RECENT UPDATES ON THE DEVELOPMENT OF VACCINES FOR COVID-19: A MINI REVIEW
HTML Full TextRECENT UPDATES ON THE DEVELOPMENT OF VACCINES FOR COVID-19: A MINI REVIEW
Jovita Kanoujia *, Sumit Kumar Tiwari and Pawan Kumar Gupta
Amity Institute of Pharmacy, Amity University of Madhya Pradesh, Maharajapura, Gwalior - 474005 Madhya Pradesh, India.
ABSTRACT: The ongoing outbreak due to SARS-CoV-2 in all over the World resulted in extreme pressure on researchers for urgent and rapid development of vaccines. In this paper, the brief introduction of viruses and various vaccine candidates under various stages of development are discussed in the context of SARS-COV-2. This paper comprises details of vaccines and clinical trials which are recently performed in different countries. Vaccines for COVID-19 Like disease thus present a unique model to standard development precepts.
| Keywords: |
Vaccines, COVID-19, DNA, RNA, Virus vector
INTRODUCTION: As we know that WHO has declared COVID-19 as a pandemic across the World. Coronaviruses are found in a variety of mammalian species cattle, chicken and swine, etc. In animals, different tissues are occupied by the corona virus and create a variety of diseases as compared to humans. In humans, coronavirus majorly affects the respiratory tract or mild upper respiratory infection. In very rare cases, it has been seen that coronavirus affects the gastrointestinal tract, which results in diarrhea in children 1, 2. COVID-19 is spherical in shape, which enveloped pleomorphic particles. The envelope is observed with projecting glycoprotein, surrounded by a core consisting of matrix protein (which is known as spike protein) within a single strain of positive sense-RNA associated within nucleoprotein 3, 4.
The spike protein is responsible for the attachment of antigen to the host cell and carried the main antigen epitopes, which neutralized the antibodies. Coronavirus occupies the respiratory tract via nose after it enters the human body, replicates and shows a symptom like the common cold, nasal obstruction, having a problem in breathing, fever, runny nose, etc. 5, 6.
SARS-COV-2 or COVID-19: Whenever a new disease occurs, which deals with the human, they are named to access the discussion on their prevention and controls. International classification of diseases (ICD) is maintained by WHOM for the classification and nomenclature of new disease7-9. Official names of the virus, which is responsible for coronavirus disease-2019 (COVID-19) is served acute respiratory syndrome coronavirus-2 (SARS-COV-2) previously known as the 2019 novel coronavirus originate on February 2020 because the COVID-19 is genetically related to the coronavirus responsible for SARS outbreak in china 2003. The COVID-19 outbreak involves larger geography with abundantly higher speed of transmission, and it is re-emergent disease 10, 11.
Human Corona Virus and Different Strains of COVID -19: As we know, that coronavirus is not new as they are a large group of viruses that have been present around for a long time. Some of them infect humans with sniffles or coughing and droplet generate during talking.
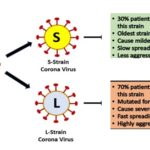
As per literature, the coronavirus is categorized into four subgroups, such as 229E (alpha), NL63 (alpha), OC43 (beta), HKU1 (beta). The rare subgroups are a beta virus that causes Middle East Respiratory Syndrome (MERS), a beta virus that causes severe acute respiratory syndrome (SARS-COV), the virus that causes severe acute respiratory syndrome-2 (SARS-COV-2 or COVID-19). As till today, scientists have found two different strains in studied of 107 cases across the World, and they found that there are two different strains available of new coronavirus (COVID-19), and these are L-strain and S-strain 12.
Challenges in Vaccine Development for COVID-19: In the case of a disease like COVID-19, still the source of the virus is not identified, and the lack of knowledge about the epidemiology and pathogenesis of infection is one of the major challenges for the development of a vaccine. The immunological protection connection of the outbreak is not completely understood. The concept of vaccines such as live, subunit, and killed virus vaccines are traditional and not able to respond to COVID-19 like virulent disease 13, 14. The newer type of vaccines, such as DNA vaccine, RNA vaccine, and virus-like particles has their technical limitation. Another aspect is clinical trials of these vaccines, which include careful concern of sample size, the involvement of control group, ethical issues, and event rate. A deep understanding of epidemiologic studies could help to develop an efficient vaccine. As per the data shared by WHO (15th May 2020), approximately 110 vaccine candidates are in preclinical assessment, and eight vaccine candidates are in clinical evaluation 15, 16.
Recent Updates of Vaccines Under Trials for COVID-19 Treatment: Several countries, academia, pharmaceutical industry, the government in a collaborative manner are trying to develop the effective vaccine for novel coronavirus (COVID-19) and some candidates are progressed into clinical developments phases, as per the data available in literature review the vaccine candidates are discussed below and summarized in Table 1.
ChAdOx1: ChAdOx1 is a vaccine developed by Oxford University, containing adenovirus as a vector to evoke the immune system against viruses. This technique utilizes the use of DNA sequence from the adenovirus to target the spike protein of the SARS-Cov-2. This vector virus is not able to replicate in the host body and enhances its safety potential for the children. The trial using this vaccine was successful in rhesus macaques with stimulated immunity and developed the antibodies against corona virus-2 17.
Ad5-nCoV: Ad5-nCoVwas the first vaccine which was undergoing human trials developed by the biotech firm Can Sino Biologics Inc, China. This vaccine is currently in Phase II clinical trials and is the farthest along in research for vaccines against the novel coronavirus. It uses a harmless virus known as adenovirus to transport DNA to the coronavirus spike proteins which present on the surface of the SARS-CoV-2. Ad5-nCoVis the combination of recombinant protein and live virus (Adenovirus type-5) to produce antigen like protein, which in turn initiate the production of antibody 18.
INO-4800: INO-4800 vaccine was announced by the US-based firm Inovio Pharmaceutical which was currently in phase-1 clinical trials. This vaccine is based on a relatively new vaccine technique involves the translation of DNA into protein after the delivery into the cells. Row, this translated protein activates the immune system to produce the antibody against SARS-CoV-2 19.
mRNA-1273: mRNA-1273was designed by US biotech firm Moderna, based on the concept of mRNA, the information molecule. The lipid nanoparticles loaded mRNA as a vaccine provokes the cells to produce the specific protein which is required to fight against infection. The instruction for producing the protein to the cell is transferred by mRNA vaccine 20.
PiCoVacc: The PiCoVacc consists of an inactivated form of SARS-CoV-2, developed by Sinovac Biotech. This vaccine was successfully used in three animal models. This animal model uses rats, mice, and rhesus macaques showing activation of an immune response in all models. In response to SARS-CoV-2, the produced neutralizing antibodies are able to fight infection 21.
BNT162: BNT162 vaccine is designed with the combined efforts of Bio N Tech, Pfizer, and Fosun Pharma by involving different types of modified RNA. This RNA include mRNA containing mRNA (uRNA), modified nucleoside mRNA (modRNA), and self self-amplifying mRNA (saRNA). Every mRNA is combined with lipid nanoparticle technology (LNP) for delivery and formulation 22.
Inactivated SARS-CoV-2 Vaccine by Wuhan Institute of Biological Products: Wuhan Institute of virology with Sinopharma is working on the development of an inactivated vaccine. The development process is in the phase-2 trial, and the results from the trials show good safety outcomes using 96 human volunteers within three age groups. The organization requires 1 year to a final conclusion about the efficacy and safety of the developed vaccines 23.
Inactivated SARS-CoV-2 Vaccine (Vero cells) by Beijing Institute of Biological Products: The Beijing Institute of Biological Products, China, is also working on the development of vaccines by using inactivated for SARS-CoV-2. The trial is in phase ½ and involves the volunteer aged above 3 years. The outcomes of the trial show the increase in serum antibody level to combat against the corona virus after the 28 days of full vaccination 24.
TABLE 1: CURRENT STATUS OF VACCINE DEVELOPMENT FOR THE COVID-19 AS PER WHO (15 MAY 2020)
| S. no. | Vaccine Name | Technology/Platform used | Developed by | Clinical trial Phase |
| 1 | ChAdOx1
|
Non-Replicating Viral (Adenovirus) Vector | Oxford University, United Kingdom | Phase 1/Phase2 |
| 2 | Ad5-nCoV | Non-Replicating Viral Vector Adenovirus Type 5 Vector | Can sino biologics Inc, China | Phase 1/Phase2 |
| 3 | INO-4800 | DNA plasmid vaccine with electroporation | Inovio Pharmaceuticals, United States | Phase 1 |
| 4 | mRNA-1273 | (Lipid nanoparticles) LNP encapsulated mRNA | ModernaInc, United States | Phase 1 |
| 5 | PiCoVacc | Inactivated SARS-CoV-2 | Sinovac, Biotech, China | Phase 1/Phase 2 |
| 6 | BNT162 | 3 LNP-mRNAs
|
BioNTech/ Pfizer/FosunPharma, United States | Phase 1/Phase 2 |
| 7 | Unnamed | Inactivated SARS-CoV-2 | Wuhan Institute of Biological Products/Sinopharm, China | Phase 1/Phase 2 |
| 8 | Unnamed | Inactivated SARS-CoV-2 | Beijing Institute of Biological Products/Sinopharm, China | Phase 1/Phase 2 |
CONCLUSION: In this paper, we have discussed the basic introduction of SARS-CoV-2 and main challenges in the vaccine development for COVID-19. We have also discussed several vaccine candidates under the development stages with their key concept and clinical stages.
ACKNOWLEDGEMENT: Nil
CONFLICTS OF INTEREST: No conflicts of interest.
REFERENCES:
- Heng Li, Shang-Ming Liu, Xiao-Hua Yu, Shi-Lin Tang, and Chao-Ke Tang: Coronavirus disease 2019 (COVID-19): current status and future perspectives. International Journal Antimicrobial Agents 2020; 105951.
- Shereen MA, Khan Khan S, Kazmi A, Bashir N and Siddique R: COVID-19 infection: Origin, transmission, and characteristics of human corona viruses. Journal Advanced Research 2020; 24: 9‐
- Chan JFW, Kok KH, Zhu Z, Chu H, To KKW and Yuan S: Genomic characterization of the 2019 novel human-pathogenic corona virus isolated from a patient with atypical pneumonia after visiting. Wuhan. Emerging Microbes & Infections 2020; 9(1): 221-36.
- Van Boheemen S, de Graaf M and Lauber C: Genomic characterization of a newly discovered corona virus associated with acute respiratory distress syndrome in humansm. Bio 2012; 3(6): 00473-12.
- Riou J and Althaus CL: Pattern of early human to human transmission of Wuhan 2019 novel corona virus (2019-nCoV), December 2019 to January 2020. Euro Surveillance 2020; 25(4): 2000058.
- Shi Y, Yi Y, Li P, Kuang T, Li L, Dong M, Ma Q and Cao C: Diagnosis of severe acute respiratory syndrome (SARS) by detection of SARS corona virus nucleocapsid antibodies in an antigen-capturing enzyme-linked immuno sorbent assay. J Clin Microbiol 2003; 41(12): 5781-2.
- Emergency use ICD codes for COVID-19 disease outbreak. https://www.who.int/classifications/icd/covid19/ en/
- Naming the corona virus disease (COVID-19) and the virus that causes it, https:// www. Who. Int / emer-gencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), https: // www. cdc. gov / nchs/icd/icd10cm.htm.
- Lai CC, Shih TP, Ko WC, Tang HJ and Hsueh PR: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Ant Agents 2020; 55(3):105924.
- Organization WH. Laboratory testing for corona virus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization 2020.
- Zhong NS, Zheng BJ and Li YM: Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong. People's Republic of China in February Lancet 2003; 362(9393): 1353‐
- Bisht H, Roberts A, Vogel L, Subbarao K and Moss B: Neutralizing antibody and protective immunity to SARS corona virus infection of mice induced by a soluble recombinant polypeptide containing an N-terminal segment of the spike glycoprotein. Virology 2005; 334(2): 160‐
- Yang ZY, Kong WP and Huang Y: A DNA vaccine induces SARS corona virus neutralization and protective immunity in mice. Nature 2004; 428(6982): 561‐
- Draft landscape of COVID-19 candidate vaccines, https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines.
- DRAFT landscape of COVID-19 candidate vaccines - 20 April 2020, https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf
- A Study of a Candidate COVID-19 Vaccine (COV001), https://clinicaltrials.gov/ct2/show/NCT04324606?term=vaccine&cond=covid-19&draw=2
- A randomized, double-blinded, placebo-controlled phase II clinical trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector), http:// www. chictr. org. cn/showprojen.aspx?proj=52006.
- INOVIO's COVID-19 DNA Vaccine INO-4800 Demonstrates Robust Neutralizing Antibody and T Cell Immune Responses in Preclinical Models, http:// ir. inovio. com/news-releases/news-releases-details/2020/INOVIOs-COVID-19-DNA-Vaccine-INO-4800-Demonstrates-Robust-Neutralizing-Antibody-and-T-Cell-Immune-Responses-in-Preclinical-Models/default.aspx
- Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19), https: // clinicaltrials. Gov / ct2 / show/NCT04283461?term=vaccine&cond=covid-19&draw=2
- Safety and Immunogenicity Study of Inactivated Vaccine for Prophylaxis of SARS CoV-2 Infection (COVID-19), https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate-vaccines
- Study to Describe the Safety, Tolerability, Immunogenicity, and Potential Efficacy of RNA Vaccine Candidates against COVID-19 in Healthy Adults, https://clinicaltrials.gov/ct2/show/NCT04368728?term=vaccine&cond=covid-19&draw=3.
- A phase I/II clinical trial for inactivated novel coronavirus (2019-CoV) vaccine (Vero cells) http: // www. chictr. org.cn/showproj.aspx?proj=53003
- A randomized, double-blind, placebo parallel-controlled phase I/II clinical trial for inactivated Novel Corona virus Pneumonia vaccine (Vero cells) http: // www. chictr. org. cn/showprojen.aspx?proj=52227.
How to cite this article:
Kanoujia J, Tiwari SK and Gupta PK: Recent updates on the development of vaccines for COVID-19: a mini review. Int J Pharmacognosy 2020; 7(6): 133-36. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(6).133-36.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
133-136
527
979
English
IJP
J. Kanoujia *, S. K. Tiwari and P. K. Gupta
Amity Institute of Pharmacy, Amity University of Madhya Pradesh, Maharajapura, Gwalior Madhya Pradesh, India.
jovita_kanoujia@rediffmail.com
06 June 2020
26 June 2020
28 June 2020
10.13040/IJPSR.0975-8232.IJP.7(6).133-36
30 June 2020