SCAFFOLDS: A NOVEL TOOL FOR BONE TISSUE ENGINEERING
HTML Full TextSCAFFOLDS: A NOVEL TOOL FOR BONE TISSUE ENGINEERING
Ashish Kadeval *, Tejas Patel, Tejal Soni, B. N. Suhagia and Mehul Patel
Faculty of Pharmacy, Dharmsinh Desai University, College Road, Nadiad - 387001, Gujarat, India.
ABSTRACT: As life expectancy increases, malfunction or loss of tissue caused by injury or disease leads to reduced quality of life in many patients at significant socioeconomic cost. Bone disorders are of significant concern due to the increase in the median age of our population. Traditionally, bone grafts have been used to restore damaged bone. Even though major progress has been made in the field of bone tissue engineering, present therapies, such as bone grafts still have limitations. Current research on biodegradable polymers is emerging, combining these structures with osteogenic cells, as an alternative to autologous bone grafts. Different types of biodegradable materials have been proposed for the preparation of three-dimensional porous scaffolds for bone tissue engineering. Among them, natural polymers are one of the most attractive options, mainly due to their similarities with the extracellular matrix, chemical versatility, good biological performance, and inherent cellular interactions. Also, synthetic biomaterials are now being used as bone graft substitutes. These biomaterials were initially selected for structural restoration based on their biomechanical properties. Later scaffolds were engineered to be bioactive or bioresorbable to enhance tissue growth. Now scaffolds are designed to induce bone formation and vascularization. These scaffolds are often porous, biodegradable materials that harbor different growth factors, drugs, genes or stem cells. In this review, we highlight recent advances in bone scaffolds and discuss aspects that still need to be improved.
| Keywords: |
Bone scaffolds, Osteoinduction, Vascularization, Tissue engineering, Biodegradation, Osteogenic
INTRODUCTION: Concerns including the aforementioned and others surrounding the utilization of autogenous chancellors bone grafts as the standard gold treatment for critical-sized defects in bone have motivated the development of a wide variety of sophisticated synthetic (tissue-engineered) bone scaffolds in recent years. Advantages of utilizing synthetic bone scaffolds include the elimination of disease transmission risk, fewer surgical procedures, a reduced risk of infection or immunogenicity, and the abundant availability of synthetic scaffold materials.
This section reviews basic principles in bone tissue engineering and the design challenges surrounding the development of a synthetic scaffold which mimics the complicated physiochemical attributes of bone. The fundamental concept behind tissue engineering is to utilize the body’s natural biological response to tissue damage in conjunction with engineering principles. As the role of cell signaling and subsequent functionality in tissue engineering emerges with greater clarity, tissue engineers are developing multifunctional bioactive scaffolds. Ideal synthetic scaffolds must be capable of presenting a physiochemical biomimetic environment while biodegrading as native tissue integrates and actively promote or prevent desirable and undesirable physiological responses, respectively 1, 2, 3. To address these biomimetic requirements specifically, a synthetic bone scaffold must:
- Provide temporary mechanical support to the affected area,
- Act as a substrate for osteoid deposition,
- Contain a porous architecture to allow for vascularization and bone in-growth,
- Encourage bone cell migration into the scaffold,
- Support and promote osteogenic differentiation in the non-osseous, synthetic scaffold (osteoinduction),
- Enhance cellular activity towards scaffold-host tissue integration (osseointegration),
- Degrade in a controlled manner to facilitate load transfer to developing bone,
- Produce non-toxic degradation products,
- Not incite an active chronic inflammatory response,
- Be capable of sterilization without loss of bioactivity, and
- Deliver bioactive molecules or drugs in a controlled manner to accelerate healing and prevent pathology 4, 5, 6.
Multiple bone tissue engineering strategies such as cell transplantation, a cellular scaffolds, gene therapy, stem cell therapy, and growth factor delivery have been applied to address the challenging requirements listed above. In practice, most bone tissue engineering approaches implemented a combination of these strategies. However, two primary tissue engineering strategies have emerged as the most promising approaches.
- Before implantation, mesenchymal stems cells (MSCs)are isolated (typically from the patient), expanded ex-vivo, seeded onto a synthetic scaffold, allowed to produce extracellular matrix (ECM) on the scaffold in controlled culture conditions, and finally implanted into the osseous defect or void in the patient.
- Implantation of a cellular scaffold immediately after injury/bone removal. MSCs are pluripotent cells capable of differentiation into some cell types. Under the influence of chemicals such as dexamethasone, ascorbic acid, and b-glycerol phosphate, MSC differentiation can be driven towards bone-forming cells, or osteoblasts, which then produce bone ECM within the scaffold ex-vivo. Transplanted scaffolds seeded with MSCs have been shown to enhance osteogenic capacity and integrate with native tissue faster than a cellular scaffold in many preclinical trials 7, 8, 9, 10.
A primary obstacle in translating this technology from the bench to the bedside is that this technique involves an additional surgery and the patient must wait for the bone graft to develop in-vitro. Therefore, to promote the rapid development of a transplant-ready cellular scaffold, a variety of novel ex vivo culture techniques have been investigated to accelerate the cellular production of ECM. Three predominant ex-vivo culture techniques utilized in bone tissue engineering are growth factor delivery, bioreactor systems, and gene therapy.
The most common ex-vivo culture technique involves supplementing the MSCs/osteoblasts with growth factors. Are view of bone-relevant growth factors and their influence on MSC and osteoblasts phenotypic behavior is provided in a later section. Briefly, growth factors such as platelet-derived growth factors (PDGFs), bone morphogenetic proteins (BMPs), insulin-like growth factors (IGFs), and transforming growth factor-bs (TGF-bs) have an indisputable role in osteoinduction and osteoconduction. In ex-vivo conditions, growth factors can be delivered by simply adding them to the culture media or encapsulated in a bio-degradable scaffold. However, the short half-life and subsequently high dose required in growth factor delivery have motivated the development of alternative technologies for enhancing MSC performance in bone scaffolds ex-vivo.
The concept of gene therapy is similar to growth factor delivery in that the goal is to increase the local concentration of osteoinductive and osteoconductive cues for surrounding cells in-vivo and thereby encourage native MSCs to migrate into the scaffold, proliferate, differentiate, and begin ECM production. There are two basic gene therapy strategies in bone regeneration: (1) deliver a gene coding for the production of growth factors or other biological cues directly into the area of interest in-vivo, and (2) seeding genetically modified cells into the scaffold ex-vivo. Both strategies have been extensively tested and proven in animal models and hold great potential for the future of bone tissue engineering. A systematic review of gene therapy in bone regeneration can be found in the literature and is not provided here.
Bioreactor systems of a variety of designs have also been utilized to enhance the in-vitro performance of osteogenic cells before implantation. Bioreactors simulate the 3D dynamic and mechanical in-vivo environment and are designed to provide cells seeded deep within a scaffold with all necessary nutrients and biological cues to survive, proliferate, differentiate, and produce ECM. Sikavits AS et al., recently demonstrated proof of this concept by showing that after 16 days of culture, MSC-produced ECM was uniformly distributed in 3D scaffolds cultured in a flow profusion bioreactor, whereas the ECM was limited to the periphery in the case of standard static culture condition 11-14.
Despite the enormous potential of this approach for bone tissue engineering, there are still some barriers to address. The first and most significant barrier is that some studies have shown that MSCs which have been extensively cultured ex vivo lose their phenotypic behavior (such as osteo-differentiation and bone-forming capacity) once implanted in-vivo. Secondary challenges confronted in this approach are related to the relatively low concentration of MSCs in bone marrow and their characteristic low proliferative capacity making it difficult to obtain sufficient cell density in a large scaffold. In addition, to the increased risk due to a second surgery, there is a need to establish rigorous sterilization techniques for the cell-seeded scaffold which has been in culture ex-vivo for up to several weeks.
The second main tissue engineering strategy involves implantation of and a cellular scaffold immediately after injury / bone removal. The governing principles of this approach are the same as the first approach, however, to ensure rapid healing, it is even more critical to design a scaffold that mimics native bone tissue by driving local MSC migration in to the scaffold, supporting and promoting osteoinduction, and providing a biodegradable matrix that enhances MSC production of ECM that eventually integrates with the native tissue and fills the void or defect (osseointegration). Some clear advantages of this approach are that cellular scaffolds are much easier to sterilize, they have a shelf-life, and they have the lowest potential for infection or immunogenicity of all the bone repair strategies discussed earlier 15, 16.
Similar to the first approach, the performance of and a cellular scaffold may be substantially enhanced through the incorporation of bioactive molecules which are released in a controlled manner as the scaffold degrades and native tissue replaces it. The focus of this review is on this second approach and the development of and a cellular scaffold which meets the requirements listed earlier. Challenges accompanying this strategy will be discussed in detail throughout this chapter. In brief, the most important challenges reviewed and addressed in this work are: (1) the design of a micro and nanoscale dimensional hierarchy representative of bone, (2) the incorporation and controlled the viable release of bioactive molecules and drugs, and (3) control of bio-erosion to match native tissue synthesis rate 17-22.
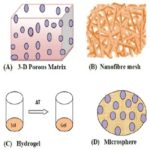
FIG. 1: DIFFERENT FORMS OF POLYMERIC SCAFFOLDS FOR CELL/DRUG/GENE DELIVERY: THREE-DIMENSIONAL POROUS MATRIX (A); NANOFIBER MESH (B); HYDRO GEL (C); AND MICROSPHERE (D) 17-22
Brief Overview of Bone Biology: Bone is a dynamic and complex tissue evolving and adapting to various stimuli throughout one’s lifetime. It plays crucial roles in both mechanical support and mineral homeostasis. Within a skeletal element, there are different morphologies of bone, such as cortical and trabecular bone.
Cortical bone is a compact structural tissue, with only 10% porosity, being 80% of the mass of an adult human skeleton. Trabecular bone is a spongy structure with 50%-90% porosity, filled with bone marrow. The majority of bones are covered by a highly vascularized fibrous connective tissue; the periosteum. Five different cell types are involved in bone maintenance and remodeling: MSCs, bone-lining cells, osteoblasts, osteocytes, and osteoblasts. Within the bone structure, MSCs are found in the bone marrow and also in the periosteum. Bone marrow is composed of a hematopoietic tissue and the supporting Struma.
Marrow stromal cells, originally thought to only contribute to the hematopoietic microenvironment, later came to the center stage with the recognition of being the stem/progenitor cells of skeletal tissues. Human autologous bone marrow associated with macroporous HA scaffolds was implanted in large bone segmental defects and shown to promote bone regeneration. After a 7-year follow-up, patients presented complete healing of their defects. Bone-lining cells are flat cells that cover all bone surfaces and are believed to arise from osteoblasts that become inactive. These cells form an important cellular barrier that divides the canalicular network (where osteocytes are present) from other fluids. Osteoblasts can be derived from MSCs that synthesize the osteoid (no mineralized organic matrix of the bone, that is, type I collagen, osteocalcin, osteopontin, bone sialoproteins, and bone morphogenetic proteins) 23-27.
Osteoblasts also have an active role in the vascularization process by secreting morphogens that activate angiogenesis by signaling endothelial cells. Osteocytes are terminally differentiated osteoblasts entrapped within the bone ECM that are involved in the maintenance of ECM and calcium homeostasis. Osteocytes also sense mechanical stress and communicate signals for bone remodeling and tissue maintenance. The fifth cell type is the osteoclast, responsible for bone resorption, which is the first stage of the bone remodeling process, followed by bone homeostasis. These cells are large multinucleated cells differentiated from a fraction of monocytes found in peripheral blood. As with many other connective tissues, one of the main components of bone is its ECM, which in this case is mineralized. Bone ECM is composed of 35% organic matrix and65% mineral matrix. The most abundant mineral in bone ECM is HA, a calcium phosphate crystallized at the surface of collagen fibrils, required to resist bending and compression stresses. The organic matrix is mainly protein composed of type I collagen (90%), and the remaining fraction includes up to 200 other noncollagenous proteins, such as glycol protein, proteoglycans, integrin-binding proteins, and growth factors 28, 29.
Bones are developed by two main processes: intramembranous and endochondral ossification. Intramembranous ossification is a process that generates flat bones and the skull structure. In this pathway, the embryonic mesenchymal condenses and develops in primary ossification centers, which will eventually fuse to form a network of anastomosing interconnected trabecular made of woven bone. After that, the periosteum is formed at the surface of trabecular, further mineralized and part of the intertrabecular connective tissue is transformed in hematopoietic tissue.
Finally, the woven bone is remodeled into a lamellar type of bone. Endochondral ossification is an osteogenic process through which long bones, vertebrae, and the pelvis are generated from precursor cartilaginous tissue. This process starts in the fetus where MSCs differentiate into chondro-cytes, converting the condensed mesenchymal into a cartilaginous model of bone that will expand in its extremities while becoming hypertrophic in the center. These hypertrophic chondrocytes will promote primary ossification by secreting molecules (such as alkaline phosphates’ [ALP], type X collagen, or vascular endothelial growth factor) that will induce calcification of cartilage. This tissue will be resorbed, becoming a structure onto which progenitor cells differentiate into osteoblasts that start to deposit osteoid. After birth, secondary ossification centers develop at the extremity of long bones, allowing the development and growth of bone structure 30.
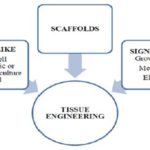
Bone Tissue Engineering Strategies: Bone has an intrinsic self-ability to regenerate, but over a large defect, inherent osseous processes are not able to repair the defect during the patient’s lifetime. Further, diseased bones do not heal properly and, under certain pathological conditions, start damaging themselves. Tissue engineering has emerged as a possible solution for these clinical conditions. Several strategies can be employed to develop new bone tissue. Those strategies may involve the use of an ECM-like structure (scaffold), cells, and growth factors. These three basic components need to be well synchronized to achieve a successful tissue engineering therapy. The strategy used for a specific bone defect must be adapted to the clinical state of the patient 30, 31.
FIG. 2: THE TISSUE ENGINEERING TRIAD 30, 31
Overall, there are primarily three approaches that have been described for tissue engineering strategies: (1) to use engineered on matrices alone, to guide tissue regeneration; (2) to inject autologous, allogeneic, or xenogeneic cells alone; (3) to develop constructs of cells seeded on these matrices. The first method involves implanting the scaffold at the site of interest, allowing host cells to migrate from the surrounding tissues to colonize the scaffold. The second strategy has the advantage of involving minimal surgical invasion and cells can be manipulated by recombinant gene technology or clonal expansion before injection or infusion.
Requirements for an Ideal Scaffold: The biomechanical system of bone is complex so that the following requirements for an ideal scaffold are diverse.
I. Biocompatibility one of the primary requirements of any bone scaffolds is biocompatibility a term which has been described in many ways. Biocompatibility of a scaffold is described as its ability to support normal cellular activity including molecular signaling systems without any local and systematic toxic effects to the host tissue. An ideal bone scaffold must be osteoconductive where the scaffold lets the bone cells to adhere, proliferate and form an extracellular matrix on its surface and pores. The scaffold should also be able to induce new bone formation through bimolecular signaling and recruiting progenitor cells, a property known as osteoinduction. Furthermore, an ideal scaffold needs to form blood vessels in or around the implant within a few weeks of implantation to actively support nutrient, oxygen and waste transport 32.
II. Mechanical properties the mechanical properties of an ideal bone scaffold should match host bone properties and proper load transfer is important as well. Mechanical properties of bone vary widely from chancellors’ to cortical bone. Young’s modulus of cortical bone is between 15 and 20 GPa, and that of chancellors bone is between 0.1 and 2 GPa. Compressive strength varies between 100 and 200 MPa for cortical bone, and between 2 and 20 MPa for cancellous bone. The large variation in mechanical property and geometry makes it difficult to design an “ideal bone scaffold” 33.
III. Pore size A must have property for scaffolds are interconnected porosity where pore size should be at least 100 μm in diameter for successful diffusion of essential nutrients and oxygen for cell survivability. However, pore sizes in the range of 200 to 350 μm are found to be optimum for bone tissue in-growth. Furthermore, recent studies have indicated that multi-scale porous scaffolds involving both micro and macro porosities can perform better than the only macroporous scaffold. Unfortunately, porosity reduces mechanical properties such as compressive strength and increases the complexity for reproducible scaffold manufacturing. Researchers have explored porous scaffolds using polymers, ceramics, composites, and metals. The strength of dense bioceramic materials matches close to the cortical bone, and different polymers to that of cancellous bone, however, ceramic-polymer composite scaffolds are typically weaker than bone. Porous metallic scaffolds meet the mechanical requirements of bone but fail to provide the necessary implant-tissue integration and add the concern related to metal ion leaching 34.
IV. Bioresorbability Bioresorbability is another crucial factor for scaffolds in bone tissue regeneration. An ideal scaffold should have not only similar mechanical properties that of the host tissue, but also be able to degrade with time in vivo, preferably at a controlled resorption rate and eventually creating space for the new bone tissue to grow. The degradation behavior of the scaffolds should vary based on applications such as 9 months or more for scaffolds in spinal fusion or 3 to 6 months for scaffolds in cranio-maxillofacial applications. Naturally, design and manufacturing of multi-scale porous scaffolds having an ideal composition including targeted bimolecular, mechanical properties and related bio-resorbability are some of the key challenges today towards their successful implementation in bone tissue engineering 35.
V. Biodegradability: The scaffold material should be biodegradable. Its degradation products should not be toxic and should be eliminated easily from the implantation site by the body, eliminating the need for further surgery to remove it. The scaffold’s degradation rate should be adjusted to match the rate of tissue regeneration so that it has disappeared completely once the tissue is repaired 36.
VI. Structure: It should have a reproducible microscopic and macroscopic structure with a high surface: volume ratio suitable for cell / drug attachment 37.
VII. Interface Adherence: Interface adherence is how cells or proteins attach to a scaffold’s surface. The scaffold should support cell adhesion and proliferation, facilitating cell-cell contact and cell migration 38.
VIII. Porosity: Porous structures allow for optimal interaction of the scaffold with cells. Specifically, pore size determines the efficiency at which cells seeded into the scaffold; small pores prevent the cells from penetrating the scaffold, while large pores prevent cell attachment due to a reduced area and, therefore, available ligand density. The scaffold should have an adequate porosity; this includes the magnitude of the porosity, the pore size distribution, and its interconnectivity. This also will allow cell in-growth and vascularisation and promote metabolite transport. A scaffold with an open and inter connected pore network and a high degree of porosity (>90%) is ideal for the scaffold to interact and integrate with the host tissue 39.
IX. Nature: Mimicking the native extracellular matrix (ECM), an endogenous substance that surrounds cells, allows them to bind into tissues and provide signals that cellular aid development and morphogenesis 40.
X. Processability: The scaffold should possess relatively easy processability and malleability into the desired shape, according to the need. They should be capable of being produced into a sterile product 41.
XI. Loading Capacity Release Kinetics: Loading capacity release kinetics is defined as the amount of drug that can be mixed into the scaffold. The scaffold should have a maximum loading capacity, so the drug is released continuously for a longer duration after insertion into the body. The drug needs to be dispersed homogeneously throughout the scaffold or in discrete areas and must avoid an initial burst effect. The drug release from the scaffold needs to be controlled to allow the appropriate dose of the drug to reach the cells over a given period of time 42.
XII. Binding Affinity: Binding affinity is defined as how tightly the drug binds the scaffold; this binding affinity must be sufficiently low to allow the release of the drug; however, low binding affinity would lead to dose dumping, which eventually may produce toxic effects 43.
XIII. Stability: The stability of the incorporated drug/cell at physiological temperature concerning physical, chemical, and biological activity is to be assessed. They should know posse’s dimensional stability, chemical stability, and biological activity over a prolonged period of 44.
Biomaterials for Scaffold Fabrication: Some different categories of biomaterials are commonly used as a scaffold for cell and drug delivery.
A. Natural Polymers: Natural polymers include alginate, proteins, collagens, gelatin, fibrins, albumin, elsinan, pectin (pectinic acid), galactan, curdlan, gellan, levan, emulsan, dextran, pullulan, gluten, elastin, fibrin, hyaluronic acid, cellulose, starch, chitosan (chitin), scleroglucan, heparin, silk, chondroitin 6-sulfate, and polyhydroxyalkanoates. They can be used as biomaterials for cell/drug/gene delivery purposes. Advantages of natural polymers include their biocompatibility, commercial availability, easy processing, and they more closely mimic the natural ECM of tissues; however, limitations are short supply, expense, batch-to-batch variation, and susceptibility to cross-contamination 45-49.
B. Synthetic Polymers: Synthetic polymers are largely divided into two categories: biodegradable and nonbiodegradable. Biodegradable polymers are polyglycolide, polylactide and its copolymer poly (lactide-co-glycolide), polyphosphazene, polyan-hydride, poly (propylene fumarate), polycyano-acrylate, polycaprolactone, polydioxanone, and polyurethanes. Nonbiodegradable polymers include polyvinyl alcohol, polyhydroxy ethyl methacrylate, and poly (N-isopropylacrylamide). Advantages of this scaffold are easily controlled physicochemical properties and quality, no immunogenicity, processing with various techniques, and consistent supply of large quantities 50-52.
C. Bioceramics: Melting inorganic raw materials to create an amorphous or crystalline solid body is known as bioceramics, and these porous final products are used mainly for scaffolds. Bioceramics classified as (1) nonresorbable (relatively inert), for example alumina, zirconia, and silicon nitride; and (2) bioactive or surface active (semi-inert), for example glass ceramics such as dense hydroxyapatites [9CaO·Ca(OH)2· 3P2O5], and biodegradable resorbable (noninert) such as calcium phosphates, aluminum calcium phosphates, coralline, tricalcium phosphates (3CaO·P2O5), zinc calcium phosphorus oxides, zinc sulphate calcium phosphates, ferric calcium phosphorus oxides, and calcium aluminates 53, 54.
D. Composites: Because of some of the problems associated with using scaffolds synthesized from a single-phase biomaterial (poor mechanical properties and biocompatibility of natural and synthetic polymers and poor degradability of Bioceramics), some researchers have developed composite scaffolds comprising two or more phases to combine the advantageous properties of each phase. Combinations of (1) synthetic–synthetic, (2) synthetic-natural and (3) natural–natural polymers can tailor mechanical, degradation, and biological properties but compromise the “best” qualities of individual polymers with properties of the overall scaffold 55-57.
Scaffold Fabrication Techniques: In the body, cells and tissue are organized into 3D architecture. To engineer this functional tissue and organs, scaffolds have to be fabricated by different methodologies to facilitate the cell distribution and guide their growth into 3D space. The conventional methods include fiber mesh, fiber bonding, melt molding, solvent casting/particulate leaching, gas foaming/particulate leaching, phase separation, and high-pressure processing. Electrospinning also has been utilized in producing a nanofibrous 3D matrix, and rapid prototyping technologies have enabled solid free-form fabrication directly from a computer-aided design (CAD) model. Many different techniques that are used to fabricate scaffolds for tissue engineering are summarized in the following sections.
A. Emulsification/Freeze-Drying Method: The freeze-drying technique is used for fabrication of porous scaffolds. This technique is based upon the principle of sublimation. Scaffolds are generally prepared by dissolving/suspending polymers/ ceramics in water or an organic solvent followed by emulsification with a water phase. After pouring this mixture into a mold, solvents are removed by freeze-drying, and porous structures are obtained. Freeze-drying is conducted by freezing the material and then reducing the surrounding pressure by applying a vacuum and adding enough heat to allow the frozen water in the material to sublime directly from the solid phase to the gas phase.
This technique is applied to some different polymers including silk proteins, PEG, poly-l-lactic acid (PLLA), PLGA/poly (propylene fumarate) blends. Emulsification/freeze-drying allows for faster preparation of highly porous structures with high pore interconnectivity’s. The main advantage of this technique is that it requires neither high temperature nor a separate leaching step. Its disadvantages, however, are limited to small pore size (porosity is often irregular) and long processing time.
The pore size can be controlled by optimizing the freezing rate and pH; a fast freezing rate produces smaller pores. Yannas et al. prepared collagen scaffolds by freezing a dispersion or solution of collagen and then freeze drying. Preparation of PLGA scaffolds of pore sizes of up to200 μm with 95% porosity was reported by Whang et al. 58-61
B. Particulate Leaching Method: Particulate leaching methods are split into two categories: (1) solvent casting–particulate leaching, and (2) melt molding–particulate leaching. In solvent casting–particulate leaching, a polymer dissolved in a solvent is mixed with salt particles in a mold; the solvent is then evaporated to make a polymer monolith embedded with the salt particles, which are then removed by washing the scaffold with water, resulting in the formation of a porous scaffold. In melt molding-particulate leaching, the polymer is cast into a mold with the embedded solid porogen. The polymer is set by applying heat and pressure, and again the porogen is leached away by washing the resulting product with water to yield a porous polymer scaffold Fig. 3. The solvent casting and particulate leaching method, first developed by Mikos et al., 159 was used primarily to manufacture composite scaffolds later the method found applications in the creation of porous scaffolds for the growth of endothelial cells. PLGA, poly (lactic acid) (PLA), collagen, poly (ortho ester), or small intestine submucosa–impregnated PLGA scaffolds have been fabricated successfully into a biodegradable sponge structure with more than 93%porosity and the desired pore size of 1000 μm 62, 63.
FIG. 3: FABRICATION OF SCAFFOLD USING THE PARTICULATE LEACHING METHOD
The advantages of the solvent casting method are that it is a simple and fairly reproducible and does not require sophisticated apparatus. A highly porous (up to 93%) scaffold of pore diameters up to 500 μm with ease of control of porosity and geometry can be prepared using this technique. The pore size can be controlled by controlling the amount of porogen added and the size and shape of the porogen. Complex geometries such as tube, nose, and specific organ types can be fabricated as nanocomposite hybrid scaffolds.
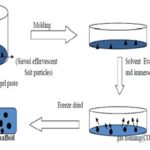
C. Gas Foaming Method: A “gas foaming” method was developed by Nam et al. using an effervescent salt as a gas foaming agent. Sieved effervescent salt particles (ammonium bicarbonate) in the form of a polymer gel paste was cast in a mold and subsequently immersed in hot water. The evolution of ammonia and carbon dioxide gas, along with the leaching out of ammonium bicarbonate particulates from the solidifying polymer matrix, resulted in the formation of pores with high interconnectivity Fig. 4 64.
Foaming techniques use gaseous porogen that is produced by chemical reactions during polymerization or are generated by the escape of gases during a temperature increase or drops in pressure; this causes a decrease in solubility of the carbon dioxide within the polymer, and as the carbon dioxide gas tries to escape it causes the nucleation and growth of bubbles, resulting in a porous microstructure 65, 66.
FIG. 4: FABRICATION OF SCAFFOLD USING THE GAS FOAMING METHOD
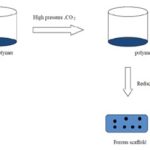
D. Supercritical Fluid Technology: Supercritical fluid technology, also known as high-pressure processing, uses a gas such as carbon dioxide (CO2) in a supercritical state at high pressure.
FIG. 5: FABRICATION OF SCAFFOLD USING SUPERCRITICAL FLUID TECHNOLOGY
The dry polymer is dissolved in supercritical carbon dioxide to form a single-phase polymer/gas solution. The pressure is then reduced to create thermodynamic instability of the dissolved CO2 and results in nucleation and growth of gas cells to generate pores within the polymer matrix Fig. 5 67, 68, 69.
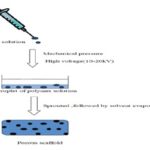
E. Electrospinning: Electrospinning is a process whereby electrical charge is used to form a mat of fine fibers. The apparatus using this method was patented by J.F. Cooly as early as 1902. In this technique, the polymer solution is passed under mechanical pressure through a high voltage (10–20 kV). The droplet of polymer solution obtained sprouts followed by solvent evaporation, leading to the formation of fine fibers that mat into the porous scaffold Fig. 6.
Electrospinning is the most widely used method for fabrication of nonwoven nanofiber matrices. Various materials can be electrospun into nanofiber-like biodegradable polymers such as PLGA and polycaprolactone, poly (ethylene oxide), polyvinyl alcohol, collagen, silk protein, and other peptides 70, 71.
FIG. 6: FABRICATION OF SCAFFOLD USING THE ELECTROSPINNING METHOD
F. Fiber Bonding: The fiber bonding method was first developed by Cima et al., who produced scaffolds made of polyglycolic acid (PGA) polymer. They took advantage of the fact that PGA was available as sutures and thus in the shape of long fibers. Mikos et al. improved the structural stability of the constructs developing a fiber-bonding technique in which the PGA fibers are joined at their cross-linking points by “sintering” above their melting point temperature. For example, PGA fibers have been bonded by embedding in PLLA solution, cooling, and subsequent removal of PLLA.
The scaffolds were fabricated by bonding a collagen matrix to PGA polymers with threaded collagen fiber stitches. The main advantage of the fiber-bonding technique is the high surface area: volume ratio, which makes them ideal for tissue engineering applications and high porosity, which provides more surface area for cell attachment and sufficient space for the regeneration of ECM. Disadvantages are poor mechanical integrity, residual organic solvents, lack of structural stability, that they can be used only to make small membranes, all the materials cannot be used for all the processes, membrane porosity is difficult to control, and morphology 72-75.
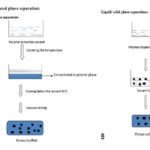
G. Thermally Induced Phase Separation: Thermally induced phase separation (TIPS) was first applied to PLA scaffolds by Schugens et al., and later several other researchers applied this technique to prepare composite scaffolds. It consists of inducing a solid-liquid or liquid-liquid phase separation. This is done by dissolving the polymer in a solvent and quenching the solution at a certain temperature. The quenching induces a phase separation into a polymer-rich phase and a poor polymer phase. In particular, TIPS uses thermal energy as the latent solvent to induce phase separation. The solvent must then be removed from the phase-separated solutions either by freeze-drying or solvent extraction. The solvent leaves behind micro structural foam (Fig. 7A, B) 77-80.
FIG. 7: (A) FABRICATION OF SCAFFOLD USING LIQUID–LIQUID PHASE SEPARATIONS. (B) FABRICATION OF SCAFFOLD USING LIQUID–SOLID PHASE SEPARATIONS.
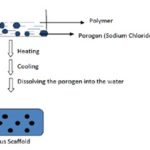
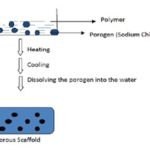
H. Melt Molding Technique: Melting polymers/ ceramics prepare scaffolds in the presence of porogen (such as sodium chloride and sugar crystals); once the mixture has cooled, porosity is achieved by dissolving the porogen in water. Finally, the porous scaffolds are usually lyophilized Fig. 8 81-83.
FIG. 8: FABRICATION OF SCAFFOLD USING THE MELT MOLDING METHOD
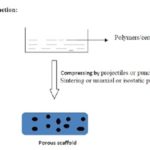
I. Powder Powder Compaction: Scaffolds are prepared by compressing the polymers/ceramics using projectiles or punch and dies; the velocity of compaction of the projectile or punch and dies is adjusted to achieve powder consolidation with the desired porosity. The process can include sintering as an alternative to using uniaxial or isostatic pressing Fig. 9 84-86.
FIG. 9: FABRICATION OF SCAFFOLD USING THE POWDER COMPACTION METHOD
J. Sol-Gel Technique: Scaffolds are prepared by dissolving inorganic metal salts or metal organic compounds in a solvent, where a series of hydrolysis and polymerization reactions allow the formation of a colloidal suspension (sol); after casting the sol into a mold a wet gel is formed and with further drying and heat treatment the gel is converted into dense ceramic or glass articles Fig. 10 87-90.
FIG. 10: FABRICATION OF SCAFFOLD USING THE SOL-GEL METHOD
Applications of Scaffold: The scaffold is used mainly to deliver the cell/drug/gene into the body, and application of scaffold is mainly categorized into those three categories: (1) cell delivery, (2) DNA/gene delivery, and (3) drug delivery.
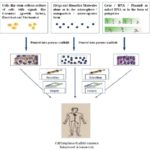
A. Matrices/Scaffold for Cell Delivery: In these, the cells with growth factor are encapsulated or seeded into the scaffold and administered into the body. Local and sustained delivery of paracrine factors, either by inducing or inhibiting cell proliferation, survival, migration, and differentiation, may greatly enhance tissue remodeling or organogenesis.
Growth factors can be incorporated into the scaffold matrix by bulk encapsulation, specific or nonspecific surface adsorption, and the addition of microspheres encapsulating them 91-93.
FIG. 11: CYCLE OF REIMPLANTATION OF CELL/DRUG/GENE SCAFFOLD CONSTRUCTS IN THE HUMAN BODY
B. Matrices/Scaffold for DNA/Gene Delivery: The gene encoding a growth factor to target cells has been suggested as an effective approach for enabling continuous expression and release of the growth factor in the local tissue site to avoid protein instability problems encountered during the harsh formulation process and the short half-life after release in the body fluid when growth factor–releasing scaffolds used. Polymer scaffolds have been designed to release the genetic material continuously as naked DNA or in the form of polyplexes, thereby transfecting to seeded cells and expressing the growth factor to stimulate morphogenesis of specific cells to form the desired tissue. Nanoparticulate polyplexes composed of DNA and polycationic-condensing agents have been incorporated within the scaffold to increase the efficiency of gene transfection. Polycationic agents such as polyethyleneimine condense plasmid DNA into positively charged nanoparticles and enhance DNA stability, intracellular uptake, and transfection efficiency. When implanted in-vivo, the seeded cells in the scaffold with the polyplexes exhibited a much higher level of gene expression than those with naked DNA. DNA polyplexes were physically immobilized onto the surface of prefabricated scaffolds by adsorption to further enhance the structural stability of the DNA through the formulation process 94, 95.
C. Matrices/Scaffold for Drug Delivery: Low molecular weight drugs that control the proliferation or differentiation of cells can be incorporated into biodegradable scaffolds to induce cellular differentiation and tissue remodeling. For example, dexamethasone, a steroidal anti-inflammatory drug, was loaded into the bulk phase of PLGA scaffolds for sustained release. It was observed that sustained release of dexamethasone effectively induced differentiation of bone marrow stem cells to osteoblasts or chondrocytes 96-99.
Challenges and Future Directions: The field of bone tissue engineering is at an exciting point, with enormous research activity focused on delivering new and improved biomimetic materials. The level of biological complexity that needs to be recapitulated within a synthetic three-dimensional environment is still uncertain. Further elucidation of the communication between cells and of the complex inters play between cells and their matrix will help focus strategies on enabling the presentation of biofactors in the correct context both chemically, temporally, and regarding their distribution.
Similarly, the clinical application of surface structuring approaches will require further understanding of the interactions occurring at the cell surface/substrate interface. Vascularization of large tissue on structs remains a significant challenge and some engineering-based approaches to try and overcome this has been discussed here. It is worth noting that advances in microsurgical techniques are also underway to allow reconstructive surgeons to generate so-called ‘axially vascularized’ tissues that can overcome some of the existing problems in achieving rapid vascularization of implanted biomaterials.
This highlights the importance of close interaction between the surgical and cell biology communities as we move from the bench closer to the bedside. The harvest of pluripotent mesenchymal cells from sources other than bone marrow, for example from the periosteum or adipose tissue, also warrants consideration. Advances in materials processing are also having a positive impact on the field. In the body, bone often has a structurally important interface with other tissues such as cartilage and ligament/tendon, for which designed scaffolds can be used to create tissue interfaces. For example, computer-aided design and SFF polymer/ceramic composites have been used to create a construct for a bone-cartilage interface by seeding chondrocytes (cartilage cells) within the cartilage portion, and BMP-7 transduced cells on the ceramic portion.
The potential to combine three-dimensional printing of scaffolds with three-dimensional printing of cells and biologics, while currently challenging, will enable the development of new designer material/biofactors hybrids. Soft material routes like sol-gel processing might also be a strategy to incorporate bimolecular during scaffold fabrication, although this is still under development. It is likely that biofunctionalization strategy will continue to receive a well-deserved focus, as will approach to integrate micron better- and nano scale features into designed scaffolds. Developments in this field will find a wealth of applications in our aging population.
CONCLUSION: Limitations with utilizing autogenous bone grafts as the standard gold treatment for critical-sized defects in bone have motivated this dynamic field of bone tissue engineering whose final goal is the design of synthetic biodegradable replacements for bone-in cases where critical-sized defects have been induced by tumor resection, orthopedic surgery, or trauma. Although, technologies and strategies reviewed in this article are progressive towards the development of a synthetic component capable of mimicking the physiochemical attributes of bone, no single tissue engineering scaffold date has demonstrated the ability to meet the comprehensive requirements outlined here.
Bone tissue engineers are aggressively pursuing solutions to remaining fundamental challenges in this field such as the: (1) development of a scaffold which presents appropriate mechanical properties throughout the course of biodegradation, (2) effective, nontoxic strategies for drug and bioactive molecule encapsulation resulting in tightly controlled temporal and spatial long-term release profiles, and (3) directing local pluripotent cell population behavior through physically mimicking the multi-scale hierarchical architecture of native bone.
The technological advances in synthetic bone scaffolds discussed herein demonstrate several obstacles that have already been overcome, and also that there is still a substantial gap to bridge before bone scaffolds can address the challenges above. Arguably, an elegant union of solutions to each of those individual challenges will likely result in the most positive patient outcomes.
In the near term, such technological unions are unlikely to be able to match the close spatial and temporal control over the bone environment that has been observed in healthy bone. The best strategies for developing bone scaffolds will be to incorporate cross-functional interdisciplinary approaches aimed at enhancing key events rather than exerting start-to-finish control over the bone development process.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Kofron MD and Laurencin CT: Bone tissue engineering by gene delivery. Adv Drug Deliv Rev 2006; 58: 555-576.
- Kimelman N, Pelled G, Helm GA, Huard J, Schwarz EM and Gazit D: Review: gene- and stem cell-based therapeutics for bone regeneration and repair. Tissue Eng. 2007; 13: 1135-1150.
- Sikavitsas VI, Bancroft GN and Mikos AG: Formation of three-dimensional cell/polymer constructs for bone tissue engineering in a spinner flask and a rotating wall vessel bioreactor. J Biomed Mater Res. 2002; 62: 136-148.
- Bancroft GN, Sikavitsas VI and Mikos AG: Design of a flow perfusion bioreactor system for bone tissue-engineering applications. Tissue Eng. 2003; 9: 549-554.
- Sikavitsas VI, Bancroft GN, Lemoine JJ, Liebschner MA, Dauner M and Mikos AG: Flow perfusion enhances the calcified matrix deposition of marrow stromal cells in biodegradable nonwoven fiber mesh scaffolds. Ann Biomed Eng 2005; 33: 63-70.
- Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R and Quarto R: Proliferation kinetics and differentiation potential of ex-vivo expanded human bone marrow stromal cells: implications for their use in cell therapy. Exp Hematol 2000; 28: 707-715.
- Banfi A, Bianchi G, Notaro R, Luzzatto L, Cancedda R and Quarto R: Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng 2002; 8: 901-910.
- Simmons PJ and Torok-Storb B: Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991; 78: 55-62.
- Bruder SP, Jaiswal N and Haynesworth SE: Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive sub cultivation and following cryopreservation. J Cell Biochem 1997; 64: 278-294.
- Cartmell S: Controlled release scaffolds for bone tissue engineering. J Pharm Sci 2009; 98: 430-441.
- Holland TA and Mikos AG: Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Eng Biotech 2006; 102: 161-185.
- Van den Dolder J, Farber E, Spauwen PH and Jansen JA: Bone tissue reconstruction using titanium fiber mesh combined with rat bone marrow stromal cells. Biomaterials. 2003; 24: 1745-1750.
- Mauney JR, Volloch V and Kaplan DL: Role of adult mesenchymal stem cells in bone tissue engineering applications: current status and prospects. Tissue Eng 2005; 11: 787-802.
- Richards M, Huibregtse BA, Caplan AI, Goulet JA and Goldstein SA: Marrow-derived progenitor cell injections enhance new bone formation during distraction. J Ortho Res 1999; 17: 900-908.
- Kadiyala S, Young RG, Thiede MA and Bruder SP: Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in-vivo and in-vitro. Cell Transplant 1997; 6: 125-134.
- Betz VM, Betz OB, Harris MB, Vrahas MS and Evans CH: Bone tissue engineering and repair by gene therapy. Front Biosci 2008; 13: 833-841.
- Phillips JE and Garcia AJ: Retroviral-mediated gene therapy for the differentiation of primary cells into a mineralizing osteoblastic phenotype. Methods Mol Biol 2008; 433: 333-354.
- Blum JS, Barry MA, Mikos AG and Jansen JA: In-vivo evaluation of gene therapy vectors in ex vivo-derived marrow stromal cells for bone regeneration in a rat critical-size calvarias defect model. Hum Gene Ther 2003; 14: 1689-1701.
- Lanza RP, Butler DH, Borland KM, Staruk JE, Faustman DL, Solomon BA, Muller TE, Rupp RG, Maki T and Monaco AP: Xenotransplantation of canine, bovine, and porcine islets in diabetic rats without immunosuppressant. ProcNatlAcadSci USA. 1991; 88: 11100-11104.
- Butler D. Poll reveals backing for xenotransplants. Nature. 1998; 391:315.
- Yang YG and Sykes M: Xenotransplantation: current status and a perspective on the future. Nat Rev Immune 2007; 7: 519-531.
- Yoo D and Giulivi A: Xenotransplantation and the potential risk of xenogeneic transmission of porcine viruses. Can J Vet Res 2000; 64: 193-203.
- Laurencin CT and El-Amin SF: Xenotransplantation in orthopedic surgery. J Am Acad Orthop Surg 2008; 16: 4-8.
- Langer R and Vacanti JP: Tissue engineering. Science 1993; 260: 920-926.
- Albrektsson T and Johansson C: Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001; 10 (S-2): S96-S101.
- Mistry AS and Mikos AG: Tissue engineering strategies for bone regeneration. Adv Biochem Eng Biotechnol 2005; 94: 1-22.
- Service RF: Tissue engineers build new bone. Science 2000; 289: 1498-1500.
- Howard D, Buttery LD, Shakesheff KM and Roberts SJ: Tissue engineering: strategies stem cells and scaffolds. J Anat 2008; 213: 66-72.
- Barrilleaux B, Phinney DG, Prockop DJ and O’Connor KC: Review: ex-vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006; 12: 3007-3019.
- Kimelman N, Pelled G, Gazit Z and Gazit D: Applications of gene therapy and adult stem cells in bone bio-engineering. Regen Med 2006; 1: 549-561.
- Logeart-Avramoglou D, Anagnostou F, Bizios R and Petite H: Engineering bone: challenges and obstacles. J Cell Mol Med 2005; 9: 72-84.
- Rı´os CN, Skoracki RJ, Miller MJ, Satterfield WC and Mathur AB: In-vivo bone formation in silk fibroin and chitosan blend scaffolds via ectopically grafted periosteum as a cell source: a pilot study. Tissue Eng Part A2009; 15: 2717.
- Gobin AS, Froude VE and Mathur AB: Structural and mechanical characteristics of silk fibroin and chitosan blend scaffolds for tissue regeneration. J Biomed Mater Res A 2005; 74: 465.
- Manjubala I, Woesz A, Pilz C, Rumpler M, Fratzl- Zelman N, Roschger P, Stampfl J and Fratzl P: Biomimetic mineral-organic composite scaffolds with controlled internal architecture. J Mater Sci: Mater Med 2005; 16: 1111.
- Zhao F, Grayson WL, Ma T, Bunnell B and Lu WW: Effects of hydroxyapatites in 3-D chitosan-gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006; 27: 1859.
- Chesnutt BM, Yuan Y, Buddington K, Haggard WO and Bumgardner JD: Composite chitosan/nanohydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in-vivo. Tissue Eng Part A2009; 15: 2571.
- Finisie MR, Josue A, Favere VT and Laranjeira MC: Synthesis of calcium-phosphate and chitosan Bioceramics for bone regeneration. An Acad Bras Cienc 2001; 73: 525.
- Zhang Y, Ni M, Zhang M and Ratner B: Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng 2003; 9: 337.
- Finisie MR, Josue A, Favere VT, and Laranjeira MC: Synthesis of calcium-phosphate and chitosan Bioceramics for bone regeneration. An Acad Bras Cienc 2001; 73: 525.
- Zhang Y, Ni M, Zhang M and Ratner B: Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng 2003; 9: 337.
- Xu HH, Takagi S, Quinn JB and Chow LC: Fast setting calcium phosphate scaffolds with tailored macropore formation rates for bone regeneration. J Biomed Mater Res A 2004; 68: 725.
- Correlo VM, Boesel LF, Bhattacharya M, Mano JF, Neves NM and Reis RL: Hydroxyapatites reinforced chitosan and polyester blends for biomedical applications. Macromol Mater Eng 2005; 290: 1157.
- Zhang Y, Xu HH, Takagi S and Chow LC: In-situ hardening hydroxyapatite-based scaffold for bone repair. J Mater Sci Mater Med 2006; 17: 437.
- Mukherjee DP, Tunkle AS, Roberts RA, Clavenna A, Rogers S and Smith D: An animal evaluation of a paste of chitosan glutamate and hydroxyapatite as a synthetic bone graft material. J Biomed Mater Res B Appl Biomater 2003; 67: 603.
- Rodan GA: Introduction to bone biology. Bone 1992; 13: S3.
- Weiner S and Wagner H: The material bone: structure mechanical function relations. Annu Rev Mater Sci 1998; 28: 271.
- Jee W: Integrated bone tissue physiology: anatomy and physiology. Bone Mechanics Handbook. Boca Raton: CRC press, Edition 2nd, 2001: 1.
- Friedenstein A, Deriglasova U, Kulagina N, Panasuk A, Rudakowa S, Luria E and Ruadkow I: Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in-vitro colony assay method. Exp Hematol 1974; 2: 83.
- Owen M: Marrow stromal stem cells. J Cell Sci 1988; 10: 63.
- Simmons P and Torok-Storb B: CD34 Expression by stromal precursors in normal human adult bone marrow. Blood 1991; 11: 2848.
- Nakahara H, Goldberg VM and Caplan AI: Culture-expanded human periosteal-derived cells exhibit osteochondral potential in vivo. J Ortho Res 1991; 9: 465.
- Bianco P, Riminucci M, Gronthos S and Robey PG: Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells 2001; 19: 180.
- Quarto R, Mastrogiacomo M, Cancedda R, Kutepov S, Mukhaev V, Lakroukov A, Kon E and Marcacci M: Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med 2001; 344: 385.
- Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, Mastrogiacomo M and Cancedda R: Stem cells associated with macro porous Bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng 2007; 13: 947.
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM and Scadden DT: Osteoblastic cells regulate the hematopoietic stem cell niche. Nature 2003; 425: 841.
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y and Li L: Identification of the hematopoietic stem cell niche and control of the niche size. Nature 2003; 425: 836.
- Robey P and Termine J: Human bone cells in-vitro. Calcif Tissue Int1985; 37: 453.
- Fuchs S, Hofmann A and Kirkpatrick CJ: Micro vessel like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng 2007; 13: 2577.
- Unger RE, Sartoris A, Peters K, Motta A, Migliaresi C, Kunkel M, Bulnheim U, Rychly J and James Kirkpatrick C: Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of micro capillary-like structures on three-dimensional porous biomaterials. Biomaterials 2007; 28: 3965.
- Santos MI, Unger RE, Sousa RA, Reis RL and Kirkpatrick CJ: Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 2009; 30: 4407.
- Nomura S and Takano-Yamamoto T: Molecular events caused by mechanical stress in bone. Matrix Biol 2000; 19: 91.
- Kronenberg HM: Developmental regulation of the growth plate. Nature 2003; 423: 332.
- Haynesworth SE, Goshima J, Goldberg VM and Caplan AI: Characterization of cells with osteogenic potential from human marrow. Bone 1992; 13: 81.
- Murray DW and Rushton N: The effect of strain on bone cell prostaglandin E2 release: a new experimental method. Calcif Tissue Int 1990; 47: 35-39.
- Chen NX, Geist DJ, Genetos DC, Pavalko FM and Duncan RL: Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone 2003; 33: 399-410.
- Toma CD, Ashkar S, Gray ML, Schaffer JL and Gerstenfeld LC. Signal transduction of mechanical stimuli is dependent on microfilament integrity: identification of osteopontin as a mechanically induced gene in osteoblasts. J Bone Miner Res 1997; 12: 1626-1636.
- Carvalho RS, Bumann A, Schaffer JL and Gerstenfeld LC: Predominant integrin ligand expressed by osteoblasts shows preferential regulation in response to both cell adhesion and mechanical perturbation. J Cell Biochem 2002; 84: 497-508.
- Lutolf MP and Hubbell JA: Synthetic biomaterials as instructive extracellular microenvironments for morpho-genesis in tissue engineering. Nat Biotech 2005; 23: 47-55.
- Athanasiou KA, Zhu C, Lanctot DR, Agrawal CM and Wang X: Fundamentals of biomechanics in tissue engineering of bone. Tissue Eng 2000; 6: 361-381.
- Glowacki J and Mizuno S: Collagen scaffolds for tissue engineering. Biopolymers. 2008; 89: 338-344.
- McCann TJ, Mason WT, Meikle MC and McDonald F: A collagen peptide motif activates tyrosine kinase-dependent calcium signaling pathways in human osteoblasts-like cells. Matrix Biol 1997; 16: 273-283.
- Maurer P, Hohenester E and Engel J: Extracellular calcium-binding proteins. Curr Opin Cell Biol 1996; 8: 609-617.
- Patel A, Fine B, Sandig M and Mequanint K: Elastin biosynthesis: the missing link in tissue-engineered blood vessels. Cardiovascular Res 2006; 71: 40-9.
- Scott AS, Patricia S, Koyal G, Jennifer M, Isaac AR and Gary LB: The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogs. Polymers 2010; 2: 522-53.
- Gibbs BF, Zougman A, Masse R and Mulligan C: Production and characterization of bioactive peptides from soy hydrolysate and soy fermented food. Food Res Int 2004; 37: 123-31.
- Yannas IV, Burke JF, Gordon PL, Huang C and Rubenstein RH: Design of artificial skin. Part II. Control of chemical composition. Biomaterials 1980; 14: 107-31.
- Schugens C, Maquet V, Grandfils C, Jerome R and Teyssie P: Polylactide macro porous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid-liquid phase separation. J Biomed Mater Res 1996; 30(4): 449-61.
- Bredt JF, Sach E, Brancazio D, Cima M, Curodeau A and Fan T: Three dimensional printing systems. US Patent 1998; 5807437.
- George M and Abraham TE: Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan-a review. J Control Release 2006; 114: 1-14.
- Khor E and Lim LY: Implantable applications of chitin and chitosan. Biomaterials 2003; 24: 2339-49.
- Abarrategi A, Lópiz-MoralesY, Ramos V, Civantos A, López-Durán L, Marco F and López-Lacomba JL: Chitosan scaffolds for osteochondral tissue regeneration. J Biomed Mater Res A 2010; 95(4): 1132-41.
- Morrison WR and Karkalas J: Starch: Carbohydrates. In: Dey PM, Harborne JB (Ed.). Methods in Plant Biochemistry. London: Academic Press Limited; 1989; 2: 323-52.
- Poutanen K and Forssell P: Modification of starch properties with plasticizers. Trends Polym Sci 1996; 4: 128-32.
- Iva P, Paula M, Helena S and Rui L: Highly porous and interconnected starch-based scaffolds: production, characterization and surface modification. Mat Sci Eng C 2010; 30: 981-9.
- Yu-Ju L, Chi-Nan Y, Yu-Chen H, Yung-Chih W, Chun-Jen L and I-Ming C: Chondrocytes culture in three-dimensional porous alginate scaffolds enhanced cell proliferation, matrix synthesis and gene expression. J Biomed Mater Res A 2009; 88A (1): 23-33.
- Liao YH, Jones SA, Forbes B, Martin GP and Brown MB: Hyaluronan: pharmaceutical characterization and drug delivery. Drug Deliv 2005; 12: 327-42.
- Nishinari K and Takahashi R: Interaction in poly-saccharide solutions and gels. Curr Opin Colloid Interface Sci 2003; 8: 396-400.
- Yadav AK, Mishra P and Agrawal GP: An insight on hyaluronic acid in drug targeting and drug delivery. J Drug Target 2008; 16: 91-107.
- Pan L, Ren Y, Cui F and Xu Q: Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J Neurosci Res 2009; 87: 3207-20.
- Chung C and Burdick JA: Influence of three-dimensional hyarulonic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A 2009; 15: 243-54.
- Chung C, Erickson IE, Mauck RL and Burdick JA: Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A 2008; 14: 1121-31.
- Lippiello L: Glucosamine and chondroitin sulfate: biological response modifiers of chondrocytes under simulated conditions of joint stress. Osteoarthr Cartil 2003; 11: 335-42.
- Lee CT, Kung PH and Lee YD: Preparation of poly (vinyl alcohol)- chondroitin sulfate hydrogel as matrices in tissue engineering. Carbohydr Polym 2005; 61: 348-54.
- Naessens M, Cerdobbel A, Soetaert W and Vandamme EJ: Leuconostoc dextransucrase and dextran: production, properties and applications. J Chem Technol Biotechnol 2005; 80: 845-60.
- Cascone MG, Barbani N, Cristallini C, Giusti P, Ciardelli G and Lazzeri L: Bioartificial polymeric materials based on polysaccharides. J Biomater Sci Polym Ed 2001; 12: 267-81.
- Hoffmann B, Seitz D, Mencke A, Kokott A and Ziegler G: Glutaraldehyde and oxidised dextran as crosslinker engineering. J Mater Sci Mater Med 2009; 20(7):1495–503.
- Rees DA: Structure, conformation and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem 1969; 24: 267-332.
- Bao L, Yang W, Mao X, Mou S and Tang S: Agar/ collagen membrane as skin dressing for wounds. Biomed Mater. 2008; 3(4): 044108.
- Mangione MR, Giacomazza D, Bulone D, Martorana V, Cavallaro G and San Biagio PL: K+ and Na+ effects on the gelation properties of carrageenan. Biophys Chemist. 2005; 113: 129-35.
How to cite this article:
Kadeval A, Patel T, Soni T, Suhagia BN and Patel M: Scaffolds: A novel tool for bone tissue engineering. Int J Pharmacognosy 2014; 1(8): 485-00. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(8).485-00.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
485-500
874
2142
English
IJP
A. Kadeval*, T. Patel, T. Soni, B. N. Suhagia and M Patel
Faculty of Pharmacy, Dharmsinh Desai University, College Road, Nadiad, Gujarat India.
ashishkadeval@gmail.com
25 March 2014
14 June 2014
28 July 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(8).485-500
01 August 2014