QUANTITATIVE AND QUALITATIVE EVALUATION OF ASHWAGANDHA CHURNA
HTML Full TextQUANTITATIVE AND QUALITATIVE EVALUATION OF ASHWAGANDHA CHURNA
Priya Mishra *, Pawan Kumar Gupta and Pushpendra Kannojia
Department of Pharmacology, IPS - College of Pharmacy, Gwalior - 474002, Madhya Pradesh, India.
ABSTRACT: Herbal formulation is a dosage form consisting of one or more herbs or processed herbs(s) in specified quantities to provide specific nutritional, cosmetic benefits, and/or other benefits meant for use to diagnose treat, mitigate disease of human beings or animals and / or to alter the structure or physiology of human beings or animals. The world health organization (WHO) estimates that 80% of the population (Asian and African countries) presently use herbal medicine for some aspect of primary health care. The present work was designed to evaluate the phytochemical potential and qualitative analysis of Ashwagandha churna. It was a simple maceration technique of extraction was followed to get the purified drug for further investigations. The chemical test was evaluated for the detection of different constitute include alkaloid, starch, flavonoid, tannins, saponins, steroid, carbohydrates, phenol, glycoside, terpenoids, resins, cardiac glycoside, coumarins. The qualitative study investigation includes HPLC and TLC. The result demonstrated the successful presence of above constitutes.
| Keywords: |
Ashwagandha churna, World Health Organization (WHO), HPLC
INTRODUCTION: Herbal formulation is a dosage form consisting of one or more herbs or processed herbs(s) in specified quantities to provide specific nutritional, cosmetic benefits, and / or other benefits meant for use to diagnose treat, mitigate disease of human beings or animals and / or to alter the structure or physiology of human beings or animals. Herbalism (also herbology or herbal medicine) is the use of plants for medicinal purposes. Plants have been the basis for medical treatments through much of human history, and such traditional medicine is still widely practiced today. Modern medicine recognizes herbalism as a form of alternative medicine, as the practice of herbalism is not strictly based on evidence gathered using the scientific method.
Modern medicine, does, however, make use of many plant-derived compounds as the basis for evidence tested pharmaceutical drugs. Phyto-therapy and phytochemistry work to apply modern standards of effectiveness testing to herbs and medicines that are derived from natural sources. The world health organization (WHO) estimates that 80 % of the population (Asian and African countries) presently use herbal medicine for some aspect of primary health care.
Pharmaceuticals are prohibitively expensive for most of the world’s population, half of whom lived on less than 52 US per day in 2002. In comparison, herbal medicines can be grown from seed or gathered from nature for little or no cost. Many of the pharmaceuticals currently available to physicians have a long history of uses as herbal remedies, including opium, aspirin, digitalis, and quinine. According to the World health organization, estimates that 25% of modern drugs used in the United States have been derived from plants 9, 10.
Extraction: Powder was taken and weighed for further evaluation. The powder form was used for extraction with a suitable solvent system after examining the solubility property of the drugs.
The hydroalcoholic solvent system was the preferable solvent system for the study as per the available literature. Simple maceration technique of extraction was followed to get the purified drug for further investigations 5, 6.
Phytochemical Investigation: 2, 5, 8
Test for Starch: Dissolved 0.015 gm of iodine and 0.075 gm of potassium iodide in 5 ml of distilled water and add 2 - 3 ml of an aqueous extract of the drug, the blue color is produced.
Test for Steroids:
A) Salkowski Test: 2 - 3 drops of concentrated sulphuric acid was added to chloroform solution, shaken and allowed to stand, the appearance of red color in the lower layer indicates the presence of sterols.
B) Liebermann-Burchard Test: Extract was mixed with the chloroform and few drops of acetic anhydride and mixed well. Concentrated sulphuric acid was added from the sides of the test tube slowly until the ring appears; the appearance of the reddish brown ring indicates the presence of steroids.
Test for Flavonoids:
A) Shinoda Test: To the extract, a few fragments of magnesium ribbon and concentrated hydrochloric acid was added. The appearance of red to pink color after a few minutes indicates the presence of flavonoids. Lead acetate test: To the extract added few drops of aqueous basic lead acetate solution. Formation of a yellow precipitate indicates the presence of flavonoids.
B) Alkaline Reagent Test/ NaOH Test: few drops of sodium hydroxide solution were added to extract. The intense yellow color disappeared after adding dilute HCl which indicates the presence of flavonoids.
Test for Alkaloids: The extract was basified with ammonia and extracted with chloroform. The chloroform solution was acidified with dilute hydrochloric acid, shaken well and filtered. The filtrate was used for testing the alkaloids.
A) Hager's Test: The filtrate was treated with a few drops of Hager's reagent. Formation of a yellow precipitate indicates the presence of alkaloids.
B) Wagner's Test (Iodine in Potassium Iodide): The acid layer was treated with few drops of Wagner's reagent. Formation of a reddish brown precipitate indicates the presence of alkaloids.
C) Mayer's Test (Potassium Mercuric Iodine Solution): The acid layer was treated with a few drops of Mayer's reagent. Formation of a creamy white precipitate indicates the presence of alkaloids.
D) Dragendorff's Reagent (Potassium Bismuth Iodide): The acid layer was treated with a few drops of Dragendorff's reagent. Formation of a reddish brown precipitate indicates the presence of alkaloids.
Test for Tannins:
A) Gelatin Test: To the extracts of the drug added 1% solution of gelatin containing 10% sodium chloride. Formation of a white precipitate indicates the presence of tannins.
B) Ferric Chloride Test: To extracts, a few drops of 1% neutral ferric chloride solution were added, the formation of blackish blue color indicates the presence of tannins.
Test for Saponins:
A) Foam Test: Small amount of extract of the drug was shaken with little quantity of water if foam produced persists for 10 min; it indicates the presence of saponins.
B) Froth Test: To 5 ml of an extract of the drug added a single drop of sodium bicarbonate solution. Shaken the mixture vigorously and left for 3 min. Formation of honeycomb-like froth indicates the presence of saponins.
Test for Carbohydrates: Small amount of extracts of the drug were dissolved in little quantity of distilled water and filtered separately. The filtrates were used to test the presence of carbohydrates.
A) Molisch's Test: The filtrate of the drug was treated with Molisch reagent and concentrated sulphuric acid was added from the sides of the test tube to form a layer. A reddish violet ring shows the presence of carbohydrates.
B) Benedicts Test: To the filtrate added 2 ml Benedict's reagent and boiled in a water bath. Formation of Green or reddish brown precipitate indicates the presence of carbohydrates.
C) Fehling’s Test: Filtrates were hydrolyzed with dilute hydrochloric acid, neutralized with alkali and heated with an equal amount of Fehling's A and B solutions. Formation of green to yellow to red precipitate indicated the presence of reducing sugars.
Test for Phenols:
Phenolic Compounds: Extract was dissolved in alcohol, and 1 drop of neutral ferric chloride was added to this. The intense color indicates the presence of the phenolic compound.
Glycosides: 0.5 ml of extract was taken in a test tube and added with 1 ml glacial acetic acid containing traces of ferric chloride. To this solution, 1 ml of concentrated sulphuric acid was added and observed for the formation of reddish brown color at the junction of two layers and the upper layer turned bluish green in the presence of glycosides.
Terpenoid: 2 ml of chloroform and 1 ml of conc. H2SO4 was added to 1mg of extract and observed for reddish brown color that indicates the presence of terpenoids.
Resins: 1 ml of extract was diluted with water. Formation on bulk black precipitate indicates the presence of resins.
Cardiac Glycoside: 100 mg of extract was dissolve in 1ml of glacial acid containing 1 drop of ferric chloride solution. This was then under layer with 1 ml of conc — sulphuric acid. A brown ring obtained at the interface indicated the presence of a deoxy sugar characteristic of cardenolide.
Coumarins: 1 ml of extract was treated with alcoholic 10% NaOH. Dark yellow color shows the presence of coumarins.
Qualitative Evaluation:
TLC (Thin Layer Chromatography): 3 Thin Layer Chromatography is the separation of a mixture into individual components using stationary phase and mobile phase. Chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction.
Procedure: 7
Preparation of Solvent:
- Mobile phase prepared by taking methanol + water (distilled water) in the ratio 6:4.
- Placed the solvent into the developing chamber for saturation.
Preparation of Sample and Spotting:
- Sample A (Ashwagandha churna powder) and Sample B (Ashwagandha extract) were dissolved in methanol, and the sample was spotted on the chromatographic plate.
- The spots were dried then chromatographic plate was placed on the chamber.
Visualization of Spots:
- Mobile phase reached 3/4th run; the plate was removed from the chamber and dried.
- Plates were placed on iodine fuming chamber for a few sec. and spots visualization cleared.
Retardation Factor Rf is Define as:
Rf = Distance travelled by solute / Distance travelled by solvent
High-Performance Liquid Chromatography (HPLC): 1, 4 High-performance Liquid Chromato-graphy (HPLC) is a specific form of column chromatography generally used in biochemistry and analysis to separate, identify, and quantify the active compounds. HPLC mainly utilizes a column that holds packing material (stationary phase), a pump that moves the mobile phase(s) through the column, and a detector that shows the retention time of the molecules.
Advantages of HPLC:
- There is ease of sample preparation and sample introduction.
- To provide a specific, sensitive and precise method for analysis of different complicated samples.
- There is the speed of analysis.
- The analysis by HPLC is specific, accurate and precise.
- Separation is fast and efficient (high resolving power).
HPLC of Ashwagandha Churna:
- Preparation of mobile phase: acetonitrile: water (6:4)
- Wavelength = 215 nm
- Flow rate = 1 ml/min
Preparation of Standard Solution: Take 100 mg (Std.) drug dissolve in 100 ml of the mobile phase, prepared the concentration of solution 1000 µg/ml. Take 1 ml of 1000 µg/ml of the solution then volume makes up to 10 ml, Prepared the concentration of solution 100 µg/ml. Take 0.5 ml of 100 µg/ml of the solution then volume makes up to 10 ml, prepared the concentration of solution 50 µg/ml.
Taken 100 mg (Std.) drug dissolve in 100 ml of mobile phase (1000 µg/ml).
Taken 1 ml of the above solution then volume makes up to 10 ml (100 µg/ml).
Taken 0.5 ml of the above solution then volume to up to 10 ml (50 µg/ml).
Preparation of Sample Solution: Take 100 mg (sample) drug dissolve in 100 ml of the mobile phase, prepared the concentration of solution 1000 µg/ml. Take 1 ml of 1000 µg/ml of the solution then volume makes up to 10 ml, prepared the concentration of solution 100 µg/ml. Take 0.5 ml of 100 µg/ml of the solution then volume makes up to 10ml, prepared the concentration of solution 50 µg/ml.
Taken 100 mg (sample) drug dissolve in 100 ml of mobile phase (1000 µg/ml)
Taken 1 ml of the above solution then volume makes up to 10 ml (100 µg/ml).
Taken 0.5 ml of the above solution then volume to up to 10 ml (50 µg/ml).
Procedure: 20 ml of standard and sample were injected to HPLC and the chromatogram calculated the content of Withaferin-A samples in comparison with a standard.
RESULTS AND DISCUSSION:
Preliminary Phytochemical Screening:
TABLE 1: PRELIMINARY PHYTOCHEMICAL SCREENING OF ASHWAGANDHA CHURNA
| S. no. | Phyto-
constituents |
Phytochemical
test |
Result |
| 1 | Starch | Iodine test | - |
| 2 | Steroids | Salkowski test | + |
| 3
|
Flavonoids
|
Ferric chloride test
Alkaline reagent text |
+
- |
| 4
|
Alkaloids
|
Hager’s test
Wagner’s test Mayer’s test Dragendorff’s test |
++
+ + ++ |
| 5 | Tannins | Ferric chloride test | + |
| 6
|
Saponins
|
Froth test
Form test |
+
- |
| 7 | Carbohydrates | Molisch’s test | + |
| 8 | Glycoside | 10% NaOH test | ++ |
| 9 | Terpenoids | Salkowski’s test | + |
| 10 | Resins | Aqueous solution test | ++ |
| 11 | Cardiac glycosides | Keller killiani’s test | ++ |
| 12 | Coumarins | 10% NaOH solution | + |
| 13 | Phenol | Ferric chloride test | - |
(+) Positive, (++) Moderated Positive, (-) Negative
Quantitative Evaluation:
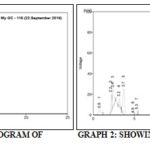
T.L.C. (Thin Layer Chromatography):
A (sample) = Ashwagandha churna
B (Standard) = Ashwagandha extract
Rf = Distance travelled by solute / Distance travelled by solvent
TABLE 2: Rf VALUE OF SAMPLE AND STANDARD
| S. no. | Samples | Rf value |
| 1 | A | 0.87 |
| 2 | B | 0.85 |
HPLC of Ashwagandha Churna:
Sample = Ashwagandha churna
Standard = Ashwagandha extract
The dried extract was taken for the chemical detection of the constitutes test for alkaloid, flavonoid, tannine, steroids, glycoside, terpenoids, etc. Phytochemical screening revealed for the presence of alkaloid, flavonoid, tannins, steroids, glycoside, terpenoids, resins, starch, carbohydrate. Above table depicts the finding of various contents of the plant. According to literature, they are useful in stress increasing body strength, treatment of body weakness anxiety, increase memory, brain power, cardiac disorders, antioxidant, cancer, etc. The phytochemical analysis is very important in the evaluation of active biological component of the plant. The phytoconstituents quantified in the present study exhibit a great deal of medicinal importance specially Alkaloid part plays a major role in the anxiolytic activity. Quantitative estimation of Ashwagandha churna T.L.C spot of the sample very nearest to the standard sample spot and HPLC analysis of herbal formulation sample value almost similar to standard sample value.
TABLE 3: RESULT ANALYSIS OF SAMPLE (50 µg/ml) BY HPLC
| S. no. | Retention time (min) | Area (mV.s) | Height (mV) | Area (%) | Height (%) | WOS (min) |
| 1 | 2.187 | 22.424 | 2.938 | 16.5 | 31.1 | 0.12 |
| 2 | 2.397 | 26.410 | 1.340 | 19.5 | 14.2 | 0.35 |
| 3 | 3.057 | 44.349 | 2.395 | 32.7 | 25.4 | 0.33 |
| 4 | 3.420 | 40.066 | 2.581 | 29.6 | 27.4 | 0.26 |
| 5 | 4.367 | 2.324 | 0.182 | 1.7 | 1.9 | 0.21 |
| Total | 135.573 | 9.435 | 100.0 | 100.0 |
TABLE 4: RESULT ANALYSIS OF STANDARD (50 µg/ml) BY HPLC
| S. no. | Retention time (min) | Area (mV.s) | Height (mV) | Area (%) | Height (%) | WOS (min) |
| 1 | 0.783 | 11.205 | 0.492 | 11.4 | 6.4 | 0.37 |
| 2 | 2.173 | 16.775 | 1.696 | 17.1 | 22.2 | 0.18 |
| 3 | 2.400 | 21.641 | 1.731 | 22.1 | 22.7 | 0.25 |
| 4 | 3.167 | 8.105 | 0.740 | 8.3 | 9.7 | 0.16 |
| 5 | 3.720 | 36.579 | 2.507 | 37.3 | 32.9 | 0.24 |
| 6 | 4.877 | 1.502 | 0.140 | 1.5 | 1.8 | 0.14 |
| 7 | 5.333 | 2.299 | 0.321 | 2.3 | 4.2 | 0.12 |
| Total | 98.106 | 7.627 | 100.0 | 100.0 |
CONCLUSION: It is one of the most researched plant of the 21st century having a huge number of valuable chemical constitutes. This study suggests the isolation of constitute of the Ashwagandha churna that could be used in the treatment of numerous ailment and the advantage of easily availability. This herbal formulation is safe.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Indian herbal pharmacopeia, Published by Indian drug manufacturers association, New edition- 2002; 467.
- Doddappa DS, Gavishidd A and Anappa H: Evaluation of phytoconstituent of methanolic root extract of Withania somnifera. Journal of Advanced scientific research 2015; V6: 1-6.
- Kushwaha R and Karanjekar S: Standardization of Ashwagandharishta formulation by TLC. International Journal of Chem Tech Research 2011; 3: 1033-1036.
- Manwar JV, Mahadik KR and Paradkar AR: Determination of withanolide from the roots and herbal formulation of Withania somnifera by H.P.L.C published by Pelagia research library 2012; 3: 41-46.
- Khandelwal KR: Practical Pharmacognosy, Nirali Prakashan, Pune 2006; 15: 146-156
- Kokate CK, Purohit AP and Gokhale SB: pharmacognosy, 45th edition, published by- Nirali Prakashan 6.17-6.19.
- Nagavali D, Vetrichelvan T and Gayathri N: Validation of Ashwagandha churna. The ancient science of life 2001; 20(4): 86-88.
- Saldulu C, Venkateshwara C and Gangadhar RS: Preliminary phytochemical studies of medicinal plant drug: somnifera L. Bio-Life Journal 2014; 2: 306-08.
- Ashwagandha.myYOG.com
- com
How to cite this article:
Mishra P, Gupta PK and Kannojia P: Quantitative and qualitative evaluation of Ashwagandha churna. Int J Pharmacognosy 2018; 5(8): 526-31. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(8).526-31.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
13
526-531
690
1383
English
IJP
P. Mishra *, P. K. Gupta and P. Kannojia
Department of Pharmacology, IPS - College of Pharmacy, Gwalior, Madhya Pradesh, India.
priya.mishra4491@gmail.com
13 April 2018
09 June 2018
13 June 2018
10.13040/IJPSR.0975-8232.IJP.5(8).526-31
01 August 2018