QUANTITATIVE ESTIMATION AND LC MS STUDIES OF SOME MEDICINAL PLANTS
HTML Full TextQUANTITATIVE ESTIMATION AND LC MS STUDIES OF SOME MEDICINAL PLANTS
A. S. Renjini *, R. V. Celestin Baboo and M. K. Sirajudheen
Department of Pharmacognosy, Jamia Salafiya Pharmacy College Pulikkal, Malappuram, Kerala, India.
ABSTRACT: This paper describes the Quantitative estimation and LC MS study of selected plants are Adenocalymma alliaceum and Turbinaria ornata. Is a plant belonging to the family Bignoniaceae commonly called as ‘Garlic vine’ and or ‘false garlic’. Turbinaria ornata (T. ornata) is one of the main seaweeds in the marine ecosystem that has been used as a source of medicine among brown seaweeds. These brown algae belong to the family Sargassaceae. This review paper discusses in detail study of quantitative estimation and LC MS study. Quantitative determination of Adenocalymma alliaceum shows the presence of alkaloid, flavonoid and tannin. Quantitative determination of Turbinaria ornata shows the presence of alkaloid, phenol, flavonoid and tannin. LCMS study reveals the presence of 1-hexamine, Apigenin and p-coumaric acid in Adenocalymma alliaceum and Tetradecanoic acid, Hydroperoxide and Nonyl trifluoroacetate in Turbinaria ornata.
Keywords: Adenocalymma alliaceum, Turbinaria ornata, Quantitative estimation, LC MS study
INTRODUCTION: The plants fulfil the variety of human requirements including food, nutraceuticals, medicine and additionally contribute to maintaining the ecosystem. According to the literature search, Adenocalymma alliaceum is a species of flowering vine in the Bignoniaceae family that is used traditionally for the treatment of analgesic, anti-inflammatory, antipyretic, insect bite, snake bite and required to prove its traditional claim. Experimental evidence suggests that the most significant medicinal agents are found in seaweeds. Also states that the extracts of seaweeds used to eradicate dreadful diseases including cancer.
A seaweed, Turbinaria ornata belongs to the family Sargassaceae widely distributed in Indian oceans has a wide variety of health benefits including antioxidant, anti-inflammatory, neuro-protective effects that require scientific investigation for proving its medicinal value.
Adenocalymma alliaceum (Mansoa alliacea): Is a plant belonging to the family Bignoniaceae commonly called as ‘Garlic vine’ and or ‘false garlic’. Leaves have pungent garlic smell and flavour when it is crushed but it does not smell if the plant is left alone 1. Fresh young and soft leaves and stems have been added into the preparation of salads, sandwiches, and other food items 2. A. alliaceum is widely used in folk medicine treatments for many ailments like cold, as an aid to fertility, commonly added to baths to treat feverish conditions, flu, body aches, cramps, fatigue, mosquito and snake repellent, epilepsy, uterine disorders 3, etc. Whole plant parts should be used in analgesic, anti-rheumatic and anti-inflammatory. Garlic vine is known for its analgesic, anti-inflammatory 4, anti-rheumatic and antipyretic properties and is beneficial as herbal medicine 5.
FIG. 1: ADENOCALYMMA ALLIACEUM
Turbinaria ornate: T. ornata is one of the main seaweeds in the marine ecosystem that has been used as a source of medicine among brown seaweeds 6. Turbinaria species, such as Turbinaria ornata and Turbinaria conoides, have been extensively distributed along the coastal waters of Tamil Nadu. These brown algae belong to the family Sargassaceae 7. Therapeutic potentials of pure compounds isolated from the Genus Turbinaria are extraordinarily promising as antiproliferative, antipyretic, anti-inflammatory, immunostimulatory, anti-diabetic, anti-obesity, antiviral, antimicrobial, cardioprotective, hepatoprotective and hypolipidemic 8. A wide range of biological properties of this seaweed, including antibacterial, anti-coagulant, anti-inflammatory and antioxidant properties, have been reported, which are used as thickening, gelling, and stabilizing agents in food and drinks, as well as in cosmetics and pharmaceutical products 9. Turbinaria ornata has a wide variety of health benefits and is being researched for pharmaceutical purposes because of its antioxidant, anti-inflammatory, antidiabetic, antiproliferative, and neuroprotective effects on humans 10. Turbinaria ornata has the proper compounds to be used as a potential source for reducing postprandial hyperglycemia in humans making it an alternative therapeutic approach in treating diabetes 11. Turbinaria ornata can be grown and used as a natural alternative wastewater treatment that would reduce untreated dangerous chemicals from being dumped into land and water bodies. Compounds found in T. ornata can also be used to restore land and bodies of water that were previously contaminated by toxic and environmentally destructive chemicals 12.
FIG. 2: TURBINARIA ORNATA
MATERIALS AND METHODS:
Quantitative Estimation of Phytoconstituents: Quantitative estimation refers to the measurement of specific chemical constituents in plant extracts. It helps identify and quantify various secondary metabolites present in medicinal plants. These include alkaloids, flavonoids, phenolic compounds, saponins, and tannins.
Estimation of Phenols 13:
Principle: The principle of the F–C assay is the reduction of the Folin–Ciocalteu reagent (FCR) in the presence of phenolics resulting in the production of molybdenum–tungsten blue which is measured spectrophotometrically at 750 nm and the intensity increases linearly with the concentration of phenolics in the reaction medium.
Procedure: From 10mg/ml stock solution 300µl of test sample was pipetted out and the volume in each tube was made up to 3.0 ml with distilled water. Folin-Ciocalteau reagent (0.5ml) and 2mL 20% Na2CO3 were added and the tubes were placed in a boiling water bath for exactly one minute. The tubes were cooled and the absorbance was read at 750nm in a spectrophotometer against a reagent blank. Standard gallic acid solutions (2.5-100µg/ml) were also treated as above.
Estimation of Flavonoids 14:
Principle: Total flavonoid content was measured by the aluminum chloride colorimetric assay. The basic principle of Aluminium chloride colorimetric method is that Aluminium chloride forms acid stable complexes with the C-4 keto group and either the C-3 or C-5 hydroxyl group of flavones and flavonols. In addition, it also forms acid labile complexes with the ortho-dihydroxyl groups in the A- or B-ring of flavonoids.
Procedure: Total flavonoid content was measured by the aluminum chloride colorimetric assay. The reaction mixture consists of 1mg of extract and 4 ml of distilled water was taken in a 10 ml volumetric flask. To the flask, 0.30 ml of 5% sodium nitrite was treated and after 5 minutes, 0.3 ml of 10% aluminum chloride was mixed. After 5 minutes, 2ml of 1M Sodium hydroxide was treated and diluted to 10 ml with distilled water.
A set of reference standard solutions of Quercetin (20, 40, 60, 80 and 100 μg/ml) were prepared in the same manner as described earlier. The absorbance for test and standard solutions were determined against the reagent blank at 510 nm with an UV/Visible spectrophotometer. The total flavonoid content was expressed as μg of QE/ mg of extract.
Estimation of Alkaloid 15:
Principle: Total alkaloid is estimated based on the reaction with Bromocresol Green (BCG). In this method alkaloid and bromocresol green (BCG) reacts to form a yellow complex and is easily extractable using chloroform. The principle is that certain organic solvent can extract the colored complex (ion pair) quantitatively, which is the combination of an acid dye and a salt ion formed by the reaction of alkaloids and hydrogen ions under some acidic conditions. Chloroform is the most commonly used organic solvent for the determination of alkaloids, having the advantages of forming hydrogen bonds with ion pair easily, high extraction rate, good selectivity and poor aqueous solubility.
Procedure: The plant extract (1mg) was dissolved in 1ml dimethyl sulphoxide (DMSO), added 1ml of 2N HCl and filtered. This solution was transferred to a separating funnel, 5ml of bromocresol green solution and 5ml of phosphate buffer were added.
The mixture was shaken with 1, 2, 3 and 4 ml chloroform by vigorous shaking and collected in a 10ml volumetric flask and diluted to the volume with chloroform. A set of reference standard solutions of atropine (20, 40, 60, 80 and 100μg) were prepared in the same manner as described earlier. The absorbance for test and standard solutions were determined against the reagent blank at 470nm with an UV/Visible spectrophotometer.
Estimation of Tannins 16:
Principle: Tannins like compounds reduce posphotungsto molybdic acid in alkaline solution to produce a blue colour complex and the colour intensity is proportional to the concentration of tannin and measured at 700nm.
Procedure: Content of tannins in sample was determined by Folin-Ciocalteu method. Colorimetric estimation of tannins is based on the measurement of blue colour formed by the reduction of phosphotungsto molybdic acid by tannin like compounds in alkaline medium. One mg/1ml of extract and standard solution of tannic acid (20-100μg) was made up to 7.5mL with distilled water. Then 0.5mL of Folin-Ciocalteu reagent and 35% 1mL sodium carbonate solution were added. The volume was made up to 10mL with distilled water and the absorbance was measured at 700nm.
Estimation of Terpenoid 17:
Principle: Terpenoids, also known as isoprenoids, are a class of chemical compounds produced from isoprene. Triterpenes are ubiquitous secondary metabolites present in plants. They can be found in both forms, as genins or conjugated as glycosides. The method is known as the vanillin- acid assay because the basic principle of the method is the reaction of sulphuric acid-oxidised triterpene saponins with vanillin, which gives a distinctive red-purple colour measured at wavelengths ranging from 473 to 560 nm using a spectrophotometer.
Procedure: A volume 200μL of extract solutions in methanol (1 mg/mL) was first mixed with 1 mL of perchloric acid and 300μL vanillin/glacial acetic acid (5% w/v) solution. 5 mL of glacial acetic acid was then added to it and the absorbance was measured at 548nm with a UV-Visible spec trophotometer. Linalool (µg/ml) in methanol was used as standard. Results were expressed as mg Linallol equivalents.
Estimation of Glycosides 18:
Principle: Cardiac glycosides develop an orange red colour complex with Baljet’s reagent (Picric acid in alkaline medium). The intensity (absorbance) of colour produced is proportional to the concentration of glycosides.Cardiac glycosides were quantitatively determined according to Solich et al. by some modifications.
Reagents: Standard digitoxin: 0.02% digitoxin is prepared in chloroform: methanol (1:1). Baljet’s reagent: Freshly prepared 95ml 1% picric acid + 5ml 10% NaOH are mixed immediately before use and filtered through a sintered glass funnel.
Procedure: 1ml of the extract and 1ml of Baljet’s reagent are taken and allowed to stand for one hour. Then dilute the solution with 2ml distilled water and mix. Read the intensity of the colour obtained against blank at 495nm using a spectrophotometer. Standard graph can be prepared using varying concentration of standard digitoxin (2-14μg/ml).
Estimation of Steroids 19:
Principle: Steroids react with ferric chloride in the presence of concentrated sulphuric acid to give a pink colour. Cholesterol gives a reddish pink red colour with ferric chloride and polar sulphuric acid. The intensity of colour developed is directly proportional to the amount of steroids present and it is read at 540 nm in a calorimeter.
Procedure: One mg/ml of extract was taken in a clean test tube. Cholesterol was used as standard and was taken at varying concentrations of (1-10µg/ml) in test tubes. To the standard and test samples, 5ml of ferric chloride reagent and 4ml of concentrated sulphuric acid were added. The reaction mixtures were incubated at room temperature for 30 minutes and OD was read at 540nm. A standard graph was plotted from which the unknown value of steroid in the test sample was determined.
LC-MS 20: Spectroscopic and chromatographic methods are one of the basic and reliable methods to identifying pharmaceutically active biomolecules from the natural sources. LC-MS (Liquid chromatography -mass spectroscopy) are the sophisticated instruments which are used to screening of bioactive secondary metabolites from the natural sources. These methods are simplest, fastest and more acceptable methods for the identification of bioactive molecules from crude extract of the medicinal plants and which only needs a small amount of plant extract. In order to detect and identify the phytochemical components present in the medicinal plant, the LC-MS technique was used in the current investigation.
Procedure 21: Adenocalymma alliaceum extract phytochemical analysis was studied by using the LC-MS. The chemical constituents of the extract were determined using LC-MS. LC-MS analysis was performed using Mariner Bio spectrometry equipped with a binary pump.
The HPLC was interfaced with a Q-TOF mass spectrometer fitted with an ESI source. Full-scan mode from m/z 100 to 1200 was performed with a source temperature of 140°C. HPLC 22 column Phenomenex 5μ C8, (150 × 2 mm i.d.) was used for the analysis. Solvent was methanol with 0.3% formic acid. Solvents were delivered at a total flow rate of 0.1 mL/min. The solvent was run by isocratic elution. The MS spectra were acquired in the positive ion mode.
Procedure 23: Turbinaria ornate extract phytochemical analysis was studied by using the LC-MS. The chemical constituents of the extract were determined using LC-MS. LC-MS 24 analysis was performed using Mariner Bio spectrometry equipped with a binary pump.
The HPLC was interfaced with a Q-TOF mass spectrometer fitted with an ESI source. Full-scan mode from m/z 100 to 1200 was performed with a source temperature of 140°C. HPLC column Phenomenex 5μ C8, (150 × 2 mm i.d.) was used for the analysis. Solvent was methanol with 0.3% formic acid. Solvents were delivered at a total flow rate of 0.1 mL/min. The solvent was run by isocratic elution. The MS spectra were acquired in the positive ion mode.
RESULT AND DISCUSSION:
Quantitative Estimation of Phytoconstituents:
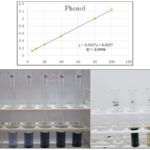
Estimation of Phenols (Gallic Acid Equivalent Method):
TABLE 1: ESTIMATION OF PHENOL
| Standards | Concentration of gallic acid (μg/ml) | OD at 750 nm |
| S1 | 5 | 0.123 |
| S2 | 10 | 0.183 |
| S3 | 20 | 0.304 |
| S4 | 40 | 0.522 |
| S5 | 80 | 0.998 |
| S6 | 100 | 1.242 |
FIG. 3: ESTIMATION OF PHENOL
TABLE 2: ESTIMATION RESULT OF PHENOL
| S. no. | Sample name | Absorbance at 750 nm | Concentration of phenol (μg/ml) |
| 1 | Adenocalymma alliaceum | 0.871 | 69.00 |
| 2 | Turbinaria ornata | 0.225 | 13.79 |
Estimation of Flavonoids (Aluminium Chloride Assay Method):
TABLE 3: ESTIMATION OF FLAVONOIDS
| Standards | Concentration of Quercetin (μg/ml) | OD at 510 nm |
| S1 | 20 | 0.101 |
| S2 | 40 | 0.163 |
| S3 | 60 | 0.241 |
| S4 | 80 | 0.344 |
| S5 | 100 | 0.421 |
| S6 | 120 | 0.543 |
| S7 | 140 | 0.614 |
FIG. 4: ESTIMATION OF FLAVONOIDS
TABLE 4: ESTIMATION RESULT OF FLAVONOIDS
| S. no. | Sample name | Absorbance at 510nm | Concentration of Flavonoid (μg/ml) |
| 1 | Adenocalymma alliaceum | 0.269 | 62.82 |
| 2 | Turbinaria ornata | 0.545 | 125.55 |
Estimation of Alkaloid (UV Spectrophotometric Method):
TABLE 5: ESTIMATION OF ALKALOID
| Standard | Concentration of Atropine (μg/ml) | OD at 470 nm |
| S1 | 20 | 0.036 |
| S2 | 40 | 0.063 |
| S3 | 60 | 0.084 |
| S4 | 80 | 0.112 |
| S5 | 100 | 0.135 |
FIG. 5: ESTIMATION OF ALKALOID
TABLE 6: ESTIMATION RESULT OF ALKALOID
| S. no. | Sample name | Absorbance at 470 nm | Concentration of Alkaloid (μg/ml) |
| 1 | Adenocalymma alliaceum | 0.107 | 79.25 |
| 2 | Turbinaria ornata | 0.060 | 40.08 |
Estimation of Tannins (Folin Ciocalteu Method):
FIG. 6: ESTIMATION OF TANNINS
TABLE 7: ESTIMATION OF TANNINS
| Standards | Concentration of tannic acid (μg/ml) | OD at 700 nm |
| S2 | 1 | 0.061 |
| S3 | 10 | 0.345 |
| S4 | 20 | 0.516 |
| S5 | 40 | 0.907 |
| S6 | 80 | 1.613 |
| S7 | 100 | 2.002 |
TABLE 8: ESTIMATION RESULT OF TANNINS
| S. no. | Sample name | Absorbance at 700 nm | Concentration of Tannins (μg/ml) |
| 1 | Adenocalymma alliaceum | 1.990 | 99.23 |
| 2 | Turbinaria ornata | 0.230 | 6.11 |
Estimation of Glycosides (UV Spectrophotometric Method):
TABLE 9: ESTIMATION OF GLYCOSIDE
| Standards | Concentration of Digitoxin (μg/ml) | Absorbance at 495 nm |
| S1 | 5 | 0.004 |
| S2 | 10 | 0.076 |
| S3 | 15 | 0.132 |
| S4 | 20 | 0.183 |
| S5 | 25 | 0.249 |
| S6 | 30 | 0.331 |
FIG. 7: ESTIMATION OF GLYCOSIDE
TABLE 10: ESTIMATION RESULT OF GLYCOSIDES
| Sl. no. | Sample code | Absorbance at 495 nm | Concentration of glycosides (μg/ml) |
| 1 | A | 0.116 | 13.81 |
Estimation of Steroids (UV Spectrophotometric Method):
TABLE 11: ESTIMATION OF STEROIDS
| Standards | Concentration Cholesterol (µg/ml) | Absorbance at 540 nm |
| S1 | 10 | 0.062 |
| S2 | 20 | 0.138 |
| S3 | 40 | 0.221 |
| S4 | 80 | 0.405 |
| S5 | 100 | 0.495 |
| S6 | 200 | 0.966 |
FIG. 8: ESTIMATION OF STEROID
TABLE 12: ESTIMATION RESULT OF STEROID
| Sl. no. | Sample code | Absorbance at 540 nm | Concentration of steroid (μg/ml) |
| 1 | T | 0.105 | 15.89 |
Estimation of Terpenoids (UV Spectrophotometric Method):
TABLE 13: ESTIMATION OF TERPENOIDS
| Standards | Concentration Linalool (μg/ml) | Absorbance at 548nm |
| S1 | 5 | 0.175 |
| S2 | 10 | 0.267 |
| S3 | 20 | 0.384 |
| S4 | 40 | 0.615 |
| S5 | 60 | 0.812 |
| S6 | 80 | 1.076 |
| S7 | 100 | 1.278 |
FIG. 9: ESTIMATION OF TERPENOIDS
TABLE 14: ESTIMATION RESULT OF TERPENOIDS
| Sl. no. | Sample Code | Absorbance at 548 nm | Concentration of terpenoids (μg/ml) |
| 1 | T | 0.308 | 14.4 |
LC-MS:
LC-MS Sudy of Adenocalymma alliaceum: The tentative assignment of compounds detected from the Adenocalymma alliaceum extractvia LC-MS analysis are presented. The compounds were detected and were identified by their fragmentation pattern and in conjunction with the PubChem and research reference article. Peak area and retention time were used for the identification of the compounds.
TABLE 15: PHYTOCHEMICAL ANALYSIS OF ADENOCALYMMA ALLIACEUM
| Sl. no. | Phytochemicals | m/z |
| 1 | 1,3-Dimethylthiourea | 103 |
| 2 | 1,3-Dihydroxyacetonedimer | 181.4 |
| 3 | Aceticacid, [(aminocarbonyl)amino]oxo- | 132.1 |
| 4 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- | 144.1 |
| 5 | 1-Hexanamine | 101.2 |
| 6 | p-Coumaric acid | 163.4 |
| 7 | Apigenin | 169 |
| 8 | 1,5-Anhydro-d-mannitol | 164 |
FIG. 10: ADENOCALYMMA ALLIACEUM EXTRACT LC-MS
LC-MS Study of Turbinaria ornate: The tentative assignment of compounds detected from the Turbinaria ornatevia LC-MS analysis are presented. The compounds were detected and were identified by their fragmentation pattern and in conjunction with the PubChem and research reference article. Peak area and retention time were used for the identification of the compounds Table 16.
TABLE 16: PHYTOCHEMICAL ANALYSIS OF TURBINARIA ORNATA
| Sl. no. | Phytochemicals | m/z |
| 1 | Tetradecanoic acid | 227.6 |
| 2 | 1,2-benzenedicarboxylic acid, butyl 2-methylpropyl ester | 278 |
| 3 | 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester | 278 |
| 4 | Hydroperoxide,1-methylbutyl | 104.3 |
| 5 | Nonyl trifluoroacetate | 241.6 |
| 6 | Dodecyl trifluoroacetate | 281.4 |
| 7 | 3 Ethoxy-1,1,1,5,5,5- hexamethyl-3 trimethyl | 341.4 |
FIG. 11: TURBINARIA ORNATA EXTRACT LC-MS
CONCLUSION: In the present investigation, Selected Adenocalymma alliaceum plant belonging to terrestrial and Turbinaria ornata seaweed from marine source. This review gives information about the quantitative estimation and LC MS study of selected plants. The plant Adenocalymma alliaceum used in folk treatments the plant parts are widely used for cold, as an aid to fertility, commonly added to baths to treat feverish conditions, flu, body aches, cramps, fatigue, mosquito and snake repellent, epilepsy, uterine disorders, etc. Turbinaria ornata (T. ornata) is one of the main seaweeds in the marine ecosystem that has been used as a source of medicine among brown seaweeds. These brown algae belong to the family Sargassaceae. Therapeutic potentials of pure compounds isolated from the Genus Turbinaria are extraordinarily promising as antiproliferative, antipyretic, anti-inflammatory, immunostimulatory, anti-diabetic, anti-obesity, antiviral, antimicrobial, cardioprotective, hepatoprotective and hypolipidemic.Quantitative determination of Adenocalymma alliaceum shows the presence of alkaloid (79.25μg/ml), phenol (69μg/ml), flavonoid (62.82 μg/ml) and tannin (99.23μg/ml) Quantitative determination of Turbinaria ornata shows the presence of alkaloid (40.08μg/ml), phenol (13.79μg/ml), flavonoid (125.55μg/ml) and tannin (6.11μg/ml)LCMS study reveals the presence of 1-hexamine, Apigenin and p-coumaric acid in Adenocalymma alliaceum and Tetradecanoic acid, Hydroperoxide and Nonyl trifluoroacetate in Turbinaria ornata.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Angelica Tasambay Salazar, Laura Scalvenzi, Andrea Stefany Piedra Lescano and Matteo Radiace: Ethnopharmacology, biological activity and chemical characterization of Mansoa alliacea. A review about a promising plant from Amazonian region. MODEC 02 International workshop of Natural Products and Agro Industrial processes in Ecuadorian Amazon Region 2017; 10(3): 1-8.
- Maria das Gracas Bichara Zoghbi, Jorge Oliveira and Giselle Maria Skelding Pinheiro Guilhon: The genus (Bignoniaceae): a source of organosulfur compounds. Brazilian Journal of Pharmacognosy 2009; 19(3): 795-804.
- Garlic Vine-Medicinal plants of India. http://www.medicinalplantsindia.com/garlic-vine.html.
- Camden M. Towne, J an F. Dudt and Durwood B. Ray: Effect of Mansoa alliacea (Bignoniaceae) leaf extract on embryonic and tumorigenic mouse cell lines. Journal of Medicinal Plants Research 2015; 9(29): 799-805.
- Hasrat JA, De Backer JP, Vanquelin G and Vlientinck AJ: Medicinal plants in Suriname: screening of plant extracts for receptor binding activity. Phytomed 1997; 4(1): 59-65.
- C Desmachelier, M Repetto, J Coussio, S Llesuy and G Ciccia: Total reactive antioxidant potential (TRAP) and Antioxidant reactivity (TAR) of medicinal Plants used in Southwest Amazonia (Bolivia and Peru). International Journal of Pharmacognosy 1997; 35(4): 288-296.
- Gogineni V and Hamann MT: Marine natural product peptides with therapeutic potential: chemistry, biosynthesis, and pharmacology,” Biochimica et Biophysica Acta (BBA) - General Subjects 2018; 1862(1): 81–196.
- Jensen PR and Fenical W: “Marine microorganisms and drug discovery: current status and future potential,” Drugs from the Sea 2000; 6–29.
- Sharma M, Singh DP and Rangappa KS: “The biomolecular spectrum drives microbial biology and functions in agrifood-environments,” Biomolecules 2020; 10(3): 401.
- Fleurence J: “Seaweed proteins: biochemical, nutritional aspects and potential uses,” Trends in Food Science and Technology 1999; 10(1): 25–28.
- Kaliaperumal N: “Present status of marine algal biodiversity in Gulf of Manner region,” Tamil Nadu Indian Hydrobiol 2007; 10(1): 53–62.
- Remya RR, Samrot AV and Kumar SS: “Bioactive potential of brown algae,” Adsorption Science and Technology 2022; 2022: 1–13.
- Meda C. E. Lamien, Romito M, Millogo J and Nacoulma OG: “Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity,” Food Chemistry 2005; 91(3): 571–577.
- Lee Wei Har and Intan Safinar Ismail: Antioxidant activity, total phenolics and total flavonoids of Syzygium polyanthum (Wight) Walp leaves. Int J Med Arom Plants 2012; 2(2): 219-228.
- Fazel Shamsa, Hamidreza Monsef, Rouhollah Ghamooshi and Mohammadreza Verdian-rizi: Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. Thai J Pharm Sci 2008; 32: 17-20.
- Quantitative estimation of total phenolic, flavonoids, tannin and chlorophyll content of leaves of Strobilanthes Kunthiana (Neelakurinji) KC CI, G Indira - Journal of Medicinal Plants Studies 2016; 4(4): 282-2.
- Solich P, Sedliakova V and Karlicek R: Spectrophotometric determination of cardiac glycosides by flow-injection analysis. Anal Chim Acta 1992; 269(2): 199-203.
- Tofighi Z, Hadjiakhoondi A and Yassa N: "Determination of cardiac glycosides and total phenols in different generations of Securigera securidaca suspension culture." Research Journal of Pharmacognosy 2016; 3(2): 23-31.
- Zak B, dickenman RC, White, EG, Burnetth and Cherney, PJ: rapid estimation of free and total cholesterol. Am J Clin Path 1954; 24: 1307.
- Beckett AH and Stenlake GH: Practical Pharmaceutical Chemistry, fourth , CBS Publishers and distributors, New Delhi 2005.
- Sharma BK: Instrumental methods of chemical analysis, twenty third ed., Goel Publishing House, Meerut, 2004.
- Arpino, Patrick. “Combined liquid chromatography mass spectrometry. Part III. Applications of thermospray”. Mass Spectrometry Reviews 1992; 11: doi:10.1002/mas.1280110103.
- Arpino and Patrick: “Combined liquid chromatography mass spectrometry. Part I. Coupling by means of a moving belt interface”. Mass Spectrometry Reviews 1989; 8: doi:10.1002/mas.1280080103.
- James J Pitt: Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry, Clinical Biochemist Reviews 2009; 30(1): 19–34.
How to cite this article:
Renjini AS, Baboo RVC and Sirajudheen MK: Quantitative estimation and LC MS studies of some medicinal plants. Int J Pharmacognosy 2024; 11(7): 363-74. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(7).363-74.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
363-374
2168 KB
716
English
IJP
A. S. Renjini *, R. V. Celestin Baboo and M. K. Sirajudheen
Department of Pharmacognosy, Jamia Salafiya Pharmacy College Pulikkal, Malappuram, Kerala, India.
2017renjinias@gmail.com
06 June 2024
21 July 2024
27 July 2024
10.13040/IJPSR.0975-8232.IJP.11(7).363-74
31 July 2024