presence of carbohydrates.
Fehling’s Test: Equal volumes of Fehling’s solution A (CuSO4 in distilled water) and Fehling’s solution B (Potassium Tartrate and NaOH in distilled water) are added to test solution and mixed. The mixture is then boiled on a water-bath. The formation of a red brick precipitate of CuO indicates the presence of reducing sugars.
Barfoed’s Test: 1 ml of Barfoed’s reagent is added to 1 ml of the test solution and then boiled on a water-bath. The formation of a red precipitate of cupric oxide indicates the presence of mono-saccharides.
Detection of Proteins and Free Amino Acids: Small quantities of alcohol and aqueous extracts were diluted separately in water and tested for the presence of proteins and free amino acids by subjecting the extracts to various tests.
Biuret’s Test: The test solution is treated with a few drops of 0.7% copper sulphate solution (Biuret’s reagent). The formation of a purplish violet color indicates the presence of proteins.
Heat Coagulation Test: Heat the test solution on boiling water-bath. Formation of non-clear solution indicates the presence of proteins, as proteins coagulate on heating.
Millon’s Test: To 2 ml of the test solution, about 2 ml of Millon’s reagent was added. The formation of a white precipitate indicates the presence of free amino acids.
Ninhydrin Test: To the test solution, a few drops of Ninhydrin solution were added and boiled. The formation of a violet color indicates the presence of amino acids.
Detection of Phytosterols and Triterpenoids: The petroleum ether, chloroform, acetone and alcohol extracts were refluxed separately with a solution of alcoholic potassium hydroxide till complete saponification took place. The saponified mixtures were diluted with distilled water and extracted with solvent ether. The ethereal extract was evaporated to dryness, and the residue subjected to Liebermann-Burchard’s and Salkowaski tests.
Liebermann-Burchard’s Test: The ethereal residues were treated with a few drops of acetic anhydride, boiled and cooled. 1 ml of sulphuric acid was added through the sides of the test column. Formation of a brown ring at the junction of two liquids and green color in the upper layer indicates the presence of steroids, and deep red color indicates triterpenoids.
Salkowaski Test: The extract is treated with few drops of concentrated sulphuric acid, red color at the lower layer indicates steroids, and yellow color at the lower layer indicates triterpenoids.
Detection of Tannins: Small quantities of acetone, alcohol, and aqueous extracts were diluted separately in water and were tested for the presence of tannins.
Ferric chloride Test: To the test solutions, a few drops of 5% freshly prepared ferric chloride solution was added. The formation of blue-black or green-black color indicates the presence of tannins.
Gelatin Test: To the test solutions, a few drops of 1% gelatin solution in 10% sodium hydroxide was added. The formation of a white precipitate indicates the presence of tannins.
Lead Acetate Test: To the test, solution adds few drops of 10% lead acetate solution. This test solution was treated with a solution of NaOH containing gelatin. The formation of a white precipitate indicates the presence of tannins.
Detection of Flavonoids: The acetone, aqueous and alcohol extracts were subjected to the following additional tests.
Shinoda Test: To the test solution, few magnesium turnings were added, and concentrated hydrochloric acid was added dropwise from the sides of the test column. Pink, scarlet, crimson red, or occasionally green to blue color appears after 5 min indicating the presence of flavonoids.
Alkaline Reagent Test: To the test solution few drops of Sodium Hydroxide solution were added. The formation of an intense yellow color that turns to less intense on the addition of acid indicates the presence of Flavanoids.
Detection of Alkaloids: Small portions of solvent-free extracts were stirred separately with a few drops of dilute hydrochloric acid and filtered. The filtrate was tested with various reagents.
Mayer’s Test: The filtrate is treated with Potassium mercuric iodide (Mayer’s reagent). The formation of a cream color precipitate indicates the presence of alkaloids.
Dragendorff’s Test: The filtrate is treated with Potassium bismuth iodide (Dragendorff’s reagent). The formation of a reddish brown precipitate indicates the presence of alkaloids.
Wagner’s Test: The filtrate is treated with a solution of iodine in Potassium Iodide (Wagner’s reagent). The formation of a brown precipitate indicates the presence of alkaloids.
Hager’s Test: The filtrate is treated with a saturated solution of Picric acid (Hager’s reagent). The formation of a yellow precipitate indicates the presence of alkaloids.
Detection of Fixed Oils and Fats:
Spot Test: Small quantities of petroleum ether and chloroform extracts were pressed separately between two filter papers. The formation of oil stains on the filter paper indicates the presence of fixed oil. A pinch of sodium hydrogen sulphate was added to a few drops of the test solution. Pungent odor emanates, indicating the presence of glycerine.
Detection of Saponins:
Foam Test: About 1 ml of alcohol and aqueous extracts were diluted separately with distilled water to 20 ml and shaken in a graduated cylinder for 15 min. The formation of any froth above the surface indicates the presence of saponins.
Chromatographic Studies: 8, 9
HPTLC Studies: HPTLC is a modified and advanced method of TLC. This technique was used to find out the fingerprint analysis of an alcoholic extract of this plant for the identification and standardization of this plant extract. In the present work, Camag HPTLC system equipped with Linomat V applicator, TLC scanner 3, Camag Reprostar 3, with 12 bit CCD camera for photo documentation, controlled by Win CATS software was used. All the solvents used were of HPLC grade obtained from Merck.
- Preparation of Alcohol Extract Solution in Methanol: A solution of the alcohol extract in methanol was prepared at a concentration of 20 mg/ml.
- Chamber used for Mobile Phase: Camag twin trough chamber (10 × 10 cm).
- Preparation of Mobile Phase for Alcohol Extract: The Mobile phase used for alcohol extract was benzene: acetic acid (4.5:0.4). The mobile is prepared by adding benzene 4.5 ml and acetic acid 0.4 in 10 × 10 cm twin troughs developing chamber and mixed well. This mobile phase is kept for saturation (about 1 h).
- Stationary Phase: Precoated plates for HPTLC, silica gel 60 F 254 manufactured by E. Merck KGaA.
- Procedure: The solution of alcohol extract of 2 µl was applied as 10 mm bands on a pre-coated silica gel G 60 F 254 for HPTLC with a Linomat V applicator using a Camag 100 µl syringe. The mobile phase used for alcohol extract was benzene: acetic acid (4.5:0.4). No pre-washing of the plates was done. The chamber saturation time was 1 h. The sample application position on HPTLC plates was 8.0 mm. The plates were kept for development, to a migration distance of 75 mm. The developed plates were dried with hot air and scanned using Camag TLC Scanner 3 at wavelength 366 nm, 254 nm; slit dimension 4.00 × 0.30 mm, Micro, scanning speed 20 mm/sec. No post derivatization was done prior to scanning. The Rf and peak areas were interpreted by using the software. The developed plates were photo-documented under 254 nm, 366 nm, and visible light, using Camag Reprostar 3.
RESULTS AND DISCUSSION:
Pharmacognostical Studies: Macro and microscopical characters of the plant part were used for the identification of the drug.
Macroscopical Characters of Aerial Part: Madras Nelli is an annual or perennial, erect to glabrous herb of to 90-120 cm tall branches angular red-brown. Leaf was arranged spirally, simple and asymmetrical, triangular, lance late. Flowers were unisexual, regular six lobes with six disc-free glands. Fruits and capsules were flattened at both ends 3 mm in diameter, shiny greenish six seeded and bitter in taste.
Microscopical Characters:
Powder Microscopy: Powder microscopy of aerial part powder of Madras nelli showed anisocytic type of stomata and with the straight cell wall. The regular cell wall was present all along the margins, and crystals were observed in clusters. There were continuous plasid tissues in the mid rib.
FIG. 1: POWDER MICROSCOPY OF AERIAL PART POWDER OF MADRAS NELLI
TABLE 1: POWDER MICROSCOPY CHARACTERS OF MADRAS NELLI
| Plant name | Stomatal type | Cell wall | Margin of lminax | Crystals | Plasid tissue in mid rib |
| P. maderasptensis | Anisocytic | Straight | Regular cell wall all along the margins | Clusters | Continuous |
Treatment of aerial parts powder of Madras Nelli showed positive test for starch, tannins and lignins wheras negative for oil globules and crystals.
TABLE 2: TREATMENT OF AERIAL PARTS POWDER OF MADRAS NELLI
| S. no. | Reagent used | Test | Reaction | Result |
| 1 | Iodine solution | Starch | Blue color | + |
| 2 | Ferric chloride | Tannin | Black color | + |
| 3 | Sudan 3 solution | Oil globule | No oil globule is seen | - |
| 4 | Conc HCl | Crystals | No effervescence is seen | - |
| 5 | Phloroglucinol + dil HCl | Lignin | Magenta colour | + |
+ = present; - = absent.
Proximate Values: Various physical constants of aerial parts of the plant were performed like a loss on drying, ash values, and extractive values, which are presented in Tables 3, 4, and 5, respectively. Loss on drying was 5%, which indicates lower quantities or absence of volatile constituents.
It also shows that the drug was dried enough to control bacterial growth. Total ash value and acid-insoluble ash values were found to be 10% and 2%, respectively. The very low values of acid-insoluble ash represent that the drug less adheres with dirt and sand, which in turn represents the purity of the drug.
The aqueous extractive value was more compared to alcohol extractive value, which may be due to tannins. Both extractive values were found to be 11.80% and 9.80%, respectively.
TABLE 3: LOSS ON DRYING OF THE AERIAL PARTS POWDER OF MADRAS NELLI
| Fresh weight (g) | Dry weight (g) | Loss on drying (g) | Loss on
drying (%) |
| 5 | 4.750 | 0.250 | 5% |
TABLE 4: ASH VALUES OF THE AERIAL PARTS POWDER OF MADRAS NELLI
| Drug weight (g) | Ash weight (g) | Total ash (%) | Acid insoluble ash (%) |
| 3 | 0.30 | 10% | 2% |
TABLE 5: EXTRACTIVE VALUES OF THE AERIAL PARTS POWDER OF MADRAS NELLI
| S. no. | Extractives | Colour | Consistency | Extractive values (% w/w ) |
| 1 | Alcohol soluble | Light brown | Powder | 9.80% |
| 2 | Water-soluble | Dark brown | Powder | 11.80% |
Phytochemical Studies:
Successive Solvent Extraction: Powdered mass of the plant was subjected to successive solvent extractions in different solvents, i.e., Petroleum ether, chloroform, alcohol, and water. The results are presented in Table 6.
TABLE 6: SUCCESSIVE SOLVENT EXTRACTIVE VALUES AND NATURE OF EXTRACTS OF AERIAL PARTS POWDER OF MADRAS NELLI
| S. no. | Solvent | Colour | Consistency | Extractive value (% w/w) |
| 1 | Petroleum ether (40 – 60 °C) | Greenish black | Sticky mass | 5.65 |
| 2 | Chloroform | Greenish black | Sticky mass | 6.23 |
| 3 | Alcohol | Dark brown | Sticky mass | 9.23 |
Preliminary Phytochemical Screening: The qualitative chemical investigations were carried out to check for the presence of various phytoconstituents. The tests revealed for the presence of carbohydrates and in ethanol and water extracts, phytosterols in ether extract, flavonoids, and tannins in ethanol extract and saponins in ethanol and water extract. The detailed results are presented in Table 7.
TABLE 7: PRELIMINARY PHYTOCHEMICAL SCREENING OF THE AERIAL PARTS POWDER OF MADRAS NELLI
| S. no. | Test | Pet. ether extract | Chloroform extract | Ethanol extract | Water extract |
| 1 | Proteins and Amino acids | - | - | + | + |
| 2 | Carbohydrates | - | - | + | + |
| 3 | Glycosides | - | - | - | - |
| 4 | Phytosterols and Triterpenoids | + | - | - | - |
| 5 | Tannins | - | - | + | - |
| 6 | Flavanoids | - | - | + | - |
| 7 | Saponins | - | - | + | + |
| 8 | Alkaloids | - | - | - | - |
| 10 | Fats & Fixed oils | - | - | - | - |
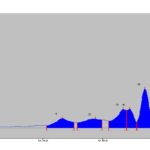
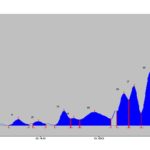
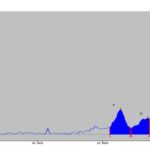
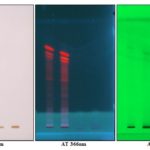
HPTLC Studies: HPTLC fingerprint profile of the alcoholic extract of P. maderaspantesis was performed. In this study the alcoholic extract revealed five phytoconstituents at Rf - 0.49, 0.60, 0.70, 0.72, 0.77. The quantification of the spots obtained was performed, and the percentage area of each spot was 10.08%, 9.65%, 19.64%, 19.69%, and 40.94%, respectively. The plate was viewed under UV Light at 254 nm, 366 nm, and 425 nm and also under normal white light. The photographs are shown in Fig. 2. The data of HPTLC was given in Table 11. Chromatogram with Rf values as shown in Graph 1, 2 and 3.
GRAPH 1: HPTLC OF EXTRACT AT 425 nm
TABLE 8: HPTLC DATA AT 425 nm
| Peak | Start position | Start height | Max position | Max height | Max % | End position | End height | Area | Area % | Assigned substance |
| 1 | 0.44Rf | 11.5AU | 0.49Rf | 46.0AU | 10.08% | 0.53Rf | 27.7AU | 2177.1AU | 15.82% | Unknown * |
| 2 | 0.54Rf | 25.2AU | 0.60Rf | 44.1AU | 9.65% | 0.63Rf | 38.7AU | 2430.8AU | 17.66% | Unknown * |
| 3 | 0.65Rf | 33.2AU | 0.70Rf | 89.6AU | 19.64% | 0.71Rf | 34.7AU | 2833.8AU | 20.59% | Unknown * |
| 4 | 0.71Rf | 84.9AU | 0.72Rf | 89.9AU | 19.69% | 0.74Rf | 29.0AU | 1781.1AU | 12.94% | Unknown * |
| 5 | 0.74Rf | 29.2AU | 0.77Rf | 186.9AU | 40.94% | 0.82Rf | 1.4AU | 4540.1AU | 32.99% | Unknown * |
Tract 1, id.
GRAPH 2: HPTLC OF EXTRACT AT 366 nm
TABLE 9: HPTLC DATA AT 366 nm
| Peak | Start position | Start height | Max position | Max height | Max %
|
End position | End height | Area | Area % | Assigned substance |
| 1 | 0.29Rf | 0.1AU | 0.33Rf | 40.8AU | 3.59% | 0.36Rf | 7.6AU | 1060.9AU | 3.41% | Unknown * |
| 2 | 0.37Rf | 14.1AU | 0.39Rf | 26.5AU | 2.33% | 0.42Rf | 11.0AU | 690.0AU | 2.21% | Unknown * |
| 3 | 0.45Rf | 3.4AU | 0.48Rf | 95.2AU | 8.38% | 0.50Rf | 46.3AU | 218 6.4AU | 7.02% | Unknown * |
| 4 | 0.51Rf | 46.4AU | 0.51Rf | 48.4AU | 4.26% | 0.53Rf | 38.3AU | 1013.0AU | 3.25% | Unknown * |
| 5 | 0.54Rf | 38.6AU | 0.59Rf | 87.0AU | 7.66% | 0.64Rf | 43.8AU | 5308.9AU | 17.04% | Unknown * |
| 6 | 0.66Rf | 91.7AU | 0.69Rf | 208.8AU | 18.38% | 0.70Rf | 68.8AU | 5135.7AU | 16.48% | Unknown * |
| 7 | 0.70Rf | 169.8AU | 0.72Rf | 257.0AU | 22.63% | 0.75Rf | 78.9AU | 5934.70AU | 19.05% | Unknown * |
| 8 | 0.75Rf | 80. 7AU | 0.78Rf | 349.4AU | 30.76% | 0.82Rf | 4.8AU | 9135.1AU | 29.32% | Unknown * |
| 9 | 0.84Rf | 5.3AU | 0.88Rf | 22.8AU | 2.01% | 0.91Rf | 0.1AU | 690.5AU | 2.22% | Unknown * |
Tract 1, id: Alc ext
GRAPH 3: HPTLC OF EXTRACT AT 254 nm
TABLE 10: HPTLC DATA AT 254 nm
| Peak | Start position | Start height | Max position | Max height | Max %
|
End position | End height | Area | Area % | Assigned substance |
| 1 | 0.62Rf | 10.6AU | 0.66Rf | 38.0AU | 27.93% | 0.69Rf | 9.8AU | 1137.6AU | 27.67% | Unknown * |
| 2 | 0.69Rf | 9.8AU | 0.7 Rf | 25.0AU | 18.41% | 0.75Rf | 24.2AU | 815.2AU | 19.82% | Unknown * |
| 3 | 0.79Rf | 23.1AU | 0.81Rf | 28.1AU | 20.68% | 0.85Rf | 1.9AU | 795.5AU | 19.35% | Unknown * |
| 4 | 0.86Rf | 0.5AU | 0.91Rf | 23.5AU | 17.28% | 0.93Rf | 19.3AU | 785.0AU | 19.09% | Unknown * |
| 5 | 0.93Rf | 19.4AU | 0.94Rf | 21.4AU | 15.69% | 0.98Rf | 1.8AU | 578.7AU | 14.07% | Unknown * |
Track 2, id: Alc ext 2
FIG. 2: HPTLC OF ALCOHOLIC EXTRACT
TABLE 11: HPTLC DATA ON THE ALCOHOLIC EXTRACT OF MADRAS NELLI
| S. no. | Parameter | Alcohol extract |
| 1 | Stationary
phase |
Precoated plates for HPTLC, silica gel 60 F 254 |
| 2 | Software | Win CATS |
| 3 | Applicator | Camag linomat V |
| 4 | Band length | 10 mm |
| 5 | Mobile phase | Benzene, acetic acid (4.5, 0.4) |
| 6 | Development chamber | Camag twin trough
(10 × 10 cm) |
| 7 | Development distance | 75 mm |
| 8 | Time of saturation | 1 h |
| 9 | Scanner | Camag TLC scanner 3 |
| 10 | Scanning speed | 20 nm |
| 11 | Lamp | Deuterium lamp and tungsten lamp |
| 12 | Data resolution | 100 μm/step |
| 13 | Photo documentation | Camag reprostar 3 |
CONCLUSION: The diagnostic characters generated from this pharmacognostic study will be useful in the proper identification of the crude drugs obtained from different parts of Madras Nelli, and they will also be helpful in quality assurance of it. The phytochemical screening tests conducted on aerial parts powder of Madras Nelli revealed the presence of pharmacologically important classes of phytochemicals like protein and amino acids, carbohydrates, Phytosterols, and terpenoids, tannins, flavonoids, and saponins. The presence of such phytochemicals in this medicinal herb clearly indicates its therapeutic properties and also validates its wide range of ethnomedicinal uses to some extent. Similarly, HPTLC analysis in alcoholic extract revealed the presence of five spots. Each spot is probably due to pure phytochemicals as each phytochemical has a specific Rf value.
ACKNOWLEDGEMENT: Nil
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Phyllanthus maderaspatensis L.: Available from: https://www.prota4u.org/database/protav8.asp?g=pe&p=Phyllanthus+maderaspatensis+L.
- Wallis T: Text Book of Pharmacognosy CBS Publishers and Distributors. Shahdara Delhi 5th Edi 1985; 434-36.
- Mukherjee PK: Quality control of herbal drugs: an approach to evaluation of botanicals. Business Horizons 2002.
- CKK: Practical Pharmacognosy. New Delhi: Vallabh Prakashan 2010.
- Pharmacopoeia I: Government of India, ministry of health and family welfare. Delhi Controller of Publications 1996; 2: 117-24.
- Organization WH: Quality control methods for medicinal plant materials 1998.
- Malins DC: Thin-layer chromatography: A laboratory handbook (Stahl Egon) 1965; ACS Publications.
- Wagner H and Bladt S: Plant drug analysis 2nd -edition springer publ. Comp. Berlin Heidelberg New York 1996.
- Bhat SVBAN and Sivakumar M: Chemistry of natural plant-Revised edition. New Delhi.
- Narasa publishing house.
How to cite this article:
Padhaya RR, Maharjan S, Khanal A, Pokhrel T and Burdipad GK: Standardization of aerial parts and phytochemical investigation of plant Phyllanthus maderaspatensis L. (Madras nelli). Int J Pharmacognosy 2019; 6(11): 365-73. doi link: http://dx.doi.org/10.13040/IJPSR. 0975-8232.IJP.6(11).365-73.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
365-373
559
928
English
IJP
R. R. Padhaya, S. Maharjan *, A. Khanal, T. Pokhrel and G. K. Burdipad
Central Institute of Science and Technology, New Baneshwor, Kathmandu, Nepal.
maharjansajan02@gmail.com
12 November 2019
26 November 2019
29 November 2019
10.13040/IJPSR.0975-8232.IJP.6(11).365-73
30 November 2019