SPECTROSCOPIC CHARACTERIZATION OF THE STEM-BARK OF IRVINGIA GABONENSIS
HTML Full TextSPECTROSCOPIC CHARACTERIZATION OF THE STEM-BARK OF IRVINGIA GABONENSIS
T. S. Malgwi *, K. Y. Musa, I. M. Maje and H. M. Mshelia
Department of Pharmacognosy, Faculty of Pharmacy, University of Maiduguri, Maiduguri, Nigeria.
ABSTRACT: Irvingia gabonensis is a medicinal plant valued in African traditional medicine for its diverse therapeutic uses. This study focused on the ethyl acetate extract of its stem bark to isolate and identify its major bioactive constituent using modern chromatographic and spectroscopic techniques. Extraction of the powdered stem bark with ethyl acetate produced a 5.30% yield. Thin-layer chromatography (TLC) suggested the presence of several phytochemical components, and subsequent column chromatography led to the isolation of a prominent compound labeled TSM 01. TLC analysis confirmed its purity by showing a single, well-defined spot when sprayed with visualizing agents. The structure of TSM 01 was elucidated using a combination of Fourier Transform Infrared (FTIR), Proton and Carbon-13 Nuclear Magnetic Resonance (¹H-NMR and ¹³C-NMR), Gas Chromatography–Mass Spectrometry (GC–MS), and Liquid Chromatography–Mass Spectrometry (LC–MS). The FTIR spectrum showed characteristic peaks for hydroxyl and carbonyl functional groups, while NMR spectra revealed features typical of a symmetrical aromatic compound. The GC–MS and LC–MS data both displayed a molecular ion peak at m/z 302, consistent with a molecular weight of 302 g/mol. Together, these results identified TSM 01 as Ellagic acid, a well-known polyphenolic compound with recognized pharmacological importance.
Keywords: Irvingia gabonensis, Ethyl acetate extract, Ellagic acid, Chromatography, Spectroscopy
INTRODUCTION: Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) Baill., commonly known as African or bush mango, is a perennial evergreen tree of the family Irvingiaceae, widely distributed across the tropical rainforests of West and Central Africa.
In traditional medicine, various parts of the plant, particularly the stem bark, have been used to treat gastrointestinal disorders, wounds, and inflammatory conditions 3, 7.
Despite its extensive ethnomedicinal use, systematic studies on the phytochemical composition and bioactive constituents of I. gabonensis stem bark remain limited. Medicinal plants represent a significant source of structurally diverse secondary metabolites with important pharmacological potential. Identifying and characterizing these compounds are essential for validating traditional claims and discovering new chemical leads. The ethyl acetate extract of I. gabonensis stem bark, in particular, has shown promise due to its richness in phenolic and flavonoid compounds 10. Chromatographic and spectroscopic techniques remain indispensable tools in natural product research. Chromatographic methods such as Thin Layer Chromatography (TLC), Column Chromatography (CC), and High-Performance Liquid Chromatography (HPLC) enable the separation and purification of complex plant extracts, facilitating the isolation of pure compounds for further analysis. Spectroscopic methods, including Fourier Transform Infrared Spectroscopy (FTIR), Proton and Carbon-13 Nuclear Magnetic Resonance (¹H-NMR and ¹³C-NMR), Gas Chromatography–Mass Spectrometry (GC–MS), and Liquid Chromatography–Mass Spectrometry (LC–MS), provide complementary structural information essential for compound identification and characterization 13.
This study focused on the ethyl acetate extract of I. gabonensis stem bark, employing chromatographic and spectroscopic analyses to isolate and elucidate phytoconstituent. The integration of these analytical approaches contributes to a clearer understanding of the plant’s chemical profile and supports its potential pharmacological relevance.
MATERIALS AND METHODS:
Chromatographic Analyses:
Thin Layer Chromatography (TLC): Preliminary chromatographic profiling of the ethyl acetate extract of Irvingia gabonensis stem bark was carried out using thin layer chromatography (TLC) to optimize solvent systems for effective compound separation using column chromatography. TLC plates (20 × 20 cm) precoated with silica gel 60 F₂₅₄ (Merck®, Germany) served as the stationary phase. The extract was reconstituted in its extraction solvent, and aliquots were applied as discrete spots 1.5 cm above the plate base using glass capillaries. Plates were developed in ascending mode within saturated chromatographic chambers using solvent systems of varying polarity: n-hexane:ethyl acetate (9:1 to 1:9 v/v), alongside pure n-hexane and pure ethyl acetate. After the solvent front migrated approximately 8 cm, plates were removed and air-dried in a fume hood. Developed chromatograms were visualized under UV light at 254 and 365 nm and further detected using specific chromogenic reagents: p-anisaldehyde–sulphuric acid (for general phytoconstituents), ferric chloride (for phenolics), aluminum chloride (for flavonoids), and Liebermann–Burchard reagent (for steroids and triterpenoids). Heating at 110 °C was used where necessary to enhance spot visualization. Retention factors (Rf values), color reactions, and spot patterns were recorded. The solvent system yielding the best resolution was adopted for subsequent column chromatography 1, 14.
Column Chromatography of the Ethyl Acetate Extract: Column chromatography was performed on the ethyl acetate extract of I. gabonensis to isolate individual phytoconstituents. Silica gel 60 (60–120 µm; Merck, Germany) served as the stationary phase. A total of 120 g of silica gel was packed into a glass column (3 cm × 60 cm) using n-hexane as the wetting solvent. 10 g of the extract were adsorbed onto silica gel to form a dry load and applied to the column surface.
A polarity-gradient elution was employed using n-hexane and ethyl acetate mixtures in the ratios 100:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, and 0:100 (v/v). Fractions of 20 mL each were collected sequentially and monitored by analytical TLC under the same chromatographic conditions described above. Fractions with similar Rf values and color reactions were pooled and labeled (ASM fractions) for further purification.
Purification of Combined Fractions: Fractions exhibiting comparable TLC profiles (ASM 264–269) were combined to yield a 320 mg residue, which was subjected to a second column chromatographic purification. The column was packed with 10 g silica gel (60–120 µm) and preconditioned with n-hexane. Gradient elution was carried out with increasing polarity using n-hexane and ethyl acetate in ratios of 100:0, 7:3, 1:1, 3:7, and 2:8 (v/v). Eluted fractions (2 mL) were collected and analyzed by TLC using hexane:ethyl acetate (2:8) as the mobile phase. Spots were visualized under UV light (254/365 nm) and stained with p-anisaldehyde reagent. Fractions showing identical TLC characteristics were combined and labeled (BSM fractions). Among these, one fraction (BSM 03) exhibited a single distinct spot, indicative of a pure compound.
This isolate was designated as TSM 01 for subsequent spectroscopic characterization.
Spectroscopic Characterization of the Isolated Compound:
Fourier Transform Infrared (FTIR) Spectroscopy: FTIR analysis of compound TSM 01 was conducted using an Agilent Cary 630 FTIR spectrometer equipped with a DTGS detector and ATR accessory. Spectra were recorded in the range 4000–650 cm⁻¹ at a resolution of 4 cm⁻¹. Characteristic absorption bands were interpreted to determine functional groups, and spectral data were compared with reference spectra from the NIST ATR–IR library.
Nuclear Magnetic Resonance (NMR) Spectroscopy: ¹H and ¹³C NMR spectra were acquired on a Bruker AVANCE III HD 400 MHz spectrometer using deuterated DMSO (DMSO-d₆) as solvent and tetramethylsilane (TMS) as internal reference. For ¹H NMR, acquisition parameters included 400 MHz frequency, 30° pulse angle, spectral width of 12 ppm, and relaxation delay (D₁) of 1–2 s with 115 scans. The ¹³C NMR spectra were recorded over 0–200 ppm with a relaxation delay of 2–5 s and 550 scans. Chemical shifts (δ, ppm), multiplicities, and coupling constants were analyzed and compared with literature values for structure elucidation.
Gas Chromatography–Mass Spectrometry (GC–MS): GC–MS analysis of TSM 01 was performed on an Agilent 7890B GC system coupled to a 5977B MSD using a DB-5MS capillary column. Helium was used as carrier gas at 1.0 mL/min in splitless mode. The oven temperature was programmed from 100 °C (hold 2 min) to 250 °C at 10 °C/min, then to 300 °C (hold 5 min). The ionization source operated under electron impact (EI) at 70 eV with a scan range of m/z 50–600. Fragmentation patterns were analyzed using Agilent MassHunter software and identified by comparison with the NIST mass spectral library.
Liquid Chromatography–Mass Spectrometry (LC–MS): LC–MS analysis was performed using an Agilent 6400 Series Triple Quadrupole LC/MS system with Electrospray Ionization (ESI) in negative mode. Separation was achieved on a C18 reverse-phase column (40 °C) with a mobile phase comprising 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile with ammonia (B), delivered at 0.5 mL/min. The injection volume was 5 µL, and the total run time was 25 min. Data were acquired over m/z 100–650 and processed using Agilent MassHunter Workstation software. Molecular ion peaks and characteristic fragmentations were used to confirm compound identity.
Physicochemical Evaluation: The isolated compound (TSM 01) was characterized for its color, solubility in various solvents (hexane, chloroform, ethyl acetate, methanol), and melting point using a digital melting point apparatus. TLC profiling with p-anisaldehyde, Liebermann–Burchard, and ferric chloride reagents further supported its chemical classification.
RESULTS:
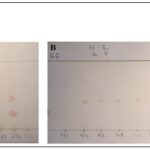
Thin Layer Chromatographic Profile of the Ethyl Acetate Extract of Irvingia gabonensis: The ethyl acetate extract of Irvingia gabonensis stem bark demonstrated a complex and diverse phytochemical profile on thin layer chromatographic (TLC) analysis. Development of the extract in hexane:ethyl acetate (9:1) revealed five distinct bands (Rf = 0.86–0.98), indicating a moderate polarity and heterogeneous composition Fig. 1A. Sequential developments in solvent systems of increasing polarity (ratios 8:2 to 1:9) revealed variable resolution patterns, reflecting solvent-dependent separation of the extract’s constituents Table 1. A total of 3–7 bands were observed per solvent system, with the highest separation recorded in the hexane:ethyl acetate (6:4) and (1:9) systems Fig. 1B–1H. Visualization with specific detecting reagents further confirmed the presence of multiple classes of secondary metabolites. Spraying with Liebermann–Burchard reagent produced five brown-to-orange bands Fig. 2A, characteristic of triterpenoids and steroids; ferric chloride treatment revealed two black spots Fig. 2C, confirming phenolic content; while aluminum chloride under UV light (365 nm) produced four fluorescent bands Fig. 2B, indicative of flavonoid constituents. Collectively, the TLC fingerprint suggests a chemically rich extract composed primarily of polyphenols, flavonoids, tannins, and terpenoids.
TABLE 1: THIN LAYER CHROMATOGRAPHIC ANALYSIS OF THE ETHYL ACETATE EXTRACT OF I. GABONENSIS STEM BARK
| Detecting Reagents | Solvent System | No. of Spots | Colour of Spots | Rf values |
| P–Anisaldehyde | H:E (9:1) | 5 | Brown, Brown, Brown, Purple, Purple | 0.86, 0.95, 0.96, 0.97, 0.98 |
| H:E (8:2) | 3 | Brown, Brown, Brown | 0.90, 0.96, 0.99 | |
| H:E (7:3) | 3 | Brown, Brown, Brown | 0.64, 0.88, 0.96 | |
| H:E (6:4) | 7 | Brown, Brown, Brown, Brown, Brown, Purple, Purple | 0.51, 0.77, 0.78, 0.87, 0.92, 0.98, 0.99 | |
| H:E (5:5) | 5 | Brown, Brown, Brown, Brown, Purple | 0.52, 0.64, 0.94, 0.95, 0.98 | |
| H:E (4:6) | 5 | Brown, Brown, Purple, Pink, Purple | 0.52, 0.76, 0.88, 0.92, 0.99 | |
| H:E (3:7) | 6 | Brown, Brown, Brown, Purple, Purple, Orange | 0.38, 0.48, 0.80, 0.92, 0.93, 0.94 | |
| H:E (2:8) | 4 | Brown, Brown, Pink, Purple | 0.33, 0.48, 0.64, 0.69 | |
| H:E (1:9) | 6 | Brown, Brown, Brown, Brown, Orange, Purple | 0.38, 0.52, 0.78, 0.90, 0.91, 0.93 | |
| Lieberman-Burchard | H:E (2:8) | 5 | Brown, Brown, Brown, Orange, Brown. | 0.33, 0.44, 0.62, 0.63, 0.64 |
| AlCl3 reagent and viewed Ultraviolent light at 365nm | H:E (2:8) | 4 | Purple, Purple, Pink, Purple | 0.33, 0.48, 0.64, 0.69 |
| Ferric Chloride | H:E (2:8) | 2 | Black, Black | 0.43, 0.48 |
Solvent system: H: E - Hexane: Ethyl acetate
FIG. 1: TLC CHROMATOGRAMS OF ETHYL ACETATE EXTRACT OF I. GABONENSIS IN VARIOUS SOLVENT SYSTEMS (HEXANE: ETHYL ACETATE 9:1 TO 1:9-A-H)
FIG. 2: TLC VISUALIZATION WITH DETECTING REAGENTS: (A) LIEBERMANN–BURCHARD; (B) AL2CL₃ UNDER UV 365 NM; (C) FERRIC CHLORIDE
Column Chromatography of the Ethyl Acetate Extract: Column chromatographic separation of the ethyl acetate extract afforded 310 eluates, which were monitored and grouped into ten major pooled fractions (ASM 01–ASM 10) based on similarities in TLC profiles and Rf values Table 2. Fraction ASM 09 exhibited a distinct chromatographic pattern, showing two prominent, well-resolved spots Fig. 3A, and was subsequently selected for further purification.
A secondary fractionation of ASM 09 yielded 62 eluates that were combined into five subfractions (BSM 01–BSM 05). Among these, BSM 03 (eluates 42–47) displayed a single sharp TLC band Fig. 3B, suggesting the presence of a major compound in near-pure form. The purified isolate, designated as TSM 01, presented a single band upon TLC development (Rf = 0.48 in hexane:ethyl acetate 2:8) Fig. 3C, and was thus selected for structural elucidation.
TABLE 2: COLUMN CHROMATOGRAPHIC FRACTIONATION OF THE ETHYL ACETATE EXTRACT OF I. GABONENSIS
| Elution ratio (H:E)* | Column fractions | Bulk fractions |
| 100 : 0 | 1 – 12 | ASM1 |
| 95 : 5 | 13 – 32 | ASM2 |
| 90 : 10 | 33 – 60 | ASM3 |
| 85 : 15 | 61 – 78 | ASM4 |
| 80 : 20 | 79 – 148 | ASM5 |
| 60 : 40 | 149 – 183 | ASM6 |
| 50 : 50 | 184 – 247 | ASM7 |
| 40 : 60 | 248 – 263 | ASM8 |
| 20 : 80 | 264 – 269 | ASM9 |
| 10: 90 | 270 – 310 | ASM10 |
*Hexane: Ethyl acetate
FIG. 3: TLC PROFILES OF COLUMN FRACTIONS: (A) ASM 09; (B) BSM 03; (C) PURIFIED COMPOUND TSM 01
Spectroscopic Characterization of Compound TSM 01:
Proton (¹H) and Carbon-13 (¹³C) NMR Spectra: The ¹H NMR spectrum of TSM 01 Fig. 4A revealed diagnostic signals characteristic of aromatic polyphenolic compounds. Singlets appeared at δ 7.4 ppm (2H) corresponding to aromatic protons, and a broad singlet at δ 10.2 ppm integrating for four protons consistent with hydroxyl functionalities. Additional resonances at δ 2.5 and δ 3.6 ppm (1H and 2H, respectively) further supported the presence of proton environments adjacent to electron-withdrawing groups. The ¹³C NMR spectrum Fig. 4B showed resonances at δ 159.9 ppm (C=O), 147.9, 139.4, and 136.1 ppm (aromatic quaternary carbons), and 112.5–107.9 ppm (aromatic methine carbons). These chemical shifts correlate closely with those of ellagic acid reported in the literature Table 3.
TABLE 3: COMPARISON OF ¹H AND ¹³C NMR CHEMICAL SHIFTS OF ELLAGIC ACID AND COMPOUND TSM01
| Ellagic acid* Compound TSM 01 | ||||
| Position | δ*1H (J Values in Hz) | δ*13C | δ 1H (J Values in Hz) | δ 13C |
| 1 | 10.67 (s) | 159.08 (C7) | 10.27 (s) | 159.90 (C7) |
| 2 | 7.45 (s) | 148.08 (C1) | 7.48 (s) | 147.90 (C1) |
| 3 | 139.55 (C2) | 139.39 (C2) | ||
| 4 | 136.35 (C3) | 136.09 (C3) | ||
| 5 | 112.27 (C5) | 112.48 (C5) | ||
| 6 | 110.21 (C4) | 110.15 (C4) | ||
| 7 | 107.59 (C6) | 107.91 (C6) | ||
| 8 | 159.08 (C7-) | 159.90 (C7-) | ||
| 9 | 148.08 (C1-) | 147.90 (C1-) | ||
| 10 | 139.55 (C2-) | 139.39 (C2-) | ||
| 11 | 136.35 (C3-) | 136.09 (C3-) | ||
| 12 | 112.27 (C5-) | 112.48 (C5-) | ||
| 13 | 110.21 (C4-) | 110.15 (C4-) | ||
| 14 | 107.59 (C6-) | 107.91 (C6-) | ||
* Subrahmanyam and Srinivasan, 2013; Hz: Hertz; S: Singlet; D: Doublet; T: Triplet; M: Multiplet
FIG. 4: NMR SPECTRA OF COMPOUND TSM 01: (A) ¹H NMR; (B) ¹³C NMR
Mass Spectrometric Analyses:
Gas Chromatography- Mass Spectrometry (GC–MS) Analysis: The GC–MS spectrum of TSM 01 Fig. 5A displayed a molecular ion peak at m/z 306 [M]⁺, corresponding to the molecular weight of ellagic acid (302–306 g/mol). Fragment ions at m/z 291 (loss of –OH), 171 (loss of CO₂), and 144–107 were consistent with typical fragmentation pathways of polyhydroxylated aromatic systems Table 4.
TABLE 4: GC–MS FRAGMENT IONS OF COMPOUND TSM 01 COMPARED WITH LITERATURE DATA FOR ELLAGIC ACID
| Ellagic acid* Compound TSM 01 | ||
| Position | m/z signals | m/z signals |
| 1 | 301.1 | 306.0 |
* Chaitra and Ravishankar, 2016; m/z mass-to-charge ratio
FIG. 5: (A) GC–MS SPECTRUM AND (B) LC–MS SPECTRUM OF COMPOUND TSM 01
Liquid Chromatography- Mass Spectrometry (LC–MS) Analysis: LC–MS analysis Fig. 5B confirmed a prominent ion peak at m/z 301 [M+H]⁺ and major fragment ions at m/z 257, 229, 185, and 159, matching the fragmentation pattern of ellagic acid Table 5. These data substantiate the GC–MS findings, confirming the molecular structure.
TABLE 5: LC–MS FRAGMENTATION DATA OF COMPOUND TSM 01 COMPARED WITH LITERATURE
| Ellagic acid* Compound TSM 01 | ||
| Position | m/z signals | m/z signals |
| 1 | 301.2 | 301.0 |
| 2 | 257.0 | 257.3 |
| 3 | 229.0 | 229.0 |
| 4 | 184.9 | 185.2 |
* Yan et al., 2014; m/z mass-to-charge ratio
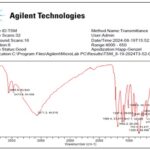
Fourier-Transform Infrared (FT-IR) Analysis: FT-IR spectroscopy of TSM 01 Fig. 6 revealed key absorption bands at 3555 and 3473 cm⁻¹ (O–H stretching), 3071 cm⁻¹ (aromatic C–H stretching), 1692 cm⁻¹ (C=O stretching), and 1617–1509 cm⁻¹ (aromatic C=C vibrations). Additional absorptions between 1192–1051 cm⁻¹ indicated ester C–O linkages, and a band at 752 cm⁻¹ was characteristic of aromatic C–H bending. The spectral pattern closely matched that of standard ellagic acid Table 6.
TABLE 6: FTIR ABSORPTION BANDS OF TSM 01 AND LITERATURE DATA FOR ELLAGIC ACID
| Ellagic acid* | Compound TSM 01 | |
| Position | Peaks | Peaks |
| 1 | 3554.84 | 3555.1 |
| 2 | 3475.85 | 3473.9 |
| 3 | 3070.30 | 3071.3 |
| 4 | 1693.53 | 1692.2 |
| 5 | 1615.59 | 1617.7 |
| 6 | 1581.60 | 1580.4 |
| 7 | 1510.30 | 1509.6 |
| 8 | 1446.62 | 1446.2 |
| 9 | 1396.62 | 1397.8 |
| 10 | 1328.48 | 1326.9 |
| 11 | 1258.65 | 1259.8 |
| 12 | 1195.60 | 1192.7 |
| 13 | 1110.49 | 1110.7 |
| 14 | 1053.50 | 1051.1 |
| 15 | 751.63 | 752.4 |
* Debby et al., 2021
FIG. 6: FTIR SPECTRUM OF COMPOUND TSM 01
Physicochemical Properties: Compound TSM 01 was obtained as an off-white crystalline powder, freely soluble in methanol, moderately soluble in chloroform and water, and exhibited a melting point of 350–354°C Table 7. When visualized with p-anisaldehyde reagent, it produced a brown spot, confirming its polyphenolic nature.
TABLE 7: PHYSICOCHEMICAL PROPERTIES OF COMPOUND TSM 01
| Parameters | Observation |
| Colour | Off white powder |
| Rf values | 0.48 (Hexane: Ethyl acetate; 2: 8) |
| Solubility | Soluble in methanol, and moderately soluble in chloroform and water. |
| Melting point | 350 – 354°C |
| Spraying Reagents | P–Anisaldehyde; Brown coloured spot was observed |
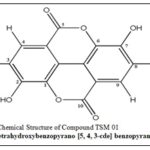
Structural Identification: The combined spectroscopic evidence ¹H and ¹³C NMR data, mass spectral fragmentation, and infrared absorption features unequivocally identified compound TSM 01 as ellagic acid (2,3,7,8-tetrahydroxy-benzopyrano [5,4,3-cde]benzopyran-5,10-dione) Fig. 7.
FIG. 7: PROPOSED CHEMICAL STRUCTURE OF COMPOUND TSM 01 (ELLAGIC ACID)
DISCUSSION: The chromatographic and spectroscopic characterization of the ethyl acetate extract of Irvingia gabonensis stem bark revealed the presence of a polyphenolic compound identified as ellagic acid. The use of complementary chromatographic techniques, Thin Layer Chromatography (TLC) and Column Chromatography proved effective in separating and monitoring constituent phytochemicals based on polarity and adsorption behavior. Preliminary TLC screening demonstrated the presence of steroids/triterpenes, tannins, and flavonoids, indicating a chemically diverse extract containing metabolites commonly associated with antioxidant, anti-inflammatory, and cytoprotective activities 8. The detection of steroids and triterpenes also corroborates earlier reports designating these compounds as chemotaxonomic markers for I. gabonensis 2, 11.
TLC was instrumental not only in identifying phytochemical groups but also in optimizing solvent systems for column elution, in accordance with the principles outlined by Parys et al. (2022) 12. The ethyl acetate extract yielded 310 primary fractions, which were monitored and pooled into ten major groups (ASM 01–ASM 10) based on TLC similarity. The ASM 09 fraction displayed two distinct bands, suggesting a mixture of closely related phenolic compounds. Subsequent re-chromatography under refined conditions afforded 62 sub-fractions, of which fractions 43–47 produced a single, sharp TLC spot, confirming the isolation of a pure compound, designated TSM 01. The efficiency of this fractionation sequence aligns with classical natural-product isolation strategies, where chromatography and solvent polarity control enhance both resolution and purity 4.
Physicochemical examination of TSM 01 revealed an off-white crystalline solid with marked solubility in methanol, moderate solubility in chloroform, and limited aqueous solubility. This pattern is consistent with amphiphilic polyphenolic molecules bearing multiple hydroxyl and aromatic moieties. The high melting range (350–354 °C) is indicative of extensive hydrogen bonding and π–π stacking, typical of highly conjugated aromatic compounds (García-Niño and Zazueta, 2015) 9. Comparable amphiphilic behavior has been reported in phenolic phyto-constituents isolated from other tropical medicinal plants 4, suggesting favorable formulation potential in both hydrophilic and lipophilic systems 16.
Spectroscopic analyses further substantiated the structural identity of TSM 01 as ellagic acid. The Fourier-Transform Infrared (FTIR) spectrum exhibited diagnostic absorption bands corresponding to hydroxyl (3555 cm⁻¹, 3071 cm⁻¹), carbonyl (1692 cm⁻¹), and aromatic (1617–1509 cm⁻¹) functional groups, consistent with literature reports for ellagic acid isolated from Terminalia catappa 6. The presence of both free and hydrogen-bonded O–H stretches reflected intramolecular hydrogen bonding, a defining feature of polyhydroxybenzoic acids. Additional bands between 1192 and 1051 cm⁻¹ confirmed C–O stretching of phenolic and ester linkages, while the absorption at 752 cm⁻¹ corresponded to aromatic C–H out-of-plane bending within the compound’s polyaromatic skeleton.
Proton Nuclear Magnetic Resonance (¹H NMR) spectroscopy in DMSO-d₆ revealed characteristic singlet resonances at δ 7.4 ppm (2H) and δ 10.2 ppm (4H), attributable to aromatic and phenolic protons respectively, in excellent agreement with the assignments reported for ellagic acid by Subrahmanyam and Srinivasan (2013) 15. The absence of splitting and integration symmetry confirms the compound’s structural equivalence and conjugated aromatic nature. The ¹³C NMR spectrum showed seven distinct resonances between δc 159.9–107.9 ppm, representing carbonyl and aromatic carbons in a symmetrical biphenyl framework. The chemical shifts correspond precisely to those reported for ellagic acid carbons (C1–C7) by Subrahmanyam and Srinivasan (2013) 15, confirming both molecular symmetry and purity. Gas Chromatography–Mass Spectrometry (GC-MS) analysis further supported this identification. The molecular ion peak at m/z 306 corresponded to the intact molecular weight of ellagic acid (C₁₄H₆O₈), with successive fragment ions at m/z 291, 171, 144, 129, and 107 reflecting typical neutral losses of hydroxyl and carbonyl groups, and rearrangements within the biphenyl core. These fragmentation pathways match the diagnostic ions previously reported by Chaitra and Ravishankar (2016) during GC-MS profiling of ellagic acid from pomegranate peel. The observed high thermal stability and reproducible fragmentation pattern affirm the compound’s identity and purity.
The LC-MS profile of TSM 01 revealed a deprotonated molecular ion at m/z 301 [M–H]⁻, corroborating the molecular weight of ellagic acid and confirming its polyhydroxylated aromatic structure. Key fragment ions at m/z 257 and 229 corresponded to the loss of hydroxyl and carbon dioxide moieties, respectively fragmentation routes characteristic of ellagic acid derivatives under electrospray ionization conditions 17. The recurrent appearance of ions at m/z 185 and 116 indicated stable, resonance-delocalized aromatic fragments, consistent with those reported by Yan et al. (2014) 17 for natural ellagic acid isolates. Collectively, the chromatographic behavior, physicochemical properties, and multi-spectroscopic evidence unequivocally establish TSM 01 as ellagic acid. The successful isolation of this compound from I. gabonensis not only validates earlier phytochemical reports but also strengthens the chemical rationale underlying its traditional medicinal applications. Ellagic acid is known for its potent antioxidant, anti-inflammatory, and cytoprotective effects activities that could contribute to the biological potential attributed to I. gabonensis stem bark 8, 9.
CONCLUSION: The ethyl acetate extract of Irvingia gabonensis stem bark demonstrated a rich phytochemical composition dominated by polyphenolic constituents. Sequential chromatographic separation and comprehensive spectroscopic analyses led to the successful isolation and structural elucidation of ellagic acid, a well-known bioactive phenolic compound. The identification of ellagic acid substantiates the plant’s traditional medicinal uses and provides a chemical basis for its reported pharmacological effects. These findings highlight I. gabonensis as a valuable natural source of antioxidant and therapeutically relevant metabolites. Further quantitative, biological, and mechanistic studies are warranted to elucidate the pharmacodynamic contributions of ellagic acid and related polyphenols to the plant’s overall bioactivity profile. Such investigations could support the rational development of standardized I. gabonensis-based phytopharmaceuticals and enhance its potential integration into evidence-based herbal medicine.
ACKNOWLEDGEMENTS: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Akhtar MS, Rafiullah M, Shehata WA, Hossain A and Ali M: Comparative phytochemical, thin layer chromatographic profiling and antioxidant activity of extracts from some Indian herbal drugs. Journal of Bioresources and Bioproducts 2022; 7(2): 128-134.
- Aliyu N, Abdulrahman E, Danmalam U, Kawu MU, Zakariya AM and Ayeni AE: Safety profile of Irvingia gabonensis (Aubry-Lecomte ex O'Rorke) Baill. Root bark extract: acute and sub-acute toxicity studies in Wistar rats. Journal of Medicinal Plants for Economic Development 2020; 4(1): 1-8.
- Awono A, Ndoye O and Eyebe A: Non-timber forest products and livelihoods in Central Africa. Forest Policy and Economics 2022; 137: 102704. https://doi.org/10.1016/j.forpol.2021.102704
- Bala I, Bhardwaj V, Hariharan S and Kumar MR: Analytical methods for assay of ellagic acid and its solubility studies. Journal of Pharmaceutical and Biomedical Analysis 2006; 40(1): 206-210.
- Chaitra NL and Ravishankar RV: Anti-HIV-1 Activity of ellagic acid isolated from Terminalia paniculata. Free Radicals and Antioxidants 2016; 6(1): 101.
- Debby S, Nirwana I and Kamadjaja M: Spectroscopy structure analysis of ellagic acid and calcium phosphate. Journal of International Dental and Medical Research 2021; 14(4): 1435-1441.
- Ekpe I, Etim O and Bassey F: Ethnobotanical and pharmacological relevance of Irvingia gabonensis: A review. Journal of Ethnopharmacology 2021; 278: 114299. https://doi.org/10.1016/j.jep.2021.114299
- Fu H, Li W, Liu J, Tang Q, Weng Z, Zhu L and Ding B: Ellagic acid inhibits dihydrotestosterone-induced ferroptosis and promotes hair regeneration by activating the wnt/β-catenin signaling pathway. Journal of Ethnopharmacology 2024; 330: 118227.
- García-Niño WR and Zazueta C: Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacological Research 2015; 97: 84-103
- Iroha IR, Okoye EI and Nwachukwu CU: Phytochemical constituents and biological activities of Irvingia gabonensis: A review. Journal of Medicinal Plants Research 2022; 16(5): 215–225. https://doi.org/10.5897/JMPR2022.7102
- Nuhu A, Abdurahman EM, Danmalam UH, Kawu MU, Adebayo SA and Bashir M: Isolation of pentacyclic triterpene, phenolic content and antioxidant activity of root bark of Irvingia gabonesis Baill. (Irvingiaceae). Journal of Pharmacy & Bioresources 2022; 19(2): 116-127.
- Parys W, Dołowy M and Pyka-Pająk A: Significance of chromatographic techniques in pharmaceutical analysis. Processes 2022; 10(1): 172.
- Sasidharan S, El-Shazly M and Loke MF: Herbal medicines: Identifying and minimizing risks of adulteration and contamination. Frontiers in Pharmacology 2022; 13: 857320. https://doi.org/10.3389/fphar.2022.857320
- Stahl E: Thin Layer Chromatography: A Laboratory Handbook. Springer India Private Limited, New Delhi, India 2005; 1041.
- Subrahmanyam Goriparti M and Sampath S: Ellagic acid- a novel organic electrode material for high capacity lithium ion batteries; electronic supplementary material (ESI) for chemical communications. The Royal Society of Chemistry 2013.
- Wilson TC and Hagerman AE: Quantitative determination of ellagic acid. Journal of Agricultural and Food Chemistry 1990; 38(8): 1678-1683.
- Yan L, Yin P, Ma C and Liu Y: Method development and validation for pharmacokinetic and tissue distributions of ellagic acid using ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Molecules 2014; 19(11): 18923-18935.
How to cite this article:
Malgwi TS, Musa KY, Maje IM and Mshelia HM: Spectroscopic characterization of the stem-bark of Irvingia gabonensis. Int J Pharmacognosy 2025; 12(11): 826-36. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.12(11).826-36.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
826-836
1286 KB
31
English
IJP
T. S. Malgwi *, K. Y. Musa, I. M. Maje and H. M. Mshelia
Department of Pharmacognosy, Faculty of Pharmacy, University of Maiduguri, Maiduguri, Nigeria.
troysm2013@unimaid.edu.ng
11 November 2025
27 November 2025
29 November 2025
10.13040/IJPSR.0975-8232.IJP.12(11).826-36
30 November 2025