PLATELET AND LEUKOCYTE-INCREASING EFFECTS OF SYZYGIUM CUMINI (L.) SKEELS (MYRTACEAE) LEAVES IN A MURINE MODEL
HTML Full TextPLATELET AND LEUKOCYTE-INCREASING EFFECTS OF SYZYGIUM CUMINI (L.) SKEELS (MYRTACEAE) LEAVES IN A MURINE MODEL
Teresa May B. Bandiola * 1 and Mary Jho-Anne T. Corpuz 1, 2, 3
The Graduate School 1, Faculty of Pharmacy 2, Research Center for Natural and Applied Sciences 3, University of Santo Tomas, Manila, Philippines.
ABSTRACT: Context: Syzygium cumini (L.) Skeels (Myrtaceae) is popularly known to have various pharmacological and traditional uses. Before this study, there were no claims reporting its potential use for dengue by increasing platelet and leukocyte levels. Objectives: The effects of the methanolic extract of S. cumini leaves on the platelet and leukocyte levels were evaluated in Sprague-Dawley rats at doses 400 mg/kg and 800 mg/kg body weight. Methodology: The bioassay utilized 24 rats that were divided into four groups (n = 6) where hydroxyurea was used to induce depletion of platelet and leukocyte levels in all groups. After induction, oral treatment of methanolic extract was given daily to the treatment groups for six days. The platelet and leukocyte counts were measured before induction to get the baseline, after induction, and at the 1st, 3rd, and 6th day of treatment. High-performance liquid chromatography (HPLC) analysis was also conducted to identify the phenolic compounds present in the extract. Results: Results revealed that the methanolic extract of S. cumini caused an increase of platelet counts at both 400 and 800 mg/kg and an increase in leukocyte counts at 800 mg/kg. HPLC data identified catechin and rutin at concentrations 759.16 ppm and 142.24 ppm, respectively. Conclusion: S. cumini is a potential candidate for further research leading to the development of an herbal therapeutic agent for dengue.
| Keywords: |
Syzygium cumini, Platelets, Leukocytes, Dengue
INTRODUCTION: Dengue, the most important viral infection transmitted among humans by arthropod-borne, has remained to be a national epidemic in the Philippines and has been one of the leading causes of mortality in children (Philippine Department of Health, 2017). There is no quick dengue infection confirmation test; however, a complete blood count (CBC) might show characteristics such as thrombocytopenia and leukopenia 1.
During dengue infection, leukopenia or depletion of leukocyte count occurs because the virus infects epidermal dendritic cells which then migrate toward the lymph node and infect macrophages, monocytes, and other types of WBC 2. On the other hand, thrombocytopenia or decrease in thrombocyte count occurs because of virus-induced bone marrow suppression, platelet destruction, and modulation of endothelial cells 2.
In a study by Rasool et al., (2012) 2, an improvement of WBC count while platelet count is still below normal showed patient recovery suggesting that WBC production is a good indicator along with a focus on platelet counts. Platelet counts decrease even after dengue virus replication has been stopped because the anti-dengue antibodies are still clearing infected platelets and because the chemokines that are produced by infected endothelial cells are still causing an effect.
In 2015, Dengvaxia® (CYD-TDV), developed by Sanofi Pasteur, became the first dengue vaccine to be licensed and approved in 19 countries. It was first licensed in Mexico for use in individuals 9 - 45 years of age living in endemic areas. CYD-TDV is a live recombinant tetravalent dengue vaccine, given as a 3-dose series on a 0/6/12 month schedule (WHO, 2016). After its approval, regional mass vaccination programs were launched in the Philippines and Brazil. Recently, however, after reassessment of data from the clinical trials, Sanofi Pasteur warned on November 29, 2017, that the vaccine is effective only to seropositive patients and that the vaccine had a significantly higher risk of more severe dengue and hospitalizations to seronegative patients. Because of this, the vaccination program in the Philippines was then suspended, with information released to World health organization by Sanofi Pasteur raising questions about the future use of the vaccine 4.
Syzygium cumini, duhat in the Philippines, belongs to the family of Myrtaceae 5, 6. There is no data available on its possible platelet- and leukocyte-increasing activity in dengue, however, one member of the Myrtaceae family, Psidium guajava, has been reported to have an anti-dengue activity and is said to be a rich source of flavonoids 7. Flavonoids are among the compounds to possess an anti-dengue activity according to Abd Kadir et al., 2013 7. In S. cumini, flavonoids represent the main group of phenolic compounds 8, 9, 10.
Phenolic compounds were reported by Apostol et al., 2012 11 to increase platelet counts in thrombocytopenic rats possibly due to antioxidant activity by using Euphorbia hirta, a traditional anti-dengue medicinal plant in the Philippines. Significantly, the total flavonoid content in the leaves of S. cumini was highest than other commonly utilized parts: seed and pulp 12. Furthermore, the HPLC data by Ruan et al., 2008 13 in the methanolic leaf extract of S. cumini showed the presence of phenolic compounds such as catechin. Rohadi et al., 2017 13 also revealed that the HPLC data of the methanolic extract contains polyphenols such as catechin and rutin, which according to their study, are correlated to the antioxidant activity.
Therefore, the presence of flavonoids in S. cumini, particularly in the leaves, and its reported antioxidant effect, laid the foundation for the development of this study.
MATERIALS AND METHODS:
Reagents and Medical Supplies: All chemicals used, from extraction to phytochemical screening, were reagent grade and were purchased from ATR Trading system (Quezon City, Philippines). Blood collection tubes, syringes, needles gauge-27, and gavage tubings that were used for bioassay were purchased from Health Craft Medical Supply (Manila City, Philippines).
Plant Material: The leaves of Syzygium cumini were collected from a single tree, free of pesticide, during the third week of August 2017 at Novaliches, Quezon City, Philippines (14. 7215120 N, 121. 0519410 E) and were authenticated and certified by Dr. Cecilia I. Banag of the University of Santo Tomas Herbarium.
Using the procedures by Kamal (2014) and Banu and Cathrine (2015), the leaves were cleaned, air-dried at room temperature in a cool, dry place away from direct sunlight, and finally ground to a coarse powder (2 kg). The 2 kg powder was then percolated and evaporated to dryness using rotary evaporator (Nanost™, Instruchem Inc.) under 40ºC.
After that, the extract was weighed giving a 7.51% yield. The extract was then stored in an amber-colored bottle and was kept at 4 ºC before succeeding experiments.
Experimental Animals: Twenty four (24) male Sprague-Dawley rats (190 - 250 g in weight) were used for in-vivo assay. They were purchased from the Department of Science and Technology (DOST), Taguig City, the Philippines on the first week of September 2017 with receipt no. 1449897 and were housed at DOST animal laboratory under the science and testing department (STD) of the said agency. The animals were sheltered under environmentally controlled conditions of 22 ± 3 ºC room temperature at 12 h light and 12 h dark cycle in a relative humidity of at least 30%.
The six rats that belonged in the same group were housed all together in one plastic cage of 2 ft. length, 1.5 ft width, and 0.5 ft height and were identified by their markings on their tail. The rats were provided with conventional rodent chow pellets (Purina Mills Ltd., Philippines) and distilled water ad libitum and their bedding (rice hulls) was sterilized before use and was changed at least once a week. Before the bioassay at DOST-STD on the 2nd to 4th week of September 2017, the rats were acclimatized first for one week and were fasted overnight before this procedure.
In-vivo Anti-Thrombocytopenic and Anti-Leukopenic Assay: Bioassay was performed following the methods by Gammulle et al., 2012 and Patil et al., 2013 14. Completely randomized design (CRD) was used in this study wherein it uses treatments that are assigned randomly to experimental subjects without restriction 15. Two doses were used in this study which is 400 mg/kg and 800 mg/kg. These were calculated based on the reported median lethal dose (LD50) of S. cumini which is 3,873 mg/kg. The calculated 1/5th and 1/10th doses based on the LD50 were 774.6 mg/kg and 387.3 mg/kg, which were rounded off to 400 mg/kg and 800 mg/kg, respectively.
The twenty-four (24) male Sprague-Dawley rats were divided into four groups (n = 6) namely: group I as negative control, group II as a positive control or toxicant group, group III as treatment group no. 1, and group IV as treatment group no.2, in which hydroxyurea (Krabinex®, Korea United Pharma, Inc.) was used to induce depletion of platelet and leukocyte levels in all the groups. Depletion was observed after 24 h of Hydroxyurea administration, as also performed by Gammulle et al., 2012 14.
After a successful decrease in platelet and leukocyte levels as indicated by a significant mean difference in platelet and WBC levels of the group's Table 1 and 2, the study continued for a 6 day treatment. The following were done to the various groups: group I was administered with distilled water (10 ml/kg p.o.) for six days; groups II was given with hydroxyurea (15 mg/kg p.o.) for the first three consecutive days; group III was treated with S. cumini extract (400 mg/kg p.o.) for a period of six days along with hydroxyurea, but hydroxyurea was only given for the first three consecutive days; and lastly, group IV was given with S. cumini extract (800 mg/kg p.o.) for a period of six days along with hydroxyurea, but hydroxyurea also was given only for the first three consecutive days. Blood samples were extracted five times through saphenous vein: before induction of thrombocytopenia and leukopenia (baseline); after the induction of thrombocytopenia and leukopenia (post-induction); 1st day of oral treatment; 3rd day of the oral treatment; and 6th day of oral treatment. Right after blood (0.5 ml) was obtained from rats, it was added to Microtainer® blood collection tubes and was brought to Advanced Diagnostic Veterinary Laboratories, Inc. at Muntinlupa City, Philippines for leukocyte and platelet counting.
Ethical and Legal Considerations: The pharmacological study protocol (DOST-IACUC reference number AR-2017-339) was approved by the institutional animal care and use committee (IACUC) of the Department of Science and Technology (DOST), and was further issued by the Philippine Bureau of Animal Industry (BAI) an Animal Research Permit with BAI Registration Number LAF-018. The experiments were performed by the rules and regulations provided by the institutions mentioned above.
All animals received humane care, and the “Animal Welfare Act” of the Philippines was strictly observed. Throughout the bioassay, Tiletamine HCl + Zolazepam HCl (Zoletil®) 60 mg/kg (Virbac S.A. Laboratories, Philippines) as the general anesthetic was administered intramuscularly before each blood extraction was performed. After the experiments, the animals were euthanized in a carbon dioxide chamber by the in-charge veterinarian of DOST and were disposed of according to the protocols of the IACUC.
Statistical Analysis: This study employed different statistical analysis using SPSS version 21. Data were reported as mean ± standard deviation and p < 0.05 was considered statistically significant at 95% confidence. Inferential statistics were used to infer the characteristics of the population based on the estimates of a sample. The inferential statistics used was a one-way analysis of variance (ANOVA). One-way ANOVA statistical treatment was used to measure the mean differences between treatments while Duncan test as post-hoc was used to determine the variant groups.
High-Performance Liquid Chromatography: Catechin, rutin, and quercetin were used as standards as performed by Rohadi et al., 2017 13 with a few modifications. Various concentrations of standards from Sigma Aldrich® were prepared from a stock solution. The wavelength for maximum absorption was 370 nm, and the flow rate was maintained at 1 ml/min using a HPLC instrument (Shimadzu™ LC-20AT/CBM-102). The plant sample was prepared using 2 mg dissolved in 10 ml HPLC grade methanol. The mixture was filtered and injected into the HPLC column (Inertsil™ ODS-3V) using a mobile phase of 75:25 methanol and distilled water at an injection volume of 20 µl.
RESULTS AND DISCUSSION:
Determination of Platelet-Increasing Effect: Table 1 shows the effect of plant extracts on thrombocytopenic rats. The data in the baseline level showed that there is no significant difference with p-value 0.836; this means that the values are the same. The data after induction also showed that there is no significant difference with p-value 0.984. The data after a day showed that there is a significant difference with p-value 0.000, based on the mean difference, and the positive control means value decreases. The data after 3 days showed that there is a significant difference with p-value 0.000, based on the mean difference, the positive control means score value decreases whereas ME 400 and ME 800 increase. Lastly, the data after 6 days showed that there is a significant difference with p-value 0.000, based on the mean difference, the positive control means score decreases whereas ME 400 and ME 800 mean scores increase.
Moreover, the data of negative control showed that there is no significant difference with p-value 0.143. The data of positive control showed that there is a significant difference with p-value 0.000, as compared to the mean difference, the values decreases after 6 days testing. The data of ME 400 showed that there is a significant difference with p-value 0.001; thus the mean score increases after 6 days but as observed Day 3 has higher mean score value than day 6. Lastly, the data of ME 800 showed that there is a significant difference with p-value 0.000; thus the mean score increases after 6 days. Hence, both ME 400 and ME 800 showed a platelet-increasing activity.
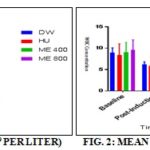
As reported by Gammulle et al., 2012 14, hydroxyurea was effective in the depletion of platelets after 24 h due to bone marrow suppression and platelet destruction. In this study, a decrease platelet count after hydroxyurea administration was observed after 24 h. Furthermore, the platelet-increasing effect of S. cumini could be attributed to the antioxidant activity of the flavonoids present. Apostol et al., 2012 11 reported that antioxidant activity counters platelet oxidation and platelet dysfunction and therefore maintains platelet lifespan and improves platelet count. Using Graphpad® Prism 7.03, the graph for the results of platelet counts is presented below:
The results of the mean platelet counts of control and experimental groups are presented in Table 1. Fig. 1 shows a significant decrease in platelet counts after induction with hydroxyurea. The results also show that both ME 400 and ME 800 increased the number of platelet levels after treatment and that the activity is best at Day 3 for ME 400 whereas Day 6 for ME 800. This platelet-increasing effect is possibly due to antioxidant activity. Also, since depletion of platelets is also caused by sequestration of platelets by the enlarged spleen 11, the platelet increasing effect of S. cumini may be due to induced contractions of the spleen since the juice of S. cumini is traditionally used in spleen enlargement 16.
TABLE 1: MEAN PLATELET COUNT (× 109 PER LITER)
| Treatment Group | Baseline X±SD | Post-induction X±SD | 1-Day X±SD | 3-Day X±SD | 6-DayX±SD | P-value |
| Negative Control | 952.67 ± 222 | 542.33 ± 330 | 553.83 ± 403b | 520.67 ± 418b | 646.17 ± 129b | 0.143 |
| Positive Control | 985.17 ± 331 | 507.67 ± 109 | 92.33 ± 30a | 66 ± 23a | 42.5 ± 24a | 0.000 |
| ME 400 | 886.83 ± 137 | 571 ± 285 | 843.17 ± 270b | 1626 ± 431c | 1417 ± 724c | 0.001 |
| ME 800 | 891.83 ± 125 | 538.83 ± 326 | 883 ± 191b | 1328 ± 263c | 1337.83 ± 281c | 0.000 |
| P-value | 0.836 | 0.984 | 0.000 | 0.000 | 0.000 |
Data are presented as mean ± SD, n = 6 per group. The values in the same column with different superscripts letters are statistically significantly (p<0.05).
TABLE 2: MEAN LEUKOCYTE COUNT (× 109 PER LITER)
| Treatment Group | Baseline X±SD | Post-induction X±SD | 1-Day X±SD | 3-Day X±SD | 6-Day X±SD | P-value |
| Negative Control | 8.97 ± 1.13 | 6.15 ± 0.67 | 7.82 ± 2.25b | 7.45 ± 1.67bc | 7.78 ± 1.96b | 0.090 |
| Positive Control | 8.42 ± 2.62 | 5.87 ± 2.34 | 4.12 ± 1.47a | 3.6 ± 1.62a | 2.62 ± 0.47a | 0.000 |
| ME 400 | 9.12 ± 2.24 | 5.53 ± 3.60 | 8.58 ± 1.91b | 5.6 ± 2.24ab | 8.1 ± 2.35b | 0.054 |
| ME 800 | 9.57 ± 2.41 | 5.52 ± 1.42 | 8.75 ± 0.77b | 8.92 ± 1.73c | 9.03 ± 3.32b | 0.021 |
| P-value | 0.836 | 0.956 | 0.000 | 0.000 | 0.000 |
Data are presented as mean ± SD, n = 6 per group. The values in the same column with different superscripts letters are statistically significantly (p<0.05)
Determination of Leukocyte - Increasing Effect: Table 2 shows the effect of plant extracts on leukocytopenic rats. The data in the baseline level showed that there is no significant difference with p-value 0.836; this means that the values are the same. The data after induction showed that there is no significant difference with p-value 0.956. The data after a day showed that there is a significant difference with p-value 0.000, based on the mean difference, and the positive control means value decreases. The data after 3 days showed that there is a significant difference with p-value 0.000, based on the mean difference, the positive control and ME 400 mean score value decreases whereas ME 800 increases. The data after 6 days showed that there is a significant difference with p-value 0.000, based on the mean difference, the positive control means score decreases whereas ME 400 and ME 800 mean scores increase.
Moreover, the data of negative control showed that there is no significant difference with p-value 0.090. The data of positive control shows that there is a significant difference with p-value 0.000, as compared to the mean difference, the values decrease after 6 days of testing. The data of ME 400 shows that there is no significant difference with p-value 0.054, thus the mean score at day 3 decreases. The data of ME 800 showed that there is a significant difference with p-value 0.021; thus the mean scores increase after 6 days. Hence, ME 800 shows an anti-leukopenic activity.
In this study, hydroxyurea, as reported by Gammulle et al., 2012 14, caused the depletion of leukocytes after 24 h due to bone marrow suppression and platelet destruction. Similar to the platelet increasing effect of S. cumini, the leukocyte-increasing effect could also be attributed to the antioxidant activity of the flavonoids present. Also, since leukocytes can be synthesized in the spleen, S. cumini may have increased leukocyte levels due to spleen contractions 16.
Using Graphpad® Prism 7.03, the graph for the results of WBC counts is presented below:
The results of the mean platelet counts of control and experimental groups are presented in Table 2. The figure above shows a significant decreased in leukocyte counts after induction with hydroxyurea. The results also show that ME 800 increased the number of leukocyte levels after treatment and that the activity is best at Day 6. Results reveal a dose-dependent effect because WBCs are produced from megakaryocytes within 6 h 2 days while 4 - 6 days for platelets 2. Hence, it is expected that a higher concentration is more effective because leukocytes have a shorter lifespan than platelets.
Flavonoids Identification and Quantification Using HPLC: In this study, catechin, rutin, and quercetin were used as standards as performed by Rohadi et al., 2017 13. Below are their calibration curves:
FIG. 3: HPLC STANDARD CURVES
From the calibration curve, the computation of standard concentration in the plant sample was done by using the linear regression equation, y = mx + c where: y = peak area, m= slope, x = concentration, and c = intercept.
TABLE 3: HPLC PROFILING
| Standard | Concentration (ppm) |
| Cathechin | 759.16 ± 129.698 |
| Rutin | 142.24 ± 10.620 |
| Quercetin | 0 |
Data are presented as mean ± SD, n = 3 per group.
All experiments were carried out in triplicates. Results revealed that cetechin and rutin were present in the methanolic leaf extract having catechin as the constituent with the highest amount followed by rutin.
On the other hand, there was no quercetin detected although numerous publications claim the presence of quercetin. This absence of quercetin may then apply to the study of Priya et al., 2017 that the active principles or individual polyphenols in S. cumini may vary in their levels and contribution to efficacy depending on the source of the plant or its geographical location.
Also, the HPLC results above were similar to the HPLC results of methanolic seed extract by Rohadi et al. 2017 13 where catechin and rutin are identified. A similar study by Ruan et al., 2008 10 had proven that the HPLC data of the methanolic leaf extract of this plant contained catechin which may be responsible for the antioxidant activity.
Based on the figures, the peaks of plant samples matched the peaks of catechin and rutin but did not match the peaks of quercetin. Hence, only catechin (759.16 ppm) and rutin (142.24 ppm) were present in S. cumini leaves.
Both catechin and rutin are flavonoids and are said to be strong antioxidants 17. Antioxidant activity, according to Apostol et al., 2012 11, counters platelet oxidation and platelet dysfunction and therefore maintains platelet lifespan and improves platelet count.
It must also be noted that in a Philippine study by De Paz et al., 2015 18, catechin, along with gallic acid, was identified in Tawa-tawa (E. hirta) using HPLC analysis. Tawa-tawa was proven to be effective in increasing platelet levels by 11, 18, 19.
Since catechin is found to be the most abundant bioactive in S. cumini methanolic leaf extract, it must be noted that catechin, compared to other flavonoids, is an aglycon and can be easily absorbed by the small intestine, while flavonoid glycosides have to be converted into aglycon form first. Also, catechin, compared to other flavonoids, is present in plasma even during conjugation metabolism. Plasma, the water part of the blood, is where WBCs and platelets are contained. Because of this, catechin can cause a significant increase in plasma antioxidant status 17.
CONCLUSION: This study significantly claimed for the first time that the leaves of S. cumini (methanolic extract) could be orally active and effective in increasing WBC and platelets having catechin (759.16 ppm) and rutin (142.24 ppm) as the main bioactive present. Hence, it is a potential candidate for further research leading to the development of an herbal therapeutic agent for dengue.
Recommendations and Future Directions: The following are recommended to meet the gaps of the study: the future researchers to isolate or identify the specific compound/s from S. cumini that exert/s the anti-thrombocytopenic and anti-leukopenic activities and since results are dose-dependent, it is recommended that a wider range of doses of S. cumini should be tested at a valid reference and to increase the number of test animals to be used.
Furthermore, it is recommended that future investigators should use other solvents and methods of extraction and to study the mechanism of action of S. cumini on how it increases platelet and leukocyte levels.
ACKNOWLEDGEMENT: The primary researcher, T. M. B. Bandiola, is deeply grateful to the Commission on Higher Education (CHED) K12 program for providing her thesis grant that sustained this study.
CONFLICT OF INTEREST: The authors declare no conflict of interest.
REFERENCES:
- Fujimoto DE and Koifman S: Clinical and laboratory characteristics of patients with dengue hemorrhagic fever manifestations and their transfusion profile. Brazilian Journal of Hematology and Hemotherapy 2014; 36(2): 115-20.
- Rasool F, Ahmad M, Masood I and Khan MS: Evaluating the relationship between white blood cells and platelets during the recovery phase in dengue hemorrhagic fever cases in Punjab, Pakistan: A Retrospective Study. International Society for Pharmacoeconomics and Outcomes Research 2012.
- WHO: World Health Organization 2016, Health Topic: Dengue [Internet]. [Cited 2016 November]. Available from: http://www.who.int/topics/dengue/en/
- WHO: World Health Organization 2018, Supplemental Statement [Internet]. [Cited 2018 April]. Available from: http://www.who.int/immunization/diseases/dengue/q_and_a_dengue_vaccine_dengvaxia_use/en/
- Quisumbing E: Medicinal plants of the Philippines, Katha Publishing House, Inc., Quezon City 1978; 110-12.
- Dacanay ATL: Characterization of the physicochemical properties of the lyophilized fruit juice of Syzygium cumini (Myrtaceae). Unpublished Thesis, University of Santo Tomas 2007.
- Kadir ASL, Yaakob H and Zulkifli RM: Potential Anti-dengue Medicinal Plants: A Review. Journal of Natural Medicine 2013; 67(4): 677-89.
- Haroon R, Jelani S and Arshad FK: Comparative analysis of antioxidant profiles of bark, leaves and seeds of Syzygium cumini (Indian Blackberry). International Journal of Research –Granthaalayah, 2015; 3(5): 13-26.
- Kamal A: Phytochemical Screening of Syzygium cumini Seeds. Indian Journal of Plant Sciences, 2014; 3(4): 1-4.
- Ruan ZP, Zhang LL and Lin YM: Evaluation of the anti-oxidant property of Syzygium cumini Leaves. Molecules 2008; 13: 2545-56.
- Apostol JG, Gan JVA, Raynes RJB, Sabado AAS, Carigma AQ, Santiago LA and Ysrael MC: Platelet-increasing effects of Euphorbia hirta Linn. (Euphorbiaceae) in ethanol-induced thrombocytopenic rat models. International Journal of Pharmaceutical Frontier Research 2012; 2(2): 1-11.
- Margaret E: Evaluation of antioxidant activity in different parts of Syzygium cumini (Linn.). International Journal of Current Microbiology and Applied Sciences 2015; 4(9): 372-379.
- Rohadi, Santoso U, Raharjo S and Falah ALI: Determination of antioxidant activity and phenolic compounds of methanolic extract of Java Plum (Syzygium cumini Linn.) Seed. Indonesian Food and Nutrition Progress 2017; 14(1): 9-20.
- Gamuelle A, Ratnasooriya WD, Fernando C, Kanatiwela C and Udagama PV: Thrombocytosis and anti-inflammatory properties, and toxicological evaluation of Carica papaya mature leaf concentrate in a murine model. International Journal of Medicinal Plants Research 2012; 1(2): 21-30.
- Caintic HE: Scientific Research Manual. Quezon City: C & E Publishing 2008.
- Ayyanar M and Subash-Babu P: Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine 2012; 2(3): 240-46.
- Kumar A and Kalakoti M: Phytochemical and antioxidant screening of leaf extract of Syzygium cumini. International Journal of Advanced Research 2015; 3(1): 371-78.
- De-Paz SLM, Sumalde AAM, Cruz CJG, Robles JAH, Pajarillo EA, Apelado PB, Bawalan RJG, Lam HY, Sia IC, Montaňo NE and Heralde, FM: Not all Taua-tauas are Alike: A morphological, molecular genetic, phytochemical, and anti-thrombocytopenic profiling of different Euphorbia hirta Linn. plants from the Philippines. Philippine Journal of Health Research and Development 2015; 19(1). pjhrd.upm.edu.ph/index.php/ main/article/view/26/0
- De-Guzman GQ, Dacanay ATL and Alejandro GJD: Ethnopharmacological studies on the uses of Euphorbia hirta in the treatment of dengue in selected indigenous communities in Pangasinan (Philippines). Journal of Intercultural Ethnopharmacology 2016; 5(3): 239-43.
How to cite this article:
Bandiola TMB and Corpuz MJAT: Platelet and leukocyte-increasing effects of Syzygium cumini (L.) skeels (myrtaceae) leaves in a murine model. Int J Pharmacognosy 2018; 5(8): 467-74. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(8).467-74.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.