RHOIFOLIN: A REVIEW OF SOURCES AND BIOLOGICAL ACTIVITIES
HTML Full TextRHOIFOLIN: A REVIEW OF SOURCES AND BIOLOGICAL ACTIVITIES
John Refaat * 1, Samar Y. Desoukey 1, Mahmoud A. Ramadan 2 and Mohamed S. Kamel 1
Department of Pharmacognosy 1, Faculty of Pharmacy, Minia University, 61519 Minia, Egypt.
Department of Pharmacognosy 2, Faculty of Pharmacy, Assiut University, 71515 Assiut, Egypt.
ABSTRACT: Flavonoids are common plant constituents used extensively in phytomedicine to treat a wide range of diseases. Many pharmacological pieces of evidence suggest that flavonoids may play an important role in the decreased risk of chronic diseases associated with a diet rich in plant-derived foods. Therefore, this article focuses on the chemistry, distribution and pharmacological properties of rhoifolin as one of the common and important flavonoids in the plant kingdom. This flavonoid has also been found in several dietary sources such as bitter orange, bergamot, grapefruit, lemon, lupinus, lablab beans, tomatoes, artichoke, bananas, and grapes. Preclinical studies have shown that rhoifolin possesses a variety of significant biological activities including antioxidant, anti-inflammatory, antimicrobial, hepatoprotective and anticancer effects. The literature search was conducted using electronic databases (e.g., Medline, Pubmed, Academic Journals and Springer Link), general web searches were also undertaken using Google applying some related search, journals and scientific theses. The bibliographies of papers relating to the review subject were also searched for further relevant references.
| Keywords: |
Apigenin 7-O-neohesperidose, Biological effects, Flavonoids, Rhoifolin, Rhoifoloside
INTRODUCTION: Medicinal plants are well-known biosynthetic laboratories of bioactive substances, thus they can magically provide us with the key to our awful health problems in life. Flavonoids constitute a large group of plant secondary metabolites that enjoy a widespread accumulation throughout the plant kingdom and are commonly found in fruits, vegetables and certain beverages 1. Chemically, flavonoids are polyphenolic molecules characterized by a diphenylpropane structure (C6-C3-C6) and are found in plants both in a free form and as glycosides.
During the last decade, flavonoids attracted extensive phytochemical attention and considerable biological interest due to their wide range of pharmacological activities and potentially beneficial effects on human health. They have been reported to have antiviral, anti-allergic, antiplatelet, anti-inflammatory, anti-tumor and antioxidant activities. Recent studies also support a protective effect of flavonoids consumption in cardiovascular diseases and cancer 2.
Apigenin is one of the most common flavonoids present in edible plants and in those used in traditional medicine to treat a wide variety of pathologies. This flavone and its glycosides are widely distributed in the plant kingdom; they are found in many plant families, e.g. Apiaceae, Asteraceae, Fabaceae, Lamiaceae, Malvaceae and Rutaceae 3. To date, a huge number of apigenin glycosides have been isolated and identified. Many of them were reported to be effective in the pathogenesis of the majority of diseases 4. Rhoifolin or rhoifoloside is a well-known tri-substituted flavone belongs to the apigenin family. This molecule was obtained for the first time from the fresh leaves of Rhus succedanea in 1952 5. Several studies have shown that this flavone possesses a variety of pharmacological activities. Accordingly, this work highlights the distribution, chemical, physical, chromatographic and spectral properties as well as the biological effects of rhoifolin.
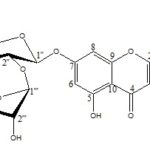
Chemical, Physical, Chromatographic and Spectral Properties: Chemically, rhoifolin is apigenin 7-O-β-neohesperidose Fig. 1 with the chemical formula C27H30O14 and the molecular weight 578.53 (exact mass: 578.1636) 3. It is usually isolated as a yellow amorphous powder or yellow needles (melting at 245-253 °C) after crystallization from methanol or 50% methanol 6, 8. Rhoifolin is soluble in methanol, hot ethanol and water (water solubility is 2.55 g/L), sparingly soluble in ethyl acetate and cold ethanol and insoluble in n-hexane and chloroform 9. It shows brown or dark purple fluorescence under UV light (254 nm) that turns to yellow upon exposure to ammonia vapors or spraying with 5% aluminum chloride reagent, in addition to yellowish brown color after spraying with 10% sulphuric acid reagent 8. Different solvent systems can be used for TLC analysis or separation of rhoifolin on silica gel e.g. ethyl acetate-methanol (8:1) [Rf 0.25], ethyl acetate-methanol (8:2) [Rf 0.625], chloroform-methanol (8:2) [Rf 0.36] butanol-acetic acid-water (4:1:1) [Rf 0.53] 6, 9. [α]D29 −110.0° (c, 0.21 in methanol) 6 and in another source −160.0° (methanol) 10.
The IR spectrum of rhoifolin shows bands [νmax (KBr)] at 3388 (OH), 1657 (α, β-unsaturated CO), 1605, 1497 and 1488 (aromatic C=C), 1249, 1178 and 1074 (glycosidic C–O) cm-1 7. UV spectral analysis of rhoifolin shows absorption bands at λmax (log ɛ) (MeOH): 266 (4.20), 336 (4.30) nm; (NaOMe): 267 (4.20), 387 (4.40) nm; (NaOAc): 257 (4.20), 266 (4.20), 391 (4.40) nm; (NaOAc + H3BO3): 268 (4.20), 340 (4.30) nm; (AlCl3): 275 (4.20), 299 (4.10), 350 (4.20), 385 (4.20) nm; (AlCl3 + HCl): 276 (4.20), 298 (4.10), 342 (4.20), 382 (4.10) nm. 7 Its positive HR-ESI-MS shows a pseudomolecular ion peak [M+H]+ at m/z 579 8, 11, whereas [M-H]+ at m/z 577 appears in the negative HR-ESI-MS spectrum 12. 1H-NMR spectrum of rhoifolin in DMSO-d6 shows the following signals (ppm): 7.91 (2H, d, J=8.8 Hz, H-2`,6`), 6.92 (2H, d, J= 8.8 Hz, H-3`,5`), 6.84 (1H, d, J= 2.0 Hz, H-8), 6.80 (1H, s, H-3), 6.33 (1H, d, J= 2.0 Hz , H-6), 5.08 (1H, singlet-like, H-1```), 5.20 (1H, d, J= 7.3 Hz, H-1``), 1.16 (3H, d, J= 6.3Hz, CH3-6```). The 13C-NMR spectrum in DMSO-d6 (ppm): 182.1 (C-4), 164.4 (C-2), 162.6 (C-7), 161.7 (C-4`), 161.1 (C-5), 157.1 (C-9), 128.7 (C-2`,6`), 120.9 (C-1`), 116.2 (C-3`,5`), 105.5 (C-10), 103.2 (C-3), 99.4 (C-6), 94.6 (C-8), sugar moiety: 100.5 (C-1``), 98.2 (C-1```), 77.6 (C-2``), 77.4 (C-3``), 76.8 (C-5``), 72.3 (C-4```), 71.0 (C-2```), 70.8 (C-3```), 71.1 (C-4``), 68.8 (C-5```), 60.9 (C-6``), 18.5 (CH3-6```) 7. The HMBC spectrum shows significant correlations between (H-3 and C-2, C-4, C-1`), (H-8 and C-6, C-7, C-9, C-10), (H-1`` and C-7), (H-2`` and C-3``), (H-3`` and C-4``), (H-1``` and C-2``, C-2```, C-5```), (H-4``` and C-5```) and (H-5``` and C-6```) 7.
In another work, small differences ranging from ~ 0.2-0.8 ppm were observed for some carbon signals of the sugar moiety in the same solvent 8. NMR data of rhoifolin were also recorded in CD3OD by Yadav et al. 11
FIG. 1: CHEMICAL STRUCTURE OF RHOIFOLIN
Plant Sources of Rhoifolin: After its first isolation from Rhus plants (Anacardiaceae) 5, rhoifolin was isolated from other plant sources belonging to different botanical families. Some edible plants were also found to be rich in this flavone, e.g. bitter orange, bergamot, grapefruit, lemon, lupinus, lablab beans, tomatoes, artichoke, bananas, and grapes. Also, different parts and juices from various Citrus spp. are reported to contain rhoifolin in high concentrations 13.
Considerable amounts (up to g/kg) of rhoifolin are also available in different organs of Chorisia spp. 6. Table 1 compiles plant species that contain rhoifolin in alphabetical order.
TABLE 1: A LIST OF PLANT SPECIES CONTAINING RHOIFOLIN
| Plant Species | Family | Part (yield) | References |
| Adinandra nitida Merrill. | Theaceae | Leaf | 14, 15 |
| Boehmeria nivea L. | Urticaceae | Leaf | 16 |
| Buddleja albiflora Hemsl. | Scrophulariaceae | 17 | |
| Carduus nutans L. | Asteraceae | 18 | |
| Chorisia crispiflora H.B.K. | Bombacaceae | Leaf (0.15%), Flower | 6, 19 |
| Chorisia insignis H.B.K. | Bombacaceae | Leaf (0.5%) | 6 |
| Chorisia pubiflora St.-Hill. Dawson | Bombacaceae | Leaf (0.24%) | 6 |
| Chorisia speciosa A. St.-Hill. | Bombacaceae | Leaf (0.27%), Flower | 6, 20 |
| Cirsium arvense | Asteraceae | 18 | |
| Cirsium bitchuense | Asteraceae | 18 | |
| Cirsium canescense | Asteraceae | 18 | |
| Cirsium undulatum | Asteraceae | 18 | |
| Citrus aurantium L (Bigarade or bitter orange) | Rutaceae | Whole plant | 3 |
| Citrus bergamia Risso. (Bergamot) | Rutaceae | Whole plant | 21 |
| Citrus campestris | Rutaceae | Shoot | 22 |
| Citrus grandis L. (C. maxima Merr.) | Rutaceae | Leaf (1.1%),
Exocarp of almost ripe fruit (0.090%) |
8
3 |
| Citrus grandis var. tomentosa | Rutaceae | Exocarp of ripe fruit (0.655%) | 3 |
| Citrus limon (Canton lemon) | Rutaceae | Leaf (9%) | 23 |
| Citrus myrtifolia | Rutaceae | Fruit | 12 |
| Citrus paradisi Macfad (Grapefruit) | Rutaceae | Leaf | 24 |
| Citrus sinensis (Sweet orange) | Rutaceae | 21 | |
| Cynara scolymus L. (artichoke) | Asteraceae | Flower head | 25 |
| Cynodon dactylon | Poaceae | 26 | |
| Cyperus alopecuroides Rottb. | Cyperaceae | Inflorescence | 27 |
| Discocleidion rufescens Franch. | Euphorbiaceae | 28 | |
| Dolichos lablab L. | Fabaceae | Flower | 29 |
| Exochorda racemosa | Rosaceae | 30 | |
| Festuca argentina Speg. | Poaceae | 31 | |
| Glechoma hederacea L. (Ground Ivy) | Lamiaceae | Whole plant | 32 |
| Gonocaryum calleryanum Baill. | Icacinaceae | Leaf | 33 |
| Ilex centrochinensis S.Y.Hu | Aquifoliaceae | Leaf | 34 |
| Jatropha curcas Linn. | Euphorbiaceae | Leaf | 35 |
| Justicia gangetica L. (Asystasia gangetica L.) | Acanthaceae | Leaf | 36 |
| Lamiophlomis rotata Benth. (Phlomis rotata) | Lamiaceae | 3 | |
| Ligustrum robustum Roxb. | Oleaceae | Leaf (0.0022%) | 37 |
| Lonicera gracilipes var. glandulosa Maxim. | Caprifoliaceae | 38 | |
| Lonicera japonica Thunb. | Caprifoliaceae | Aerial part, flower buds | 39, 40 |
| Lupinus spp. | Fabaceae | 30 | |
| Lupinus luteus (Yellow lupin) | Fabaceae | Seedlings | 41 |
| Mallotus nanus Airy Shaw. | Euphorbiaceae | Leaf | 42 |
| Musa acuminate (Banana) | Musaceae | 43 | |
| Marrubium deserti De Noè | Lamiaceae | 44 | |
| Ononis campestris (Cammock) | Fabaceae | Shoot | 16 |
| Ononis spinosa | Fabaceae | 45 | |
| Oxytropis varians | Fabaceae | 46 | |
| Paeonia suffruticosa Andrews. | Paeoniaceae | Flower | 47 |
| Poncirus trifoliata L. | Rutaceae | 48 | |
| Prosthechea michuacana W.E. Higgins | Orchidaceae | Bulbs | 49 |
| Rhus succedanea L. (Toxicodendron succedaneum L.) | Anacardiaceae | Leaf | 5 |
| Rhus sylvestris Siebold. & Zucc | Anacardiaceae | 3 | |
| Sabal serratula (Serenoa or Sabal fruit) | Arecaceae | Whole plant | 45 |
| Santalum insulare | Santalaceae | Leaf | 50 |
| Saussurea gossypiphora D. Don. | Asteraceae | 51 | |
| Saussurea medusa Maxim. | Asteraceae | 3 | |
| Scabiosa comosa Fisch. | Dipsacaceae | 52 | |
| Scutellaria barbata Don. | Lamiaceae | 53 | |
| Scutellaria polyodon | Lamiaceae | 10 | |
| Serenoa repens W. Bartram (Small saw palmetto) | Arecaceae | Fruit | 45 |
| Solanum lycopersicum (Tomatoes) | Solanaceae | 54 | |
| Terminalia arjuna | Combretaceae | Leaf | 55 |
| Tilia mongolica Maxim. | Tiliaceae | 56 | |
| Trachelospermum difforme | Apocynaceae | 57 | |
| Trachelospermum jasminoides (Lindl.) Lem. | Apocynaceae | 57 | |
| Uraria picta | Fabaceae | Aerial parts | 11 |
| Veronica francispetae M. A. Fischer | Plantaginaceae | 58 | |
| Veronica persica Poir. | Plantaginaceae | 58 | |
| Veronica polita Fries | Plantaginaceae | 58 | |
| Veronica siaretensis Lehmann | Plantaginaceae | 58 | |
| Vitis vinifera | Vitaceae | 59 |
Isolation of Rhoifolin from Chorisia spp.: Different organs of Chorisia spp. (Bombacaceae) are a well-known and rich source of rhoifolin. Substantial amounts of this glycoside can be directly obtained from the total alcoholic and aqueous extracts of these plants. Four Chorisia spp. growing in Argentina provided rhoifolin in different yields including C. insignis (0.5%), C. speciosa (0.27%), C. pubiflora (0.24%) and C. crispiflora (0.15%) 6. According to the method described by Coussio, 6 1 kg of fresh leaves of C. insignis was boiled for 15 min with 2.6 l of water, and the extract was filtered on hot. Rhoifolin was then crystallized on cooling the filtrate. Further purification was achieved by several recrystallization steps from 50% methanol to provide yellow needles sintering at 202-205 °C and melting at 245 °C 6.
In another work by Eldahshan, the air-dried powdered leaves of C. crispiflora (1kg) was extracted with 70% ethanol. The extract was then entirely dried and dissolved in a small amount of distilled water and partitioned with n-hexane, ethyl acetate, and butanol, successively. The aqueous residue was dried and extracted with methanol at 40 ºC. The methanol extract upon concentration yielded yellow crystals of rhoifolin (8.3 g) that was purified by further crystallization 7.
HPLC Analysis and Quantification of Rhoifolin: In a study by Scordino et al. to investigate the identity and relative distribution of flavonoids and furocoumarins in pulp and peel tissues of the unripe Citrus myrtifolia by HPLC/PDA/ESI/MS-MS, rhoifolin was identified and quantified as 0.4% in the pulp and 1.6% in the peel. It also showed retention time of 40.0 min in HPLC analysis using a binary gradient of 0.3% formic acid in water and 0.3% formic acid in acetonitrile on an analytical column (Luna C18 250 × 4.6 mm, 5 μm i.d. (Phenomenex)) and photodiode-array detector 12.
On the other hand, a method for determining flavonoids in human plasma was presented for application to pharmacokinetic studies of rhoifolin. Isocratic reversed-phase HPLC was used with genistin as an internal standard and solid-phase extraction using a Sep-Pak C18 cartridge. A mobile phase of acetonitrile-0.1M ammonium acetate solution (20:80 v/v) was used 59.
In another work, an LC method was developed for quantitation of rhoifolin in Uraria picta. Rhoifolin showed a retention time of 14.74 min in the isocratic RP-LC method using a C18 column and a mobile phase of acetonitrile-water containing 1.0% trifluoroacetic acid (TFA) (20:80 v/v). A flow rate of 1.0 ml min-1 and column temperature at 30 °C were maintained throughout the run. The quantitation was performed at 265 nm 11.
Biological Activities:
Anti-inflammatory Activity: In a study by Eldahshan and Azab, rhoifolin was shown to possess potent anti-inflammatory activity at low doses. It caused a time- and reverse the dose-dependent reduction of carrageenan-induced rat paw edema. Following 4 hr of treatment, rhoifolin at doses of 2.5, 25 and 250 mg/kg caused a significant inhibition of rat paw edema volume by 14, 25 and 45%, respectively in comparison to the control group (74%). In addition to significantly abrogating prostaglandin E2 level, increasing doses of rhoifolin significantly diminished the TNF-α release in the inflammatory exudates. In the same animal model, rhoifolin increased the total antioxidant capacity in a reverse dose order, with the highest capacity obtained with the lowest dose tested 61.
Anticancer Activity: Rhoifolin exhibited potent in-vitro cytotoxicity with great selectivity against human epidermoid larynx (Hep 2) (IC50 = 5.9 μg/ml) and human cervical (HeLa) carcinoma cell lines (IC50 = 6.2 μg/ml). Promising activities were also obtained against hepatocellular (Hep G2) (IC50 22.6 μg/ml), colon (HCT-116) (IC50 34.8 μg/ml) and fetal human lung fibroblast (MRC-5) (IC50 = 44.6 μg/mL) carcinoma cell lines. The effects were nearly similar to those of vinblastine. Results also showed no cytotoxic activity against healthy normal mammalian cells (Vero cells) indicating a high degree of selectivity 7.
Anti-diabetic Activity: In differentiated 3T3-L1 adipocytes, rhoifolin showed a dose-dependent insulin-mimetic effect within the concentration range 0.001-5 μM. At 0.5 μM, rhoifolin showed a nearly similar response to that of 10 nM of insulin on adiponectin secretion level. Furthermore, 5 μM of rhoifolin showed equal potential with 10nM of insulin to increase the phosphorylation of insulin receptor-β, in addition to its positive effect on GLUT4 translocation. These findings indicated that rhoifolin might be beneficial for diabetic complications through enhanced adiponectin secretion, tyrosine phosphorylation of insulin receptor-β and GLUT4 translocation 8.
Hepatoprotective Activity: Rhoifolin isolated from Chorisia crispiflora H.B.K. leaves showed 80.3% protection against CCl4-induced hepatotoxicity in mice at 20 mg/Kg. The liver showed its normal architecture and the serum levels of ALT and AST were kept close to normal 62. In another study, pretreatment of CCl4-treated rats with rhoifolin reduced the enhanced serum levels of hepatic enzymes. AST, ALT, TB, ALP and total serum protein were reduced by 60%, 59%, 51%, 39%, and 43%, respectively, indicating good anti-hepatotoxic activity. Also, the elevated level of lipid peroxidation products (TBARS), an indicator of oxidative stress in CCl4-intoxicated mice, was depressed by oral administration of rhoifolin at 20 mg/kg. The effect was comparable to that of silymarin 49.
Antihypertensive and Hemodynamic Effects: It was reported that rhoifolin exhibited important antihypertensive effects in conscious spontaneously hypertensive rats 3. In another study, the in-vitro ACE inhibitory activity of 17 flavonoids belonging to five structural subtypes were evaluated at two concentrations (500 and 100 µM) by a fluorimetric method. Among them, rhoifolin exhibited IC50 value of 183 µM. The catechol group of ring B, the double bond between C-2 and C-3 of ring C and the ketone group at C-4 of ring C were found to be important structural requirements for such activity 63.
On the other hand, the acute effects of luteolin, apiin and rhoifolin on the pulmonary vascular circuit in two experimental models of pulmonary hypertension, produced by hypoxia and by prostaglandin F2α (PGF2α) in anesthetized dogs, were studied in comparison with nifedipine. Rhoifolin at 5 mM/kg/i.v. produced no change in hypoxic pulmonary vasoconstriction but decreased cardiac output and aortic pressure. The response of pulmonary hypertension induced by PGF2α to flavonoids and nifedipine was nearly identical to that of hypoxia-induced pulmonary hypertension 64. In another comparative study of the hemodynamic effects of rhoifolin and vitexin in anesthetized dogs, rhoifolin caused a decrease of mean aortic pressure, of the arterial and pulmonary capillary pressure and heart rate 65.
Antimicrobial Activity: Rhoifolin exhibited certain inhibitory activity against Escherichia coli 28. This flavone was also found to cause 13% inhibition of coxsackievirus B3 infection with IC50 of 569.05 μM, whereas it reduced the viability of untreated cell cultures by 50% at >1000 μM in MTT assay with a calculated selective index of 1.8. Its antiviral mechanism may be due to the prevention of virus adsorption onto the cell surface, inhibition of protein kinase, viral DNA synthesis or virus-associated reverse transcriptase 66.
A composition comprising ligustroflavone, rhoifolin, and hyper in was found to potentially inhibit the influenza virus neuraminidase from hydrolyzing the sialic acid on the cell surface, prevent the virus from combining with the cell surface receptors and entering into the cells and reduce the generation of the virus within the cells, thus effectively and specifically inhibiting influenza virus replication. Besides, this composition overcomes the side reactions of the existing drugs 67.
Other Activities: In work to evaluate the inhibition of quinine 3-hydroxylation (CYP3A4 activity) in two human liver microsomes (HL1 and HL2) by grapefruit flavonoids, furanocoumarins and coumarins; rhoifolin did not inhibit the metabolism of quinine at 10 and 100 μM. Only a moderate inhibition (18% for HL1 and 26.1% for HL2) was observed at 200 μM 68. On the other hand, rhoifolin at 100 μmol/l inhibited CCl4- and FeSO4+cysteine-induced lipid peroxidation by 37.9% and 70.1%, respectively, with IC50= 66.1 μmol/l. Additionally, it exhibited inhibitory effects on AAPH-induced hemolysis of RBCs with IC50= 95.9 μmol/l indicating its potential antioxidant properties 3. It was also reported that rhoifolin possess xanthinoxidase inhibitory effect (12.9%) at 50 μg/ml 3.
CONCLUSION: Due to the growing demand for safe, natural pharmaceuticals to face the everyday challenging diseases and in light of the considerable interest in the chemistry and pharmacological properties of flavonoids, we have undertaken this review to summarize the chemistry, distribution and biological activities of rhoifolin. The available literature data have shown that this flavone enjoys a wide distribution in several plant families and can also be obtained in considerable amounts from some species, e.g. Citrus and Chorisia spp. Moreover, numerous preclinical studies have shown that rhoifolin possesses a wide range of biological activities and several possible mechanisms of action have been elucidated.
These pharmacological findings strongly recommend that rhoifolin could be developed into widely used remedies especially for its potent anti-inflammatory, hepatoprotective, insulin-mimetic actions and the highly selective cytotoxic effects. Hence, further investigation of the molecular mechanisms of these effects along with detailed clinical studies will be necessary for the future.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- López-Lázaro M: Distribution and biological activities of the flavonoid luteolin. Mini-Reviews in Medicinal Chemistry 2009; 9(1): 31-59.
- Tanwar B and Modgil R: Flavonoids: Dietary occurrence and health benefits. Spatula DD 2012; 2(1): 59-68.
- Zhou J, Xie G and Yan X: Encyclopedia of traditional Chinese medicines: Molecular structures, pharmacological activities, natural sources and applications. Springer Heidelberg Dordrecht, New York 2011; 1: 167-71, 203.
- Andersen OM and Markham KR: Flavonoids: Chemistry, biochemistry and applications. Taylor and Francis Group, London 2006: 632.
- Hattori S and Matsuda H: Rhoifolin, a new flavone glycoside, isolated from the leaves of Rhus succedanea. Archives of Biochemistry Biophysics 1952; 37(1): 85-89.
- Coussio JD: Isolation of rhoifolin from Chorisia species (Bombacaceae). Experientia 1964; 20(10): 562.
- Eldahshan OA: Rhoifolin; A potent antiproliferative effect on cancer cell lines. British Journal of Pharmaceutical Research 2013; 3(1): 46-53.
- Rao YK, Lee MJ, Chen K, Lee YC, Wu WS and Tzeng YM: Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) Osbeck leaves enhanced adiponectin secretion and insulin receptor phosphorylation in 3T3-L1 cells. Evidence-Based Complementary and Alternative Medicine 2011; 624375: 1-9.
- Refaat J: Phytochemical and biological studies of Chorisia chodatii and Chorisia speciosa A. St.-Hil. Family Bombacaceae cultivated in Egypt. A Thesis for the Doctor Degree submitted to Faculty of Pharmacy, Minia University, Egypt 2014.
- http://www.springerreference.com/docs/html/chapterdbid/351096.html, accessed in June 2013.
- Yadav AK, Deepti Y, Karuna S, Ram KV, Ajit KS and Madan MG: Flavone glycoside based validated RP-LC method for quality evaluation of prishniparni (Uraria picta). Chromatographia 2009; 69(7-8): 653-658.
- Scordinoa M, Sabatino L, Belligno A and Gagliano G: Characterization of polyphenolic compounds in unripe Chinotto (Citrus myrtifolia) fruit by HPLC/PDA/ESI/MS-MS. Natural Products Communications 2011; 6(12): 1857-1862.
- Gattuso G, Barreca D, Gargiulli C, Leuzzi U and Caristi C: Flavonoid composition of Citrus juices. Molecules 2007; 12(8): 1641-1673.
- Zhang LY, Zhang J and Wang H: Analysis of flavonoids in leaves of Adinandra nitida by capillary electrochromatography on monolithic columns with stepwise gradient elution. Journal of Separation Sciences 2005; 28(8): 774-779.
- Zhang J, Yang J, Duan J, Liang Z, Zhang L, Huo Y and Zhang Y: Quantitative and qualitative analysis of flavonoids in leaves of Adinandra nitida by high-performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Analytica Chimica Acta 2005; 532(1): 97-104.
- http://en.wikipedia.org/wiki/Rhoifolin, accessed in May 2013.
- Liang T, Cheng HJ, Ping ZY and Chong L: Chemical constituents in Buddleja albiflora. Zhongguo Zhong Yao Zazhi 2009; 34(23): 3043-3046.
- Jordon-Thaden IE and Louda SM: Chemistry of Cirsium and Carduus: A role in ecological risk assessment for biological control of weeds?. Biochemical Systematic and Ecology 2003; 31(12): 1353-1396.
- Ashmawy AM, Azab SS and Eldahshan OA: Effects of Chorisia crispiflora ethyl acetate extract on P21 and NF-κB in breast cancer cells. Journal of American Sciences 2012; 8(8): 965-972.
- Hafez SS, Abdel-Ghani AE and El-Shazly AM: Pharmacognostical and antibacterial studies of Chorisia speciosa Hill. flower (Bombacaceae). Mansoura Journal of Pharmaceutical Sciences 2003; 19(1): 40-43.
- Kawaii S, Tomono Y, Katase E, Ogawa K and Yano M: HL-60 differentiating activity and flavonoid content of the readily extractable fraction prepared from Citrus juices. Journal of Agricultural and Food Chemistry 1999; 47(1): 128-135.
- http://www.liberherbarum.com/Minor/UK/IN2516.htm, accessed in June 2013.
- Berhow M, Tisserat B, Kanes K and Vandercook C: Survey of phenolic compounds produced in Citrus. USDA ARS Technical Bulletin 1998; 1856: 1-154.
- Kanes K, Tisserat B, Berhow MA and Vandercook CE: Phenolic composition of various tissues of Rutaceae species. Phytochemistry 1993; 32(4): 967-974.
- Mostafa NM, El-Shamy A, Mohamed T, El-Toumy S, Abdel-Lateef A and Farrag A: Chemical constituents and antiulcerogenic activity of Cynara scolymus Heads 13th Congress of the International Society for Ethnopharmacology in collaboration with the Society for Medicinal Plant and Natural Product Research and Eurasia-Pacific Uninet, Graz, Austria 2012.
- Kaneko T, Sakamoto M, Ohtani K, Ito A, Kasai R, Yamasaki K and Padorina WG: Secoiridoid and flavonoid glycosides from Cynodon dactylon. Phytochemistry 1995; 39(1): 115-120.
- Sayed HM, Mohamed MH, Farag SF, Mohamed GA, Ebel R, Omobuwajo ORM and Proksch P: Phenolics of Cyperus alopecuroides Inflorescences and their biological activities. Assiut Bulletin of Pharmaceutical Sciences 2006; 29(1): 9-32.
- Tian Y, Tang H, Wang X, Qiu F, Xue G and Li J: Studies on antibacterial constituents of Discocleidion rufescens (2). Zhongguo zhongyao zazhi 2009; 34(11): 1377-1380.
- Li LQ: Research on Bian Dou Hua chemical composition. Journal of University of Pharmacology of China 1996; 27(4): 205-207.
- Plouvier V: Cephalotaxoside, a new apigenin heteroside isolated from Cephalotaxus. Presence of rhoifolin in Exochorda racemosa and Lupinus. Comptes Rendus de L'academie des Sciences Serie D: Sciences Naturelles 1966; 263(1): 1529-1532.
- Casabuono AC and Pomilio AB: Flavonoids of Festuca argentina. Fitoterapia 1990; 61(3): 284-285.
- Kikuchi M, Goto J, Noguchi S, Kakuda R and Yaoita Y: Glycosides from whole plants of Glechoma hederacea L. Journal of Natural Medicine 2008; 62(4): 479-480.
- Kaneko T, Sakamoto M, Ohtani K, Ito A, Kasai R, Yamasaki K and Padorina WG: Secoiridoid and flavonoid glycosides from Gonocaryum calleryanum. Phytochemistry 1995; 39(1): 115-120.
- Li-dong L, Guo-wei Q, Ren-sheng X, Xian-rong W, Hong-ping W, Ueda S and Fujita T: Studies on chemical constituents of Ilex centrochinensis. Acta Botanica Sinica 1994; 36(5): 393-397.
- Abd-Alla HI, Moharram FA, Gaara AH and El-Safly MM: Phytoconstituents of Jatropha curcas leaves and their immunomodulatory activity on humoral and cell-mediated response in chicks. Z Naturforsch C 2009; 64(7-8): 495-501.
- Kanchanapoom T and Ruchirawat S: Megastigmane glucoside from Asystasia gangetica (L.). Journal of Natural Medicine 2007; 61(4): 430-433.
- He ZD, Lau KM, But PP, Jiang RW, Dong H, Ma SC, Fung KP, Ye WC and Sun HD: Antioxidative glycosides from the leaves of Ligustrum robustum. Journal of Natural Products 2003; 66(6): 851-854.
- http://www.plant-expert.com/plant-2052.html, accessed in June 2013.
- Son KH, Park JO, Chung KC, Chang HW, Kim, HP, Kim JS and Kang SS: Flavonoids from the aerial parts of Lonicera japonica. Arch Pharm Res 1992; 15(4): 365-370.
- Lee EJ, Kim JS, Kim HP, Lee JH and Kang SS: Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chemistry 2010; 120(1): 134-139.
- Katagiri Y, Hashidoko Y and Tahara S: Localization of flavonoids in the yellow lupin seedlings and their UV-B-absorbing potential. Z Naturforsch 2002; 57c: 811-816.
- Kiem PV, Mai NT, Minh CV, Khoi NH, Dang NH and Thao NP: Two new C-glucosyl benzoic acids and flavonoids from Mallotus nanus and their antioxidant activity. Archives of Pharmacal Research 2010; 33(2): 203-208.
- http://gohelle.cirad.fr:1555/MUSA/NEW-IMAGEtype= COMPOUND object=Apigenin 7-O-neohesperidose, accessed in May 2013.
- Zaabat N, Hay AE, Michalet S, Darbour N, Bayet C and Skandrani I: Antioxidant and antigenotoxic properties of compounds isolated from Marrubium deserti de Noé. Food and Chemical Toxicology 2011; 49(12): 3328-3335.
- http://www.extrasynthese.com/products./rhoifolin-p2029403-c1137-s.html, accessed in June 2013.
- Mao XL, Zhi HL, Li LW, Wen JZ, Ru XZ and Zheng PJ: Phytochemical and biological studies of plants from the genus Oxytropis. Records of Natural Products 2012; 6(1): 1-20.
- Wang X, Cheng C, Sun Q, Li F, Liu J and Zheng C: Isolation and purification of four flavonoid constituents from the flowers of Paeonia suffruticosa by high-speed counter-current chromatography. Journal of Chromatography A 2005; 1075(1-2): 127-131.
- Rajkumar S and Jebanesan A: Bioactivity of flavonoid compounds from Poncirus trifoliata (Family: Rutaceae) against the dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitology Research 2008; 104(1): 19-25.
- Gutierrez RMP, Anaya SI, Vadillo CH and Victoria TC: Effect of flavonoids from Prosthechea michuacana on carbon tetrachloride-induced acute hepatotoxicity in mice. Pharmaceutical Biology 2011; 49(11): 1121-1127.
- Butaud J-F, Raharivelomanana P, Bianchini JP, Faure R and Gaydou EM: Leaf C-glycosylflavones from Santalum insulare (Santalaceae). Biochem Sys Ecol 2006; 34(5): 433-435.
- Sheng-zhen Z, Jian-hua Y and Xu-Wei S: Studies on the chemical constituents of Saussurea gossypiphora Don. Chemical Journal of Chinese Universities 1991; 12(12): 1613-1616.
- http://baike.baidu.com /view/853879.htm, accessed in June 2013.
- Wang WS, Zhou YW, Ye YH and Du N: Studies on the flavonoids in herb from Scutellaria barbata. Zhongguo zhongyao Zazhi 2004; 29(10): 957-959.
- http://pathway.gramene.org/LYCO/NEW-IMAGEtype= COMPOUND&object=Apigenin7-O-neohesperidose, accessed in June 2013.
- Chauhan SMS, Mishra MK, Parkash S and Kaushik R: Isolation of phenolics from the leaves of Terminalia arjuna. Jou of the Ind Chemi Soc 1998; 75(5): 328-329.
- Iwashina T and Kokubugata G: Flavone and flavonol glycosides from the leaves of Triumfetta procumbens in the Ryukyu Islands. Bulletin of the National Museum of Nature and Science Series B 2012; 38(2): 63-67.
- Akushima A, Hisada S, Agata I and Nishibe S: The constituents of Apocyanaceae plants, flavonoids from jasminoides var. pubescens and Trachelospermum difforme. Shoyakugaku Zasshi 1982; 36(1): 82-87.
- Mehrvarz SS, Mahmoodi NO, Asadian R and Khaniki GB: Iridoid and flavonoids patterns of the genus Veronica sect. Alsinebe subsect. Agrestis (Benth.) Stroh (Lamiales) and their systematic significance. Australian Journal of Crop Sciences 2008; 1(1): 1-5.
- http://pmn.plantcyc.org/GRAPE/NEW-IMAGEtype= COMPOUND&object= rhoifolin, accessed in June 2013.
- Ishii K, Urano S, Furuta T and Kasuya Y: Determination of rhoifolin and daidzin in human plasma by high-performance liquid chromatography. Journal of Chromatography B Biomedical Applications 1994; 655(2): 300-304.
- Eldahshan OA and Azab SS: Anti-inflammatory effect of apigenin 7-neohesperidoside (rhoifolin) in carrageenin-induced rat edema model. Journal of Applied Pharmaceutical Sciences 2012; 2(8): 74-79.
- Hassan AA: Phytochemical and biological investigation of certain plants containing pigments. A Thesis for the Doctor Degree submitted to Faculty of Pharmacy, Mansoura University, Egypt 2009.
- Guerrero L, Castillo J, Quiñones M, Garcia-Vallvé S, Arola L, Pujadas G and Muguerza B: Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. Plos One 2012; 7(11): e49493.
- Occhiuto F and Limardi F: Comparative effects of the flavonoids luteolin, apiin and rhoifolin on experimental pulmonary hypertension in the dog. Phytotherapy Research 1994; 8(3): 153-156.
- Occhiuto F, Circosta C, De Pasquale A and Briguglio F: Comparative haemodynamic effects of the flavonoids rhoifolin and vitexin in the dog. Phytotherapy Research 1990; 4(3): 118-120.
- Cantera JL, Chen W and Yates MV: A fluorescence resonance energy transfer-based fluorometer assay for screening anti-coxsackievirus B3 compounds. Journal of Virological Methods 2011; 171(1): 176-182.
- Tang C, Xie N, Yang X, Lv W, Li Z and Ye J: Composition comprising ligustroflavone, rhoifolin and hyperin and its pharmaceutical application. Patent number: 20130131000, 2013.
- Ho P-C, Saville DJ and Wanwimolruk S: Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins, and related compounds. Journal of Pharmacy and Pharmaceutical Sciences 2001; 4(3): 217-227.
How to cite this article:
Refaat J, Desoukey SY, Ramadan MA and Kamel MS: Rhoifolin: A review of sources and biological activities. Int J Pharmacognosy 2015; 2(3): 102-09. doi: 10.13040/IJPSR.0975-8232.2(3).102-09.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
102-109
695
3014
English
IJP
J. Refaat *, S. Y. Desoukey, M. A. Ramadan and M. S. Kamel
Pharmacognosy Department, Faculty of Pharmacy, Minia University, Minia, Egypt.
Johnrefaat82@yahoo.com
29 December 2014
25 January 2015
23 February 2015
10.13040/IJPSR.0975-8232.IJP.2(3).102-09
01 March 2015