REPURPOSING AND PHYTOCHEMICAL INVESTIGATION OF SALACIA OBLONGA ROOT EXTRACT RESPONSIBLE ANTICANCER ACTIVITY AGAINST BREAST CANCER CELL LINE (MCF-7)
HTML Full TextREPURPOSING AND PHYTOCHEMICAL INVESTIGATION OF SALACIA OBLONGA ROOT EXTRACT RESPONSIBLE ANTICANCER ACTIVITY AGAINST BREAST CANCER CELL LINE (MCF-7)
Ganesh Barkade * 1, Ramesh Sawant 1 and Jyoti Wadekar 2
Department of Pharmaceutical Chemistry 1, Department of Pharmacognosy 2, Dr. Vithalrao Vikhe Patil Foundation’s College of Pharmacy, Ahmednagar - 414111, Maharashtra, India.
ABSTRACT: Salacia oblonga wall, a native shrub, also known as Saptrangi and Ponkoranti, belongs to the family Celastraceae, is distributed across the world. A large number of chemical constituents such as salacinol, kotalanol, neokotalanol, neosalacinol, and mangiferin are isolated from S. oblonga, which show various pharmacological activities. Salacia oblonga wall is being used in several herbal preparations for treating diabetes and obesity. It possesses anti-inflammatory, antihyperlipidemic, antiperoxidative, antimicrobial, antimutagenic, nephroprotective, and antimutagenic activities. Salacia oblanga has a long history of use as a treatment for diabetes in Ayurveda, traditional Indian medicine. Mugs made from Salacia wood are used by people with diabetes to drink water. In addition to treating diabetes, Salacia is used for treating gonorrhea, asthma, itchiness, joint pain (rheumatism), obesity, thirst, and menstrual problems. Salacia oblonga, an inhabitant of tropical regions, has been used in traditional Indian medicinal systems. Phytochemicals were extracted in methanol from the plant and analyzed for various anticancer activity. This research focuses on extraction, phytochemical investigation of Salacia, and the potential of Salacia oblonga wall in breast cancer by using in-vitro anti-cancer testing on breast cancer cell line (MCF-7).
| Keywords: |
Repurposing, Salacia oblonga, Saptrangi, Phytochemical investigation, Anticancer activity
INTRODUCTION: Salacia is a climbing shrub distributed in South-West India, Peninsula, Ceylon, Java, Thailand, and Philippines 1. Within India, it is distributed in Karnataka (rare in semi-evergreen forests of Western Ghats), Kerala (coastal forests of Kollam, Western Ghats of Pathanamthitta and Idukki districts) and Southern Orissa 2. In the Traditional System of Medicine, the plants of this genus are being used as acrid, bitter, thermogenic, urinary, astringent, anodyne, anti-inflammatory, depurative, emmenagogue, vulnerary, liver tonic and stomachic.
They are useful in vitiates conditions of vata, diabetes, hemorrhoids, inflammation, leucorrhoea, leprosy, skin diseases, amenorrhoea, dysmenorrhoea, wounds, ulcers, hyperhidrosis, hepatopathy, dyspe-psia, flatulence, colic and spermatorrhoea 3. The present aim is to give a comprehensive review of macroscopical characteristics, phytochemical and pharmacological activities reported so far from this genus. The genus Salacia comprises of 100 species, out of which, in India, Salacia reticulata and Salacia oblonga are predominant species 4.
Taxonomical / Scientific Classification: 5, 6, 7
| Kingdom | : | Plantae - Planta, plantes, plants, vegetal |
| Subkingdom | : | Tracheobionta - vascular plants |
| Division | : | Magnoliophyta - angiosperms |
| Class | : | Magnoliopsida - dicots, dicotylédones, dicotyledons |
| Subclass | : | Rosidae |
| Order | : | Celastrales |
| Family | : | Celastraceae - bittersweet |
| Genus | : | Reticulata oblonga; campestris; Hainanensis madagascariensis; chinesis; petensis; krausii; fruticosa; macrosperma.
Salacia oblonga roots extract contains the following chemical constituents: Salacinol, kotanolol, kotalagenin-16-acetate |
FIG. 1: SALACIA OBLANGA PLANT WITH FRUITS
FIG. 2: SALACIA OBLANGA ROOTS
MATERIAL AND METHOD: 8, 9, 10, 11
Preparation of Extracts: The plant material was collected from Botanical garden of Shree Swami Samarth Seva Kendra Dindori Pranit from Nashik region, Maharashtra. The plant collection was done in the month of June. At the time of collection, various plant parts were collected. The various available plant parts were supplied for authentication. The plant was authenticated at Department of Botany, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar (Maharashtra). A herbarium specimen was prepared and deposited in a pharmacognosy laboratory for future use. The phytochemicals were extracted in methanol with the help of a Soxhlet apparatus and concentrated using a rotatory evaporator under reduced pressure.
Preliminary Phytochemical Screening: Following chemical tests are used for investigation of various chemical constituents in the extract.
Test for Alkaloids: Few mg of extract was taken separately in 5 ml of 1.5% v/v HCl filtered. The filtrate was then subjected to the following tests.
Dragendroff’s Test: To 2-3 ml filtrate, few drops of Dragendroff’s reagent were added. The development of orange-red precipitate indicated the presence of alkaloids.
Hager’s Test: To 2-3 ml of filtrate, few drops of Hager’s reagent were added. The development of yellow precipitate indicated presence of alkaloids.
Test for Glycosides: To 2.0 ml of extract, 0.2 ml of β - Napthol was added gently. Few drops of conc. H2SO4 was added to it, appearance of the violet ring indicated the presence of glycosides.
Test for Flavonoids:
Shinoda Test: About 2.0 ml of extract was dissolved in 5 ml of 95% ethanol and treated with a few drops of conc. HCl and 0.5 mg of magnesium turnings. A pink color indicates the presence of flavonoids.
Lead Acetate: To 2.0 ml of extract, a few drops of Lead acetate solution were added. Development of yellow precipitate indicated presence of flavonoids.
Test for Tannins:
With FeCl3: To 2.0 ml of extract, few drops of FeCl3 solution were added. The appearance of the blue colour indicated the presence of tannins.
With Lead acetate: To 2.0 ml of extract, few drops of lead acetate solution were added. The appearance of white precipitate indicated the presence of tannins.
Test for Sterols (Salkowaski Test): To 2.0 ml of extract, 2.0 ml of chloroform, and 2.0 ml of conc. H2SO4 was added from the side of the test tube. Was shaken for few mins. The development of the red color of the chloroform layer indicated the presence of sterols.
Physicochemical Analysis: Air-dried material was used for the quantitative determination of phytochemical values.
- Total, water-soluble ash, acid insoluble ash.
- Water soluble and alcohol soluble extractive.
- Moisture conten
Determination of Ash value:
Determination of Total Ash Value:
Procedure:
- Weigh and ignite flat, thin, porcelain dish or a tared silica crucible.
- Weigh about 2 gm of the powdered drug into the dish/crucible.
- Support the dish on a pipe – clay triangle placed on a ring of retort stand.
- Heat with a burner, using a flame about 2 cm high and supporting the dish about 7 cm, above the flame, heat till vapors almost cease to be evolved; then lower the dish and heat more strongly until all the carbon is burnt off.
- Cool in a desiccator.
- Weigh the ash and calculate the percentage of total ash with reference to the air-dried sample of the crude drug.
Determination of Acid - Insoluble Ash value:
Procedure: Proceed as per the steps mentioned in the procedure for the determination of the total ash value of a crude drug. Further
- Using 25 ml of dilute hydrochloric acid, wash the ash from the dish used for total ash into a 100 ml beaker.
- Place wire gauze over a Bunsen burner and boil for five minutes.
- Filter through an ‘ashless’ filter paper, wash the residue twice with hot water.
- Ignite a crucible in the flame, cool and weigh.
- Put the filter - paper, and residue together into the crucible; heat gently until vapors ceases to be evolved and then more strongly until all the carbon has been removed.
- Cool in a desiccator.
- Weigh the residue and calculate acid-insoluble ash of the crude drug with reference to the air-drieded sample of the crude drug.
Determination of Water-Soluble Ash: This is determined in a similar way to acid insoluble ash, using 25 ml of water, in place of dilute hydrochloric acid.
Determination of Extractive Values:
Water - Soluble Extractives: This method is applied to drugs, which contain water-soluble active constituents of crude drugs, such as tannins, sugars, plant acids, mucilage’s, glycosides, etc.
Procedure: 5 gm of the powdered drug was accurately weighed in the glass stoppered conical flask and was macerated with 25 ml of distilled water for 6 h with frequent shaking. It was then allowed to stand for 18 h. After completion of 18 h, the contents were filtered and transferred in a tarred flat bottom porcelain dish. Then the filtrate was evaporated to dryness on a water bath and dried at 105 °C for 6 h cooled in a desiccator for 30 min and weighed. Contents were calculated of extractable matter in milligrams per gram of air-dried material.
Alcohol - Soluble Extractives:
Procedure: Accurately weighed 5 gm of powdered drug placed in the glass stoppered conical flask and Macerated with 25 ml of ethanol 95% for 6 h with frequent shaking, mixture allowed to stand for 18 h.
After completion of 18 h, it was filtered rapidly, taking care not to lose any solvent. Transferred the filtrate in tarred flat bottom porcelain dish. Filtrate was evaporated to dryness on a water bath, dried at 105 °C for 6 h cooled in a desiccator for 30 min and weighed. Calculated content of extractable matter in milligrams per gram of air-dried material.
RESULT AND DISCUSSION:
Yield of Extract:
TABLE 1: YIELD OF EXTRACT
| Extraction solvent | Extract yield (w/w) | Extract
description |
| Methanol | 07.3% | Dark brown, thick consistency |
The yield was calculated for dry extract by using the following equation:
% of Dry extract yield = Mass of extract / Mass of Plant material × 100
07.3gm / 100gm × 100 = 07.3%
The extraction procedure determines both the quantity and quality of the crude extracts obtained from extraction solvent in this study, maceration using methanol, crude extract with yields ranging from 12%.
Preliminary Phytochemical Screening:
TABLE 2: PHYTOCHEMICAL ANALYSIS OF SALACIA OBLANGA ROOTS
| S. no. | Class of compounds | Plant part | Tests performed |
| roots | |||
| 1 | Alkaloids | + | Dragendorff’s test, Mayers test |
| 2 | Carbohydrates | + | Molish test, Fehling test |
| 3 | Glycoside | + | Keller killiani test |
| 4 | Phenolic compounds/tannins | + | Ferric chloride test |
| 5 | Proteins and amino acids | + | Xantho protein test |
| 6 | Flavanoids | + | Ammonia test, shinoda test |
| 7 | Saponins | + | With water With sodium bicarbonate |
| 8 | Sterols | + | Liebermann-Burchard test salkowski reaction Hesse’s reaction |
| 9 | Acid compounds | + | With sodium bicarbonate With litmus paper |
| 10 | Resins | + | With double distilled water With acetone and |
| 11 | Peroxides | - | Potassium Iodide test |
| 12 | Polyuronoids | - | Haemotoxylin test |
The qualitative chemical investigation for the above extract was carried out to detect the presence of various phytoconstituents in the extracts. The extract showed the presence of different constituents such as alkaloids, proteins, tannins, flavonoids, and phytosterols.
Physicochemical Analysis:
TABLE 3: THE ANALYTICAL VALUES IN RESPECT OF PHYSICOCHEMICAL CONSTANT
| S. no. | Particulars | Results (% w/w) |
| 1 | Total ash | 4.82 |
| 2 | Acid-insoluble ash | 3.5 |
| 3 | Water-soluble ash | 2.75 |
| 4 | Alcohol soluble extractives | 1.85 |
| 5 | Water-soluble extractives | 03.75 |
| 6 | Moisture content (LOD) | 09.70 |
The total ash value was 4.82% w/w, while water-soluble ash value was found to be 2.75% w/w. Acid insoluble ash value was found to be 3.5% w/w. Also, water-soluble extractive and alcohol soluble extractive values were found to be 3.75% w/w and 1.85% w/w, respectively. Ash value was found to be high. The water-soluble extractive value was found to be greater than the alcohol-soluble extractive value. This indicates that there are more polar compounds present in leaves that can be extracted maximum into the water than the alcohol.
Isolation of Constituent:
TABLE 4: Rf VALUE OF COMPOUND
| S. no | Rf value | Color of spot |
| 1 | 0.82 | Brown |
| 2 | 0.89 | Brown |
| 3 | 0.74 | Brown |
| 4 | 0.79 | Brown |
TABLE 5: YIELD OF ISOLATED CONSTITUENT
| Compound code | Isolation solvent | Isolated compound yield (w/w) | Isolated compound description |
| A1 | Methanol | 1.25% | Light yellow, thick consistency |
| A2 | Methanol | 1.09 % | Brown, thick consistency |
| A3 | Methanol | 1.25 % | Yellow, Powder |
| A4 | Methanol | 1.85 % | Brown, Powder |
The developed plate was then subjected to the isolation of the flavonoid constituent. The glass plates coated with silica gel G paste were dried naturally (atmospheric). Subsequently, they were activated at 100 °C for 30 min and were cooled at room temperature (≈ 25 °C). The extract was separated by TLC with the following mobile phases: benzene: toluene: ethyl acetate (30:30:40 v/v) along with the solvent system movement.
The plate was kept in the UV Chamber at 366 nm, and the flavonoid band was marked on the scales. The selected area was then scratched out along with the silica, with a sharp scalpel and collected in the Eppendorf tube. The flavonoid constituent was then eluted from the silica gel with methanol, and the content was pooled. The pooled content was concentrated by evaporating the methanol at room temperature, and the final volume was kept.
Anticancer Activity: 12, 13 Anticancer activity of the isolated constituents was done on the breast cancer cell lines (MCF-7) from Anti-cancer drug screening facility (ACDSF), Advanced Centre for Treatment, Research, and Education in Cancer, (ACTREC), Tata Memorial Centre, Kharghar, Navi Mumbai – 410210.
Experimental Procedure for SRB Assay: The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mm L-glutamine. For the present screening experiment, cells were inoculated into 96 well microtiter plates in 100 µL at plating densities, as shown in the study details above, depending on the doubling time of individual cell lines. After cell inoculation, the microtiter plates were incubated at 37 °C, 5% CO2, 95% air, and 100% relative humidity for 24 h prior to the addition of experimental drugs.
Experimental drugs were initially solubilized in dimethyl sulfoxide at 100 mg/ml and diluted to 1mg/ml using water and stored frozen prior to use. At the time of drug addiction, an aliquote of frozen concentrate (1mg/ml) was thawed and diluted to 100 μg/ml, 200 μg/ml, 400 μg/ml and 800 μg/ml with complete medium containing test article. Aliquots of 10 µl of these different drug dilutions were added to the appropriate microtiter wells already containing 90 µl of medium, resulting in the required final drug concentrations i.e.10 μg/ml, 20 μg/ml, 40 μg/ml, 80 μg/ml. After compound addition, plates were incubated at standard conditions for 48 h and assay was terminated by the addition of cold TCA. Cells were fixed in-situ by the gentle addition of 50 µl of cold 30% (w/v) TCA (final concentration, 10% TCA) and incubated for 60 min at 4 °C. The supernatant was discarded; the plates were washed five times with tap water and air-dried. Sulforhodamine B (SRB) solution (50 µl) at 0.4% (w/v) in 1% acetic acid was added to each of the wells, and plates were incubated for 20 min at room temperature. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid.
The plates were air dried. Bound stain was subsequently eluted with 10 mm trizma base, and the absorbance was read on a plate reader at a wavelength of 540 nm with 690 nm reference wavelength. Percent growth was calculated on a plate – by - plate basis for test wells relative to control wells. Percent growth was expressed as the ratio of average absorbance of the test well to the average absorbance of the control wells × 100. Using the six absorbance measurements [time zero (Tz), control growth (C), and test growth in the presence of drug at the four concentration levels (Ti)]; the percentage growth was calculated at each of the drug concentration levels. Percentage growth inhibition was calculated as:
[Ti / C] × 100%
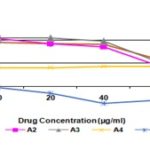
FIG. 3: GROWTH CURVE: HUMAN BREAST CANCER CELL LINE MCF-7
TABLE 6: HUMAN BREAST CANCER CELL LINE MCF-7 % CONTROL GROWTH AND DRUG CONCENTRATION
| 1.00 | Human breast cancer cell line MCF-7 | |||||||||||||||
| % Control growth | ||||||||||||||||
| Drug concentrations (µg/ml) | ||||||||||||||||
| Experiment 1 | Experiment 2 | Experiment 3 | Average Values | |||||||||||||
| 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | |
| A1 | 90.0 | 81.4 | 86.5 | 78.2 | 100.1 | 95.7 | 100.7 | 33.8 | 96.2 | 99.8 | 88.1 | 67.2 | 95.4 | 92.3 | 91.8 | 59.7 |
| A2 | 100.4 | 81.0 | 73.1 | 54.5 | 109.0 | 974 | 94.8 | 33.2 | 102.8 | 101.3 | 91.0 | 50.8 | 104.1 | 93.2 | 86.3 | 46.2 |
| A3 | 91.6 | 95.1 | 95.8 | 67.7 | 111.4 | 113.6 | 106.2 | 40.3 | 113.3 | 106.3 | 92.5 | 53.9 | 105.4 | 105.0 | 98.2 | 53.9 |
| A4 | 38.9 | 37.1 | 37.7 | 44.8 | 43.3 | 46.7 | 48.0 | 49.4 | 40.9 | 38.3 | 44.6 | 36.2 | 41.0 | 40.7 | 43.4 | 43.5 |
| ADR | -2.6 | -20.6 | -35.2 | -27.9 | 1.2 | -11.8 | -34.7 | -31.9 | -6.9 | -12.9 | -40.0 | -31.5 | -2.7 | -15.1 | -36.6 | -30.4 |
TABLE 7: HUMAN BREAST CANCER CELL LINE MCF-7 DRUG CONCENTRATION AND % INHIBITION
| Drug concentrations (µg/ml) calculated from graph | |||
| MCF-7 | LC50 | TGI | GI50 |
| A1 | NE | NE | >80 |
| A2 | NE | NE | 77.8 |
| A3 | NE | NE | >80 |
| A4 | NE | NE | <10 |
| ADR | NE | NE | <10 |
TABLE 8: DESCRIPTION OF ABBREVIATIONS
| Definitions |
| LC50 = Concentration of drug causing 50% cell kill |
| GI50 = Concentration of drug causing 50% inhibition of cell growth |
| TGI = Concentration of drug causing total inhibition of cell growth |
| ADR = Adriamycin, Positive control compound |
| The residual compound with ACTREC will be retained for one month from the date of this report. Inquiries regarding the report will not be entertained after this date. |
| NE Non- evaluable data. The experiment needs to be repeated using a different set of drug concentrations. |
| Note: Erratic data can result due to less solubility of the compound. |
| GI50 value of ≤ 10^-6 molar (i.e. 1 µmolar) or ≤ 10µg/ml is considered to demonstrate activity in the case of pure compounds. For extracts, GI50 value ≤ 20µg/ml is considered to demonstrate activity |
| Yellow highlighted test values under GI50 column indicate activity |
In-vitro Anti-Cancer Activity:
TABLE 9: RESULTS OF IN-VITRO ANTI-CANCER ACTIVITY
| S. no. | Compound code | GI50 (µmol) |
| 1 | A-1 | >80 |
| 2 | A-2 | 77.8 |
| 3 | A-3 | >80 |
| 4 | A-4 | <10 |
| 5 | STD | <10 |
DISCUSSION: Isolated 04 analogs were subjected to in-vitro anticancer activity. In the in-vitro anti-cancer activity compound, A-4 shows good anti-cancer activity on breast cancer cell line MCF-7.
CONCLUSION: In the present study can be concluded that phytoconstituents isolated from Salacia oblanga roots extract may be the potent anticancer compounds against breast cancer.
However, further investigations are needed to identify the active compounds responsible for biological activity and to elucidate the structure of compounds and mechanisms at the molecular level, which can show the way in the rational design of effective molecules for use as therapeutic agents.
ACKNOWLEDGEMENT: The authors are thankful to Dr. Vitthalrao Vikhe Patil Foundations College of Pharmacy, Ahmednagar - 414111, for providing laboratory facilities.
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Saldanha CJ: Flora of Karnataka, Oxford and IBH Publishing Co. Pvt Ltd: New Delhi 1996; 92.
- http : // envis.frlht.org.in/sreticulata.html
- Indian Medicinal Plants - a Compendium of 500 Species Orient Longman Ltd.: Hyderabad 1996; 5: 47.
- Lawerence GHM: Taxonomy of vascular plants, oxford and IBH publishing co. Pvt Ltd: New Delhi 1951; 578.
- http:// ayurvedicmedicinalplants.com/plants/ 4502.html.
- Husain A and Virmani OP: Dictionary of Indian medicinal plants. CIMAP Lucknow 1992; 400.
- Nadkarni KM: The Indian MateriaMedica, Vol-1, Popular Prakashan Pvt Ltd.: Bombay; 1089.
- Kirtikar KR and Basu BD: Indian Medicinal Plants, Periodical Experts Book Agency: New Delhi; 1993: 1: 580-85.
- Matsuda H, Murakami T, Yashiro K, Yamahara J and Yoshikawa M: Antidiabetic principles of natural medicines. IV. Aldose reductase and qlpha-glucosidase inhibitors from the roots of Salacia oblonga (Celastraceae): structure of a new friedelane-type triterpene, kotalagenin 16-acetate. Chemical and Pharmaceutical Bulletins 1999; 47(12): 1725-29.
- Stephen O and Majekodunmi R: Review of extraction of medicinal plants for pharmaceutical research Merit Research J of Medicine and Med Sci 2015; 3(11): 521-27.
- Raginee V, Satsangi G and Shrivastava N: Analysis of phytochemical constituents of the ethanolic and chloroform extracts of Calotropis procera using gas chromatography-mass spectroscopy (GC-MS) technique. J of Medicinal Plant Research 2013; 7(40): 2986 -91.
- Nane IP and Hutt AJ: Kings’ College London, UK L. A. Damani, Chinese University of Hong Kong, Hong Kong, Appendix 1/Essential Guides for Isolation/Purification of Drug Metabolites 1995: 4539-47.
- Vichai V and Kirtikara K: Sulforhodamine b colorimetric assay for cytotoxicity screening. Na Pro 2006; 1: 1112 -16.
- Skehn P, Storeng R, Scudiero A, Monks J, McMohan, D, Vistica D, Jonathan TW, Bokesch H, Kenney S and Boyd MR: New colorimetric cytotoxicity assay for anticancer drug screening. J Natl Cancer Inst 1990; 82: 1107.
How to cite this article:
Barkade G, Sawant R and Wadekar J: Repurposing and phytochemical investigation of Salacia oblonga Wall. responsible anticancer activity against breast cancer cell line (MCF-7). Int J Pharmacognosy 2019; 6(11): 353-59. doi link: http://dx.doi.org/10.13040/IJPSR. 0975-8232.IJP.6(11).353-59.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
353-359
520
1069
English
IJP
G. Barkade *, R. Sawant and J. Wadekar
Department of Pharmaceutical Chemistry, Dr. Vithalrao Vikhe Patil Foundation’s College of Pharmacy, Ahmednagar, Maharashtra, India.
ganeshbarkade7@gmail.com
06 November 2019
25 November 2019
27 November 2019
10.13040/IJPSR.0975-8232.IJP.6(11).353-59
30 November 2019