PHYTOPHARMACOGNOSTICAL INVESTIGATION OF SAMASARKARA CHURNA
HTML Full TextPHYTOPHARMACOGNOSTICAL INVESTIGATION OF SAMASARKARA CHURNA
Vaishali D. Naphade* 1, Atul R. Bendale1, Sushil P. Narkhede1, Sachin B. Narkhede2 and Anil G. Jadhav1
Sandip Institute of Pharmaceutical Sciences 1, Nashik - 422213, Maharashtra, India.
Smt. B. N. B. Swaminarayan Pharmacy College 2, Salvav (Vapi) - 396191, Gujarat, India.
ABSTRACT: Introduction: Samasarkara churna is the Ayurvedic medication preferred treatment of dyspepsia, loss of appetite and piles, formulated by mixing powder of cardamoms, long piper, black piper, flowers of Mesua ferrea, cinnamon and Sugar. Method: With the help of reported composition and standard procedure, formulated Samasarkara churna was compared with the market formulation. Efforts have been made to developed quality control parameters of Ayurvedic formulation Samasarkara churna by observing of organoleptic features, microscopical characters, and physicochemical properties. Results: Ash values and volatile oil content of standard and test sample were found to be 4.75, 2.25 and 0.2% w/v and 0.3% w/v respectively. Crude fiber content, pH of 1% w/v solution of churna and loss on drying were found to be (0.11, 0.34), (6.24, 6.29) and (0.7, 0.8) respectively. Calculated extractive values confirm that water-soluble contents are more in the Samasarkara churna. Discussion: After analyzing samples of Samasarkara churna by different parameters such as total ash, water, and alcohol soluble extractive values, lipid and volatile oil content, microscopic and phytochemical investigation showed reproducible results between batches. Conclusion: Parameters used therewith can be utilized for the evaluation and standardization of various polyherbal formulations.
| Keywords: |
Samasarkara churna, Ayurvedic formulation, Phytopharmacognostical
INTRODUCTION: Indian healthcare consists of medical diversity, and Ayurveda remains prevailing compared to modern medicine, particularly for the treatment of a variety of chronic disease conditions 1. To overcome the disease many Ayurvedic formulations are used like asava, arista, arka, avleha, kvatha, churna, lepa, vatika, gutika, netrabindu, sattva, grita, taila, bhasma, etc., but the churna has the unique place in all the formulation. Churna is the fine powder of drug and drugs intended for oral administration.
The World health organization (WHO) in 1999, has given a detailed protocol for the standardization of herbal drugs comprising of a single content, but very little literature is available for the standardization of polyherbal drugs. By considering the increasing demand for Ayurvedic formulations, proper documentation regarding their standardization is more important to assure the quality, purity, safety, and efficacy.
Keeping these things in mind, efforts have been made in developing quality control parameters for Ayurvedic formulation ‘Samasarkara churna’ using organoleptic features, microscopical characters, and physicochemical properties. It serves quality control and quality assurance aspects of the formulation. The standards of in house formulation of Samasarkara churna were determined and compared with market formulation 2, 3.
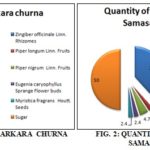
Based upon the composition given in the book of Bhaisajyaratnavali, the formula of the Samasarkara churna is composed as in Fig. 1, as follows:
MATERIAL AND METHODS:
Collection and Identification of Crude Drugs: All the plant crude drugs required for the preparation of the standard formulation of Samasarkara churna were collected from the local store in November 2015.
Preparation of Churna: All the crude drugs were examined for the presence of foreign matters and were weighed as prescribed under the formula of the Samasarkara churna in the book Bhaisajyaratnavali. All of the drugs were separately ground. All the powdered drugs were mixed using the homogenizer. One market formulation of Samasarkara churna was purchase from the local Ayurvedic pharmacy of town, details of market formulation is as follows: (Batch no. D-11 and mfg. Date: Nov. 14): The ingredients and their respective quantity; which is given on label: sunth (95.3 gm), long piper (5.9 gm), black piper (12 gm), lavang (5.9 gm), jayphal (5.9 gm), sugar (125 gm). All powdered crude drug ingredients; standard and market formulations of Samasarkara churna were examined for their morphological and microscopical characters and quantitative microscopical studies. That gives detail idea about Pharmacognostical evaluation 4-10.
Phytochemical Evaluation: Organoleptic characters, loss on drying, ash value, water-soluble extract, alcohol soluble extract, and pH in 5% aqueous suspension were assessed 11-13.
OBSERVATIONS AND RESULTS:
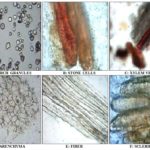
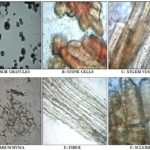
A) Microscopy of Standard Samasarkara Churna:
B) Microscopy of Test Samasarkara Churna:
TABLE 1: MORPHOLOGICAL CHARACTERS OF STANDARD AND MARKET FORMULATIONS OF SAMASARKARA CHURNA
| Parameters | Formulations | |
| Std. | Test | |
| State | Fine | Very fine |
| Color | Creamish brown | Brown |
| Odor | Aromatic and pungent | Aromatic and pungent |
| Taste | Aromatic and sweet | Aromatic and sweet |
TABLE 2: SCREENING OF PHYTOCONSTITUENTS IN STANDARD AND MARKET FORMULATIONS OF SAMASARKARA CHURNA
| Phyto-
constituents |
Formulations | |
| Std. | Test | |
| Alkaloids | + | + |
| Anthraquinone Glycosides | - | - |
| Phenolics | + | + |
| Carbohydrates | + | + |
| Flavonoids | + | + |
| Tannins | + | + |
| Saponins | - | - |
| Coumarin s | - | - |
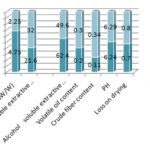
FIG. 5: PHYSICOCHEMICAL PARAMETERS OF STANDARD AND MARKET FORMULATIONS OF SAMASARKARA CHURNA
TABLE 3: ESTIMATION OF PHYTOCONSTITUENTS IN STANDARD AND MARKET FORMULATIONS OF SAMASARKARA CHURNA
| Phyto-
constituents |
Formulations | |
| Std. | Test | |
| Total tannin content (% w/w) | 1.6 | 1.7 |
| Total flavonoid content (% w/w) | 0.236 | 0.254 |
| Na+ ion salts (% w/w) | 0.9 | 1.1 |
| K+ ion salts (% w/w) | 1.2 | 1.4 |
TABLE 4: FLUORESCENCE ANALYSIS OF STD AND MKT FORMULATIONS OF SAMASARKARA CHURNA
| Treatment of
Powder |
Std. Formulation | Test Formulation | ||
| Daylight | UV light | Daylight | UV light | |
| 1N HCl | PY | FLG | YBR | FG |
| 1N H2SO4 | PY | FLG | YBR | FLG |
| 1N HNO3 | PY | FLG | YBR | FG |
| 1 N NaOH (aq.) | RBR | FG | BR | FDG |
| 1N NaOH (alcoholic) | YBR | FDG | BR | FDG |
| I2 | GBR | FDG | GBR | FDG |
| KOH | YBR | FG | RBR | FG |
| NH3 | YBR | FG | RBR | FDG |
PY-pale yellow, YBR- yellowish brown, GBR- greenish brown, RBR-reddish brown, BR- brown, FLG- fluorescence light green, FG- fluorescence green, FDG- fluorescence dark green.
TABLE 5: CONTENT AND DETAILS OF CHURNA 14-35
| Botanical name | Family | Taxonomy | Vernacular
name |
Distribution | Chemical Constituents | Medicinal Properties and uses | |||
| Ginger | Z.
officinale Wild |
Zingiber-aceae | Kingdom | Plant | Sans. | Ardraka, Sunthi | West Indies, India, Nigeria, and West Africa. Madras Cochin, Travancore, somewhat less extent in Bengal and Punjab | Gingerol, fats and waxes, volatile oil
Contains zingiberene, β- sesquiphell-andrene, and arcurcumene
|
Thermogenic, carminative, laxative, digestive, emollient, appetizer, stoma-chic, expectorant, anthelmintic, anti-ulcer, antifungal useful in asthma, cough, diarrhea, cholera, nausea, vomiting |
| Division | Phanerogams | Eng. | Ginger | ||||||
| Sub division | Angiosperm | Hin. | Adrak, Sunth | ||||||
| Class | Monocoty-
ledons |
Guj. | Sunth, Adu | ||||||
| Order | Scitamineae | Ben. | Adu | ||||||
| Species | Officinale | Tam. | Allamu, Sunti | ||||||
| black Piper | P. nigrum Linn. | Piperaceae | Kingdom | Plant | Sans. | Milagu | South Africa, Indonesia, Brazil, Malasia, and Sri Lanka, In india cultivation and collection carried out in Kerala, Karnataka, and Maharashtra | Piperine, volatile oil contains l-phellandrene, caryophyllene, limonene, sabinene, β/ α-pinene, myrecene, p-cymeme | Aromatic, stimulant, stomachic and carminative, oil can be used to help in the treatment of pain relief, rheumatism, chills, flu, colds, increase circulation, exhaustion, muscular aches |
| Division | Phanerogams | Eng. | Black pepper | ||||||
| Sub
division |
Angiosperm | Guj | Golmirch | ||||||
| Class | Dicot | Tam | Maricha | ||||||
| Subclass | Archichl
amydeae |
Beng. | Golmorich | ||||||
| Order | Piperales | ||||||||
| Genus | Piper | ||||||||
| Species | nigrum | ||||||||
| Long Piper | P. longum | Piperaceae | Kingdom | Plant | Eng. | Long
pepper |
Indonesia, India and the Philippines.piperlongum is available in Tamil Nadu, Andhra Pradesh and Kerala states | Volatile oil
piperine, piperattine resin, piperidine and starch, volatile oil contains l-phellandrene and caryophyllene |
Immunomodulating, antiallergic, antiasthmatic, fruits are used as aromatic, stimulant, stomachic and carminative |
| Division | Phanerogams | Hin. | Pippal | ||||||
| Sub
division |
Angio-sperm | Sans. | Pippali | ||||||
| Class | Dicot | Guj | Pippal | ||||||
| Subclass | Archichla-mydeae | ||||||||
| Order | Piperales | ||||||||
| Genus | Piper | ||||||||
| Species | longum | ||||||||
| Clove
|
E. caryophyllus
Sprange |
Myrtaceae | Kingdom | Plant | Sans. | Lavangaha | Zanzibar, Pemba, Madagascar, Carribian islands, Sri Lanka. In India, cloves are grown jn Nilgiri, Tenkasi hills and Tamil Nadu | Volatile oil, tannins, various triterpene acids and esters, and glycosides, eugenol, iso-eugenol, farnesol, nerolidol, sitosterol, and campesterol | Potential anticarcinogenic, used as a dental analgesic, flavoring agent, antiseptic and carminative. The oil is used in perfumery and in the preparation of vanillin |
| Division | Phanerogams | Eng. | Clove | ||||||
| Subdivision | Angio-sperm | Hin. | Laung | ||||||
| Class | Dicot | Guj. | Laving | ||||||
| Subclass | Archichia-mydeae | Ben. | Lavang | ||||||
| Order | Myrtiflorae | Tam. | Lavanga-patti | ||||||
| Genus | Eugenia | ||||||||
| Species | caryophyllus | ||||||||
| Nutmeg | M. fragrans Houtt. | Myristica-ceae | Kingdom | Plant | Sans. | Jatiphalam | Indonesia, Malasia and Carribian islands, in India it is cultivated in Kerala, Tamil Nadu
|
Volatile oil, fat, phytosterin, starch, amylodextrin, coloring matter, and saponin
|
Used as aromatic, stimulant and carminative, used in soap industries, the treatment of infantile diarrhea |
| Division | Phanerogams | Mar. | Jayphal | ||||||
| Subdivision | Angio-sperm | Hin. | Jayphal | ||||||
| Class | Dicot | Guj. | Jayphal | ||||||
| Subclass | Archichia-mydeae | Ben. | Jayphal | ||||||
| Order | Magnoli-ales | Tam. | Jajikaya | ||||||
| Genus | Myristica | ||||||||
| Species | fragrans | ||||||||
DISCUSSION & CONCLUSION: Samasarkara Churna; an Ayurvedic formulation has been standardized by the intervention of contemporary scientific quality control actions in the traditional research described in conventional texts. Pharmacognostic appeals established for the raw materials could be employed as Q.C. standards for evaluating its identity and can be used for repetitive analysis. Purity and potency of the materials and formulations; following the procedure given could be performed in QC/QA laboratory of a pharmaceutical firm. In the present study, two different polyherbal formulations of Samasarkara churna was taken, and they were evaluated as per Indian Pharmacopoeia and WHO guidelines for their different properties like - organoleptic, extractive values (alcohol and water), Ash values (Total ash), physical characteristics (tapped and bulk density, Hausner’s ratio and Carr’s index), phytochemical evaluation, fluorescence analysis. Organoleptic studies revealed that altogether batches (Standard and Test) of Samasarkara Churna were brown with a pungent odor and sweet taste. More than 90% of these samples (Standard and Test) passed through 60- mesh sieve.
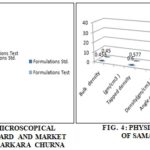
The extractive values (% w/v) of Samasarkara Churna (std. and test) in water and alcohol were found to be (62.4%, 49.6%); (21.6%, 32%); respectively, Fig. 5 it confirms that water-soluble contents are more in the Samasarkara churna. Ash values (% w/w) of Samasarkara churna (std. and test) were found to be 4.75 and 2.25 respectively and volatile oil content of std. and test for mutations were found to be 0.2% w/v and 0.3% w/v respectively. And as per Table 2 crude fiber content, pH of 1% w/v solution of churna and loss on drying were found to be (0.11, 0.34), (6.24, 6.29) and (0.7, 0.8) respectively.
The data of Table 5 shows that; physical characteristics of Samasarkara Churna like-Bulk density (g/ml), tapped density (g/ml), angle of repose, Hausner’s ratio and Carr’s index is found to be (0.453,0.45); (0.6,0.577); (32.14, 33.10); (1.32, 1.28); (24, 28.5) respectively. Low values of angle of repose show the poor flowability for all samples. As per Table 5; UV light and fluorescence were observed in both of the std. and test formulation. It is clear from Table 3 that active constituents like glycosides, carbohydrates, steroids, tannins, and saponins are present and the total tannin content in Samasarkara churna (std. and test) was found to be 1.6 and 1.7 %w/w respectively. Total flavonoid content estimated for both std. and Test Samasarkara churna which were found to be 0.236 and 0.254% w/w respectively. Na+ ion salts and K+ ion salts were quantified which were found to be (0.9, 1.1% w/w) and (1.2, 1.4% w/w) respectively.
After analysis of a sample of Samasarkara churna by different parameters such as total ash, insoluble acid ash, water-soluble extractive, alcohol-soluble extractive, lipid content, volatile oil content, and microscopic analysis, the phytochemical analysis showed reproducible results between batches. So it can be concluded that these parameters can be used for the evaluation of Samasarkara churna. The same protocol may be applied for as regular quality control and standardization for various polyherbal formulations
SUMMARY: Two consignments of different polyherbal formulation Samasarkara churna which purchased from the local market were evaluated as per Indian Pharmacopoeia and WHO guidelines. Different parameters like - organoleptic characteristics, extractive value, ash value, physical characteristics, phytochemical evaluation, fluorescence analysis, pH value, etc. were evaluated and compared. The result of Samasarkara churna was found nearby. This study on Samasarkara churna was precise, reproducible and may be considered as a protocol for its evaluation. Present methods can draw a parallel for evaluation for other Ayurvedic formulations. The same protocol may be applied for as regular quality control and standardization for polyherbal formulations like churna.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Patwardhan B, Vaidya A and Chorghade M: Ayurveda and natural products drug discovery. Current Science 2004; 86(6): 789-99.
- Waxler M: Plural medicine in India and Sri Lanka: do Ayurvedic and Western medical practices differ. Soc Sci Med 1988; 27: 531-544.
- Ray DR: Ayurdiya Kriyasharir, Sh. Vednath Ayurved Bhawan Ltd, Edition Ist, 1953; 547-675.
- Vaidhya PL: Ayurved kamahangranth Shree Bhishjyaratnavali, Savitri thakur prakashan, Varanasi, 169.
- Raval MK, Shah VK, Zalavadia VI, Seth NR, Dudhrejiya AV and Golawala K: Standardization and assessment of preformulation parameter of rasayana tablet. International J of Pharmaceutical Science and Drug Res 2010; 2(1): 58.
- Jain S, Koka S, Gupta A, Barik R and Malavia N: Standardization of Chopchiniyadi churna: An Ayurvedic formulation. Journal of Pharmacognosy 2010; 2(5): 60.
- Kokate CK, Purohit AP and Gokhle SB: Pharmacognosy Nirali prakashan, Mumbai, Edition 39th, 2007: 566-571.
- Pattnayak P, Hardel DK and Mahapatra P: Standardization of Vaisvanara churna: A Polyherbal formulation. Journal of Pharmacognosy 2010; 2(5): 50.
- Vaidhya PL: Shree Bhaisajyaratnavali. Savita Prakashan. 179.
- Kokate CK, Purohit AP and Gokhle SB: Pharmacognosy. Nirali prakashan, Mumbai, Edition 39th, 2007: 408.
- Evans WC: Trease and Evans Pharmacognosy, Elsevier, a reed Elsevier India Pvt Ltd., Edition 15th, 2005: 20-30.
- Indian Medicinal Plants. International Book Distributor, Dehradun, Edition 2nd, 1999; 4: 2435.
- Kokate CK, Purohit AP and Gokhle SB: Pharmacognosy Nirali Prakashan, Mumbai, Edition 39th, 2007: 409
- Wallis TE: Text Book of Pharmacognosy, CBS Publication, Delhi, Edition 5th, 2005; 392.
- Indian Medicinal Plants. International Book Distributor, Dehradun, 3122 Anonymous. Indian Herbal Pharmacopoeia Revised new edn, Published by Indian drug manufacturer’s association: Mumbai, 1999, Edition 2nd, Vol. III, 2002; 439.
- Evans WC: Trease and Evans Pharmacognosy, W. B. Saunders: London, Edition 15th, 2002; 27.
- Ratnam V: Indian medicinal plants, a compendium of 500 species, Orient Longman, P.S. Varier’s Aryavaidyasala: Kottakal, 1996; 5: 263.
- Handa SS and Kapoor VK: Pharmacognosy, Vallabh Prakashan: Delhi; 115 Basu BD. (1975) Indian Medicinal Plants: Plates. Part-II, M/S Periodical Experts: New Delhi 1989; 413.
- Kirtikar KR and Basu BD: Indian Medicinal Plants. Lalit Mohan Basu Prakashan: Allahabad, India. Edition 2nd, Vol. II, 1975: 1020-1023.
- Pharmacopoeia of India, Ministry of health and family welfare, Government of India: New Delhi 1966; 471.
- The Ayurvedic Pharmacopoeia of India, Part-I, Govt. Of India, Ministry of health and family welfare, Dept. of health: New Delhi, Edition 1st, 1986; 1: 47-48.
- The Wealth of India: National Institute of Science Communication and Information Resources, New Delhi, Edition 1st, 2004; 4: 319.
- Evans WC: Trease and Evans Pharmacognosy. Elsevier, a reed Elsevier India Pvt Ltd., Edition 15th, 2005; 353-354.
- Indian Medicinal Plants. International Book Distributor, Dehradun, Edition 2nd, 1999; 3: 3128.
- The Wealth of India: National Institute of Science Communication and Information Resources, New Delhi, Edition 1st, 2004; 4: 318.
- Evans WC: Trease and Evans Pharmacognosy. Elsevier, a reed Elsevier India Pvt. Ltd., Edition 15th, 2005; 353-354.
- Bhaskaran S: Physical Pharmaceutics. Birla Publication Pvt. Ltd., Delhi, Edition 1st, 2006-07: 107.
- Lachman L and Lieberman HA: Theory and Practice of Industrial Pharmacy, Indian Edition, CBS Publication and Distributors, New Delhi, 2009; 67-68.
- Subramanyam CVS and Vasanthraju SG: Laboratory Manual of Physical Pharmacy, Vallabh Prakashan, New Delhi, Edition 2nd, 2005: 65-67.
- The Ayurvedic Pharmacopoeia of India, Part: I, Published By Controller of Publication, Delhi, Edition 1st, 2: 190-191.
- Jain S, Koka S, Gupta A, Barik R and Malavia N: Standardizations of Chopchiniyadi churna: An Ayurvedic formulation. Journal of Pharmacognosy 2010; 2(5): 61.
- Khandelwal KR: Practical Pharmacognosy technique and experimental, Edition 5th, 2007: 149-153.
- Kokate CK, Purohit AP and Gokhle SB: Pharmacognosy Nirali Prakashan, Mumbai, Edition 39th, 2007: 461.
- Winter CA, Risley EA and Nuss GW: Proc Soc Exp Biol Med 1962; 111: 544-547.
- Pattnayak P, Hardel DK and Mahapatra P: Standardization of Vaisvanara Churna: A Polyherbal formulation. Journal of Pharmacognosy 2010; 2(5): 52.
How to cite this article:
Naphade VD, Bendale AR, Narkhede SP, Narkhede SB and Jadhav AG: Phytopharmacognostical investigation of Samasarkara churna. Int J Pharmacognosy 2018; 5(4): 249-55. doi link: http://dx.doi.org/10.13040/ IJPSR.0975-8232.IJP.5(4).249-55.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
249-255
769
1577
English
IJP
V. D. Naphade *, A. R. Bendale, S. P. Narkhede, S. B. Narkhede and A. G. Jadhav
Sandip Institute of Pharmaceutical Sciences, Nashik, Maharashtra, India.
vaishalinaphade587@gmail.com
06 December 2017
05 January 2018
13 February 2018
10.13040/IJPSR.0975-8232.IJP.5(4).249-55
01 April 2018