PHYTOCHEMISTRY AND ETHNOPHARMACOLOGY OF LIPPIA GENUS WITH A STATEMENT ON CHEMOTAXONOMY AND ESSENTIAL OIL CHEMOTYPES
HTML Full TextPHYTOCHEMISTRY AND ETHNOPHARMACOLOGY OF LIPPIA GENUS WITH A STATEMENT ON CHEMOTAXONOMY AND ESSENTIAL OIL CHEMOTYPES
Samuel Ehiabhi Okhale *, Ezekwesiri Michael Nwanosike, Omolola Temitope Fatokun and Oluyemisi Folashade Kunle
Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, P. M. B. 21, Garki, Abuja, Nigeria.
ABSTRACT: Lippia is a genus of flowering plants belonging to the Verbenaceae family. It contains about 220 species with diverse ethnopharmacological applications. Myriad of biologically active phytoconstituents abound in Lippia. The essential oil chemotypes found in Lippia species included myrcenone rich-type, carvone rich-type, piperitenone rich-type, ipsenone rich-type, linalool rich-type, citral rich-type, carvacrol rich-type, thymol rich-type and lippiol rich-type. Other constituents apart from essential oils isolated and chemically characterized were highlighted. β-caryophyllene and iridoid glycosides were notable as chemotaxonomic marker compounds which were common to many of Lippia species.
| Keywords: |
Lippia, Phytochemistry, Essential oils, Classification, Chemotypes, Chemotaxonomy
INTRODUCTION: Chemotaxonomy refers to the investigation of the distribution of groups of biosynthetically related chemical compounds in a series of related or supposedly related plants 1. It can also be loosely defined as the identification and classification of organisms by comparative analysis of their biochemical composition. A wealth of information can be sorted out by placing plant genera in the chemotaxonomic context. Chemotype is generally defined as a distinct population within the same species (plant or microorganism) that produces different chemical profiles for a particular class of secondary metabolites 2.
Lippia is one of 41 genera of flowering plants belonging to the family Verbenaceae 3. It contains roughly 220 species of tropical shrubs, herbs, and trees that are widely distributed around the world 4. Some of these species include adonensis (kere), multiflora (bush tea), lugosa, organoides, graveolens, chevelieri, alba, javanica 5, sidiodes, gracilis, citriodora 6, among others. Below are herbarium samples of Lippia chevalieri Fig. 1 and Lippia multiflora Fig. 2.
In this review, essential oils and non-essential oil constituents were investigated toward identification of essential oil chemotypes and chemotaxonomic markers in the Lippia genus. Essential oils showed great abundance and overwhelming chemical diversity in Lippia.
Chemotypes from other classes of secondary metabolites have not been extensively isolated, studied and documented when compared to the essential oils. By and large, this review seeks to classify various species of Lippia into chemical groups, using essential oils to show chemotypic variation.
Ethnopharmacology: Plants belonging to the Lippia genus have been widely used in ethnobotany throughout South and Central America and in tropical Africa as foods, medicines, sweeteners and in beverage flavoring 7. The Lippia species have a long history of use in traditional medicinal applications some of which have been scientifically validated. They are mostly used in the treatment of respiratory and gastrointestinal disorders. Additionally, they exhibit anti-malarial, spasmolytic, sedative, hypotensive and anti-inflammatory activities 8, 9, 10. The essential oils of L. alba had been reported to possess anti-spasmodic, digestive, anti-hemorrhoidal and anti-asthmatic activities 11. Also, atifungal, antibacterial, antiviral, anti-inflammatory and cytotoxic activities have been reported for L. alba 12, 13, 14, 15, 16.
The cytotoxic and antitumor effects of L. alba extracts and some significant components of its essential oils, such as limonene and citral, have been demonstrated in HL-60 human promyelocytic leukemia cells, K562 human erythroleukemic cells, HepG2 human hepatocellular liver carcinoma cells and HeLa human cervix epithelioid carcinoma cells 17, 18, 19. L. gracilis also exhibits antitumor activity 20. L. multiflora leaves and inflorescence are widely used as spices in cooking and traditional medicine.
The leaf is used as a vegetable, and the flower with seed are used for soup. It has been used in many ethnopharmacological applications to ameliorate bronchial inflammation, malaria fever, conjunctivitis, gastrointestinal disturbance, enteritis, coughs and colds 21. L. multiflora possesses hypotensive, fatigue relieving, and diuretic properties 22.
It has also been used as a substitute for tea and as a mouth disinfectant 23. Analgesic and antipyretic activities have also been documented for L. multiflora. Lippia adoensis extracts were used medicinally by a variety of indigenous people for treatment of skin infection 24. Lippia species are also useful in culinary seasoning and as insect repellants; L. dulcis has demonstrated anti-proliferative activity in vitro in different cancer cells 25. Virucidal activity has also been demonstrated L. dulcis 26. It was recently reported that three compounds from L. javanica were able to inhibit the HIV-1 reverse transcriptase enzyme 27. The table below comprehensively captures the ethnopharmacological applications of Lippia species Table 1. Meanwhile, the medicinal effects of L. multiflora have been attributed to essential oils, glycosides and other phytochemical components 28.
TABLE 1: TRADITIONAL USES AND PHARMACOLOGICAL ACTIVITIES OF LIPPIA SPECIES
| Species | Traditional uses | Pharmacology | References |
| L. affinis sidoides Cham. ;
L. alba (Mill.) N. E. Brown |
A, B, C, D, E, G, H, K, Q, R, S |
O
A, B, D, M, N, T, V |
29
30, 31, 32, 33 34, 35, 36, 38 |
| L. geminata H. B. K | A, B, D, G, K, Q, S | A | 33, 37, 38 |
| L. aristata Schau. | O | 29 | |
| L. chevalieri Moldenke | B,P, R, S | P | 38 |
| L. citrodora (Ort) HBK | A, B, C, G, I, Q | A, B, Y | 39, 40, 41, 42 |
| L. dulcis Trevir | A, C, D, G, I, K, R, W | C, M | 30, 43,44,45, 46 |
| L. formosa T. S Brandgee | M | 47 | |
| L. gracilis HBK | E | M | 48 |
| L. grandifolia Hochst. | L,M | 59 | |
| L. grata Shau | Q | 50 | |
| L. graveolens HBK | A, C,D, K, Q, R, U, W | 30, 47, 51 | |
| L. javanica (N.L Burm) Spreng. | A, Q, R | L, M | 52, 43 |
| L. micromera Shau | C, G, I, R | 30 | |
| L. multiflrora Moldenke | H, J, P, R | F, J, N, P | 53, 39, 28, 54, 9 |
| L. nodiflora (L.) Michx | A, I, K, M, P, R, Q, M, S | M | 55, 38 |
| L. organoides H.B.K | C, G, R | 30 | |
| L. palmeri S. Wats | F, M | 49 | |
| L. reptans H.B.K | D, G | 56 | |
| L. sidoides Cham | B, F, J, M, Q, Z | 57, 52, 50 | |
| L. somalensis Vatke | L | 51 | |
| L. turbinata Griseb | G | 58 | |
| L. ukambensis Vatke | L, M | 51 |
A: Analgesic/anti-inflammatory/antipyretic; B: Sedative; C: Culinary seasoning; D: Remedy for diarrhea and dysentery; E: Cutaneous diseases treatment; F: Antifungal; G: Remedy for gastrointestinal disorders; H: Remedy for hepatic/cholerectic/vesicle disorders; I: Diuretic; J: Antihypertensive; K: Menstrual disorders remedy; L: Larvicidal/repellant; M: Antimicrobial; N: Antiviral; O: Molluscicidal; P: Antimalarial; Q: Antispasmodic; R: Respiratory diseases treatment; S: Treatment of syphilis and gonorrhea; T: Cytostatic; U: Antidiabetic; V: Anticonvulsant; W: Abortifacient; X: Stimulant; Y: Pro-convulsant; Z: Local anesthetic.
Phytochemistry: The genus Lippia consists of nearly 220 species of herbs, shrubs and small trees which are often aromatic. Of these, thirty-nine species have had some previous work done on their essential oil compositions. Some species of Lippia are composed of a wide variety of chemically variable, volatile compounds that present biological properties 21, 59, 60. The phytoconstituent which was found to occur in the highest frequency in Lippia essential oils was limonene. Other components found in these oils, in order of decreasing frequency, were: p-cymene, α-pinene, camphor, β-caryophyllene, linalool, thymol and carvacrol 61. Essential oils are aromatic, or odoriferous, oily liquids, sometimes semi-liquid or solid, obtained from plant material, for example, flowers, buds, seeds, leaves, twigs, bark, herbs, woods, fruits, and roots. Depending on the kind of oils and the quality, essential oils can be used in different industries.
Essential oils are applied in the food industry as a flavoring, the perfume industry for fragrances, and the pharmaceutical industry for adding taste or smell or suppressing the less desirable medicated flavor. The use of essential oils in health care are called ‘aromatherapy.’ The knowledge of chemical constituents of essential oils is of fundamental importance to the pharmaceutical, food, and perfumery industries. As the use of aromatic compounds requires detailed chemical characterization and evaluation of possible modifications within their compositions, which are due to the different geographical origins and climatic conditions and different population genetics that can lead to the formation of different chemotypes 62, 63, 64.
Phytochemical studies by several researchers have shown the presence of essential or volatile oil in the aerial part of L. Multiflora, which has been extracted and characterized by some workers using instrumental methods like hydro-distillation and Gas Chromatography-Mass Spectrometry (GC-MS). Analysis of the oil by GC-MS revealed, among others, the presence of terpineol, α- and β-pinene which are known to be lethal to lice 65. Others have also used traditional classical methods of maceration in organic solvents to isolate the oil or components of the oil and then characterize by using GC-MS and other spectrometric methods like Nuclear Magnetic Resonance (NMR) and Infra-Red spectrometry 66, 10.
Investigators have widely studied the oil which is believed traditionally to possess some pharmacotherapeutic activities in the field. The chemical composition has necessitated the classification of the oil into different chemotypes to guide its identification and application 67. Some chemotypes could be seen along the monoterpenoid (rich in thymol and its derivatives, ρ-cymene, and carvacrol) and sesquiterpenoid (rich in ipsdienone and ocimenone isomers) 68. Another classification placed the analyzed samples into five chemotypes namely, linalool (29%) and germacrene D (28%) rich oil, 1,8-cineole (43-47%) and sabinene (12-15%) rich oil, high farnesol (camphoraceous) rich oil, high sesquiterpenes (45-70%) rich oil and high monoterpenes rich oil (ρ-cymene 14-19%, thymol 30-40%, thymol acetate 14-17%) 69. It as clear that the variation in chemical composition could be due to factors bordering on environmental stress and genetics.
Additionally, GC analysis of extracts L. alba revealed most constituents belonging to the terpene class of hydrocarbons: Chemotype 1 (citral, β-myrcene, limonene), Chemotype 2 (citral, limonene), Chemotype 3 (carvone, limonene) 70. Non-essential oil constituents found in L. alba include iridoids and phenylpropanoids in the roots (theveridoside, muscaveroside). Essential oils of L. javanica are classified into 5 chemotypes based on the mono and sesquiterpene contents 71. Some iridoid glycosides including theveridoside were isolated from L. javanica and were considered as chemotaxonomic markers for the genus Lippia 72.
According to Oliviera and co-workers 83, Essential oils from L. organoides show a high content of oxygenated monoterpenes (66.0%), monoterpene hydrocarbons (20.7%), sesquiterpene hydrocarbons (9.0%) and oxygenated sesquiterpenes (1.1%). The two major compounds among monoterpenes were carvacrol (38.6%) and thymol (18.5%); among sesquiterpene hydrocarbon was (E)-caryophyllene (5.9%).
The major constituents of L. sidoides are thymol and carvacrol. Other important ones include the oxygenated monoterpene 1,8-cineole, isoborneol and bornyl acetate 63. On a another note, the main constituents of L. citriodora include geranial, neral, and limonene constituting 66.3% of the total essential oil yield in May and increasing to 69% in September 73, 74, 75, 76. In the separate study, the essential oil of Lippia citriodora revealed cis-sabinene hydrate (38.99%), spathulenol (10.4), cuparene (6.81%), α-terpineol (5.05%), geranyl acetate (3.91%), β-pinene (3.46%) and E citral (3.4%) as the major Compounds identified. Oxygenated monoterpene group was predominant in the essential oil of Lippia citriodora 6. Citral, a mixture of the E- and Z-isomers, was found to be the main constituent of the L. rehmannii essential oils, while borneol, camphor, neryl acetate, isocaryophyllene, p-cymene, β-caryophyllene, and β-caryophyllene oxide were other major compounds identified 77. L. gracilis essential oils had thymol is a major bioactive component 20.
L. graveolens essential oil contained 45 chemical compounds, and the main components were carvacrol, thymol, eugenol, ocimene, pinene, and linalool, among others 78. On the other hand, essential oil of the leaves of L. chevalieri is composed mainly of thymol (27.4%), p-cymene (21.1%), and 2-phenyl-ethyl-propionate (12.6%), while the oil from the flower is composed of β-elemene (33%), ethyl cinnamate (30.3%) and α-amorphene (12.4%) 79. Aromatic volatile oil of L. microphylla was rich in monoterpenes, especially cineole, terpineol and thymol 80.
TABLE 2: OTHER CHEMICAL CONSTITUENTS OF LIPPIA SPECIES
| Species | flavonoids | Other compounds | References |
| L. alba (Mill.) N.E Brown | Flavonoid 4-sulphates | Tannins (low), geniposide (iridoid), triterpenic saponins, resin, mucilage, alkaloids, saponins, sterols | 31, 32, 37, 38 |
| L. canescens Kunth | Flavone aglycones, flavones mono- and di-sulphates | 81 | |
| L. citroidora (Ort.) H.B.K | Salvigenin, eupatorin, eupafolin, hispidulin, 6-hydroxyluteolin, 7- O- β -glucoside, luteolin, cismaritin, diosmetin,apygenin | Verbacosides | 82, 83 |
| L. dulcis Trevir. | Verbacosides, alkaloids | 48 | |
| L. graveolens H.B.K | Naringenin and pinocembrin | lapachenol | 53 |
| L. javanica (N.L Burm.) Spreng. | icterogenin | 43 | |
| L. multiflora Moldenke | Flavonoids | Verbacoside, isoverbacoside, sterols, carotenoids | 54, 55 |
| L. nodiflora (L.) Michx | Nepetin, jaceocidin and hispidulin aglycones, lippiflorin A and B glycosides, nodiflorin A and B, | Alkaloids, resin, sugars, stigmasterol, β-sitosterol | 83, 38 |
| L. rehmanii H.H.W. Pearson | Tritrerpenic compounds, icterogenin, rehmannic acid | 41 | |
| L. sidoides Cham | 6,7- dimethoxy-5,4I-dihydroxyflavone | Naphthoquinoids, lapachenol, isocatalponol | 58 |
| L. turbinata Griseb | Leucoanthocyanidins, steroidic and triterpenic compounds, alkaloids and cardenolides (traces) | 59 |
Oliviera and co-workers 83 described the preliminary stage for chemotyping of Lippia species to involve collection and drying of identified aerial parts of plant specimens. This was followed by extraction of essential oil from the leaves by hydrodistillation in a Clevenger-type apparatus for 4 hours with 1.5 L of water; with a good yield of about 1.0% v/w. The essential oil was dried with anhydrous sodium sulfate and stored at 4 ºC. Analysis of extract was also carried out using the GC-MS technique 67.
Chemically, essential oil constituents belong to the following classification namely hydrocarbons, monoterpenes, oxygenated monoterpenes, sesqui-terpenes, oxygenated sesquiterpenes, diterpenes, oxygenated diterpenes, triterpenes, oxygenated triterpene, aromatic compounds, alcohols, fatty acids, ketones and heterocyclic compounds 85, 86, 87, 88, 89, 90. Common chemotypes for Lippia essential oil are compiled in Table 3.
TABLE 3: MAIN ESSENTIAL OIL CHEMOTYPES OF LIPPIA SPECIES
| Plants | Monoterpenes | Sesquiterpenes | References |
| L. affinis aristata Schau | Sabinene, limonene, p-cymene,
α-pinene, γ-terpinene |
β-caryophyllene,
α-cadinene, γ-elemene |
29
62 |
| L. adonensis Hochst | α-terpineol, β-pinene, γ-terpinene, carvone, 1,8-cineole, p-cymene, limonene, linalool, thymol | δ-cadinene,
β-caryophyllene, nerolidol, germacrene-D |
62 |
| L. alba (Mill.) N. E. Brown | Borneol, camphor, 1,8-cineole, geranial, myrcene, linalool, neral, sabinene | β-caryophyllene,
β-elemene, γ-cadinene, α-muurolene |
29
30 62
|
| L. citroidora Kunth. | Citral- A, citral-B, geraniol,
1,8-cineole, linalool, limonene |
Caryophyllene oxide | 62
85 |
| L. dulcis Trevir. | Camphor, camphene, limonene, terpinolene, α-pinene, lippiol | α-copaene,
β-caryophyllene, δ-cadinene, (+)-hernandulcine |
48
45,48 62 91 |
| L. gracilis HBK | Thymol, cavacrol, p-cymene,
4-terpenil-acetate |
α-copaene,
β-cubebene |
50 |
| L. graveolens H.B.K | β-phellandrene, cavacrol,
p-cymene, methylthymol, thymol |
α-humulene,
β-caryophyllene, β-bisabolene, aromadendrene |
49
|
| L. javanica (N.L Burm.) Spreng. | Myrcene, myrcenone, ocimene, (E)-tagetenone, cis-tagetone | β-caryophyllene | 62 |
| L. multiflora Moldenke | 1,8-cineole, linalool | Nerolidol
β-farnesene, β-caryophyllene, germacrene-D |
28 |
| L. nodiflora (L.) Greene | 2-phenethyl alcohol, 1-octen-3-ol, linalool, 2,6-dimethyloctane, methylsalicylate, p-cymen-8-ol | Calamenene,
β- caryophyllene, α-copaene, δ-cadinene, α-bergamotene, β-bisabolene, β-caryophyllene, umbellulone |
62
|
| L. organoides H.B.K | 1,8-cineole, α-terpinene,
γ-terpinene, p-cymene |
β-caryophyllene, umbellulone | 62
|
| L. sidoides Cham
|
p-cymene,
cavacrol, α-terpinene, thymol
|
α-copaene
β-caryophyllene, α-humulene |
50
62 |
| L. turbinata Griseb | α-thujone, carvone, limonene, bornyl acetate, camphor | β-caryophyllene oxide,
β-cubebene, spanthulenol, β-caryophyllene germacrene-D |
62 |
| L. ukambensis Vatke | Terpineol, δ-3-carene, camphene, camphor, 1,8-cineole, p-cymene, trans-sabinene hydrate,
terpinen-4-ol |
β-cubebene | 62
51 |
The range of major volatile constituents reported in Lippia were hydrocarbons (pinene, limonene, isabolene), alcohols (linalol, santalol), acids (benzoic acid, geranic acid), aldehydes (citral), cyclic aldehydes (cuminal), ketones (camphor), lactones (bergaptene), phenols (eugenol), phenolic ethers (anethole), oxides (1,8 cineole) and esters (geranyl acetate) 92. Most phytochemical studies of Lippia have concentrated on the chemistry of the volatile constituents, resulting in limited information being available on the non-volatile secondary metabolites 93.
The species of Lippia contain a varying number of chemotypes (depending on the geographical source), some of which contain more of particular chemotypes than the other. For example, Lippia alba has about 12 chemotypes, and GC analysis of essential oils from this specie reveals the predominance of monoterpene compounds such as citral, β-myrcene, limonene, and carvone. Three of the 12 chemotypes are iridoides; others include flavonoid glycosides and phenylethanoid glycosides 94.
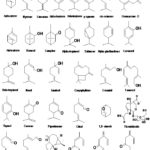
Meanwhile, Monoterpenes such as limonene, carvone, citral, β-caryophyllene, tagetenone, myrcene, γ-terpinene, camphor, 1,8-cineole, and estragole are frequently found in the essential oils of L. alba 95. Similarly, the essential oils of Lippia multiflora were characterized by richness in 1,8-cineole, sabinene, α-terpineol and α-pinene (out of at least 13 distinct chemotypes), most of which are distributed across Nigeria, Ghana and Togo 67. Kunle and Egharevba 66 gave a comprehensive review of the essential oil chemotypes found in Lippia multiflora. L. organoides was characterized by different chemical types: p-cymene, α- and β-phellandrene and limonene (chemotype A) 96, carvacrol (chemotype B) 97, 87, thymol chemotype C) 98, 100, 1,8-cineole (chemotype D) 99. Lately (E)-methyl cinnamate and (E)-nerolidol (chemotype E) 100 were identified by GC-MS analysis of Lippia ukambensis Vatke essential oil. Two chemotypes were identified by the camphor and 1,8-cineole in Lippia ukambensis Vatke on examination by GC/MS. On a separate note, the major compound in the oil of L. somalensis was 1, 8-cineole (31.9%) 51. Structure of some compounds found in Lippia species is shown in Fig. 1.
It is noteworthy that some iridoid glycosides including theveridoside were isolated from L. javanica and were considered as chemotaxonomic markers for the genus Lippia. However, the reasons for chemotaxonomy included the following: variable composition and structure of given determined chemical constituents; the percentage/ composition of any given compound in a plant would give the progression of a plant, species or genius; variation in chemical constituents can be exactly described in terms of definite structural configuration, and provides a way to understand their biosynthesis 101.
FIG. 3: STRUCTURE OF SOME COMPOUNDS FOUND IN LIPPIA SPECIES
CONCLUSION: The chemical compositions of Lippia species essential oils vary markedly giving rise to chemotypes. These depend on geographical factors, genetic factors, environmental conditions, nutritional status and the effects of mechanical damage or herbivory. By and large, limonene, p-cymene and β-caryophyllene cut across the essential oils of known species of Lippia, and could be considered chemotaxonomic markers. The chemical characterization of the oil is vital to determining the commercial value and potential application. Chemotypic variability is an essential factor in selecting essential oil-bearing medicinal plant for commercial development, especially in terms of chemical fingerprinting often required in quality control.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Vidita VB, Shashank CP and Kruti SD: Importance of Terpenoids and Essential Oils in Chemotaxonomic Approach. International Journal of Herbal Medicine 2013; 1(2): 14-21.
- Djilani A and Dicko A: The Therapeutic Benefits of Essential Oils, Nutrition, Well-Being and Health, Dr. Jaouad Bouayed (Ed.), InTech.
- Pooley E: A field guide to wildflowers: Kwazulu-Natal and the Eastern region. Durban: Natal Flora Publications Trust 1998.
- Arthur H, Joubert E, De Beer D, Malherbe CJ and Witthuhn RC: Phenylethanoid glycosides as major antioxidants in Lippia multiflora herbal infusion and their stability during steam pasteurization of plant material. Food Chemistry 2011; 127: 581-588.
- Retief E and Lippia L. In: Germishuizen, G., Meyer, N.L., Steenkamp, Y., Keith, M. (Eds.), A Checklist of South African Plants. South African Botanical Diversity Network Report No 41. Sabonet, Pretoria 2006.
- Yousefzadeh N and Meshkatalsadat MH: A quantitative and qualitative study of bioactive compounds of essential oils of plant Lippia citriodora by use of GC-MS technique. Journal of Novel Applied Sciences 2013; 2(2S): 964-968.
- Funari CS, Eugster PJ, Martel S, Carrupt PA, Wolfender JL and Silva DH: High-resolution ultra-high pressure liquid chromatography-time-of-flight mass spectrometry dereplication strategy for the metabolite profiling of Brazilian Lippia species. J Chromatogr A 2012; 1259: 167-78.
- Chanh PH, Koffi Y and Chanh APH: Comparative hypotensive effects of compounds extracted from Lippia multiflora Planta Med 1988; 54: 294-296.
- Abena AA, Atipo-Ebata JK, Hondi AT and Diatewa M: Psychopharmacological properties of crude extract and essential oil of Lippia multiflora. Encephale 2001; 27(4): 360-364.
- Jigam AA, Akanya HO, Ogbadoyi EO, Dauda BEN and Egwim CE: In-vivo antiplasmodial, analgesic and anti-inflammatory activities of the leaf extract of Lippia multiflora Journal of Medicinal Plants Research 2009; 3(3): 148-154.
- Sousa DG, Sousa SDG, Silva RER, Silva-Alves KS, Ferreira-da-Silva FW, Kerntopf MR, Menezes IRA, Leal-Cardoso JH and Barbosa R: Essential oil of Lippia alba and its main constituent citral block the excitability of rat sciatic nerves. Braz J Med Biol Res 2015; 48(8): 697-702.
- Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV and Filho BPD: Screening of some plants used in Brazilian folk medicine for the treatment of infectious diseases. Mem Inst Oswaldo Cruz 2002; 97: 1027-1031.
- Costa MCCD, Aguilar JS, do Nascimento SC. Atividade citotóxica de extratos brutos de Lippia alba (Mill.) N.E. Brown (Verbenaceae). Acta Farm Bonaerense 2004; 23: 349-352.
- Andrighetti-Frohner CR, Sincero TCM, da Silva AC, Savi LA, Gaido CM, Bettega JMR, Mancini M, de Almeida MTR, Barbosa RA, Farias MR, Barardi CRM, Simoes CMO. Antiviral evaluation of plants from Brazilian Atlantic tropical forest. Fitoterapia 2005; 76: 374-378.
- Oliveira DR, Leitao GG, Santos SS, Bizzo DHR, Lopes D, Alviano CS, Alviano DS and Leitão SG: Ethnopharmacological study of two Lippia species from Oriximina, Brazil J Ethnopharmacol 2006; 108: 103-108.
- Paik SY, Koh KH, Beak SM, Paek SH and Kim JA: The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol Pharm Bull 2005; 28: 802-807.
- Ji J, Zhang L, Wu YY, Zhu XY and Sun XZ: Induction of Apoptosis by d-limonene is mediated by caspase-dependent mitochondrial death pathway in human leukemia cells. Leuk lymphoma 2006; 47: 2617-2624
- Wang XS, Yang W, Tao SJ, Li K, Li M, Dong JH and Wang MW: Effect of delta-elemene on Hela cell lines by apoptosis induction. Yakugaku Zasshi 2006; 126: 979-990.
- Melo JO, Fachin AL, Rizo WF, Jesus HCR, Arrigoni-Blank MF, Alves PB, Marins MA, França SC and Blank AF: Cytotoxic effects of essential oils from three Lippia gracilis Schauer genotypes on HeLa, B16, and MCF-7 cells and normal human fibroblasts. Genetics and Molecular Research 2014; 13(2): 2691-2697.
- Pascual ME, Slowing K, Caretero E, Mara KD and Villar A: Lippia: Traditional uses, chemistry and A Review Journal of Ethnopharmacology 2001; 76: 201-214.
- Kanco C, Koukoua G, N’Guessan YT, Fournier J, Pradère JP and Toupet L: Contribution à l’étude phytochimique de Lippia multiflora (Verbenaceae). C R Chemie 2004; 7: 1029-1032.
- Menut C, Lamaty G, Samaté D, Nacro M and Bessière JM: Contribution à l'étude des Lippia africaines: Constituants volatils de trios espèces du Burkina Faso. Rivista Italiana Eppos 1993; 11: 23-29.
- Wasihun Y, Adraro T and Ali S: Evaluation of antibacterial activity and phytochemical constituents of leaf extract of Lippia adoensis. Asia Pacific Journal of Energy and Environment 2014; 1(1): 45-53.
- Patricia E, Sandra ML, Laura VH, Jairo RM and Elena S: Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem Inst Oswaldo Cruz 2010; 105(2): 185-190.
- Ocazionez RE, Meneses R and Torres FA: Virucidal activity of Colombian Lippia essential oils on Dengue virus replication in-vitro. Mem Inst Oswaldo Cruz 2010; 105(3): 304-309.
- Mujovo SF, Hussein AA, Meyer JJM, Fourie B, Muthivhi T and Lall N: Bioactive compounds from Lippia javanica and Hoslundia opposite. Natural Product Research 2008; 22: 1047-1054.
- Valentin A, Pelissier Y, Benoit F, Marion C, Kone D, Mallie M, Bastide JM and Bessière JM: Composition and antimalarial activity in-vitro of volatile components of Lippia multiflora. Phytochemistry 1995; 40: 1439-1442.
- Craveiro AA, Alencar JW, Matos FJA, Andrade CHS and Machado MIL: Essential oils from Brazilian Verbenaceae genus Lippia. Journal of Natural Products 1981; 44: 598-601.
- Morton: Atlas of Medicinal Plants of Middle America, Springfield, Illinois, USA 1981; 1: 745-750.
- Wanzala W, Ahmed H, Wolfgang RM and Takken W: Repellent activities of essential oils of some plants used traditionally to control the Brown Ear Tick, Rhipicephalus appendiculatus. Journal of Parasitology Research 2014; 2014: 1-10.
- Chies CE, Branco CS, Scola G, Agostini F, Gower AE and Salvador M: Antioxidant Effect of Lippia alba (Miller) N. E. Brown. Antioxidants (Basel) 2013; 2(4): 194-205.
- Zamora-Martı´nez MC and Nieto de Pascual C: Medicinal plants used in some rural populations of Oaxaca, Puebla and Veracruz, Mexico. Journal of Ethnopharmacology 1992; 35: 229-257.
- Hennebelle T, Sahpaz S, Joseph H and Bailleul F: Ethnopharmacology of Lippia alba. J Ethnopharmacol 2008; 116(2): 211-22.
- Matos FJDA, Machado MIL, Craveiro AA and Alencar JW: Essential oil composition of two chemotypes of L. alba grown in Northeast Brazil. Journal of Essential Oil Research 1996; 8: 695-698.
- Klueger PA, Daros MR, Silva RM, Farias MR and De Lima TCM: Neuropharmacological evaluation of crude and semipurified extracts from Lippia alba N.E. Br. (Verbenaceae). Abstracts. International Joint Symposium. Chemistry, Biological and Pharmacological Properties of Medicinal Plants from the Americas. Poster Session 1997; 2: B23.
- Forestieri AM, Monforte MT, Ragusa S, Trovato A and Iauk L: Antiinflammatory, analgesic and antipyretic activity in rodents of plant extracts used in African medicine. Phytotherapy Research 1996; 10: 100-106.
- Gasquet M, Delmas F, Timo´n-David P, Keita A, Guindo M, Koita N, Diallo D and Doumbo O: Evaluation in-vitro and in-vivo of a traditional antimalarial ‘Malarial-5’. Fitoterapia 1993; 64: 423-426.
- Mamun-Or-Rashid ANM, Sen MK, Jamal MAHM and Nasrin S: A comprehensive ethnopharmacological review on Lippia alba International Journal of Biomedical Materials Research 2013; 1(1): 14-20.
- Schauenberg P and Paris F: Guı´a de las Plantas Medicinales. Ediciones OMEGA, Barcelona 1977: 266.
- Schauenberg and Paris. Guide to Medicinal Plants, Lutterworth Press, London
- Hutchings A and Van Staden J: Plants used for stress-related ailments in traditional Zulu, Xhosa and Sotho medicine. Part 1: Plants used for headaches. Journal of Ethnopharmacology 1994; 43: 89-124.
- Ballero M, Poli F and Santus M: Plants used in folk medicine of Monteleone (Northern Sardinia). Fitoterapia 1998; 69: 52-64.
- Compadre CM, Pezzuto JM, Kinghorn AD and Kamath SK: Hernandulcin: an intensely sweet compound discovered by a review of ancient literature. Science 1985; 227: 417-419.
- Compadre CM, Robbins EF and Kinghorn D: The intensely sweet herb, Lippia dulcis Trev: Historical uses, field inquiries, and constituents. Journal of Ethnopharmacology 1986; 15: 89-106.
- Compadre CM, Hussain RA and Enrı´quez RG: Volatile constituents of Montanoa tomentosa and Lippia graveolens. Volatile constituents of Montanoa tomentosa and Lippia graveolens. Planta Medica 1987; 53: 495-496.
- Kaneda N, Lee IS, Gupta MP, Soejarto DD and Kinghorn D: (+)-4_-Hydroxy-hernandulcin, a new sweet sesquiterpene from the leaves and flowers of Lippia dulcis. Journal of Natural Products 1992; 55: 1136-1141.
- Dimayuga RE and Keer Garcı´a S: Antimicrobial screening of medicinal plants from Baja California Sur, Mexico. Journal of Ethnopharmacology 1991; 31: 181-192.
- Lemos TLG, Matos FJA, Alencar JW, Craveiro AA, Clark AM and McChesney JD: Antimicrobial activity of essential oils of Brazilian plants. Phytotherapy Research 1990; 4: 82-84.
- Mwangi JW, Addae-Mensah I, Munavu RM and Lwande W: Essential oils of two Lippia ukambensis Vatke Chemotypes and Lippia somalensis Vatke in Kenya. Journal of Essential Oil Research 2011; 3(6): 413- 417.
- Souza-Brito ARM and Souza-Brito AA: Forty years of Brazilian medicinal plant research. Journal of Ethnopharmacology 1993; 39: 53-67.
- Domı´nguez XA, Sa´nchez H, Sua´rez M, Baldas JH and Gonza´lez MR: Chemical constituents of Lippia graveolens. Planta Medica 1989; 55: 208-209.
- Pham HC, Koffi Y and Pham HCA: Comparative effects on TXA2 biosynthesis of products extracted from Lippia multiflora Moldenke leaves. Prostaglandins, Leukotrienes and Essential Fatty Acids 1988; 34(2): 83-88.
- Taoubi K, Fauvel MT, Gleye J, Moulis C and Fouraste I: Phenyl propanoid glycosides from Lantana camara and Lippia multiflora. Planta Medica 1997; 63: 192-193.
- Mukherjee T: Antimalarial herbal drugs. A review. Fitoterapia 1991; 62: 197-204.
- Giron LM, Freire V, Alonzo A and Caceres A: Ethnobotanical survey of the medicinal flora used by the Caribs of Guatemala. Journal of Ethnopharmacology 1991; 34(2-3): 173-187.
- Macambira LMA, Andrade CHS, Matos FJA and Craveiro AA: Naphthoquinoids from Lippia sidoides. Journal of Natural Products Lloydia 1986; 49: 310-312.
- Bandoni AL, Mendiondo ME, Rondina RVD and Coussio JD: Survey of Argentine medicinal plants. I. Folklore and phytochemical screening. Journal of Natural Products 1972; 35: 69-81.
- Rahmatullah M, Jahan R, Azam FMS, Hossan S, Mollik MAH and Rahman T: Folk medicinal uses of Verbenaceae family plants in Bangladesh. Afr J Tradit Complement Altern Med 2011; 8(5 Suppl): 53-65.
- Mesa AC, Montiel J, Zapata B, Durán C, Betancur L and Stashenko E: Citral and carvone chemotypes from the essential oils of Colombian Lippia alba (Mill.) N.E. Brown: composition, cytotoxicity and antifungal activity. Mem Inst Oswaldo Cruz 2009; 104: 878-884.
- Terblanché FC and Kornelius G: Essential Oil Constituents of the Genus Lippia (Verbenaceae)-A Literature Review. Journal of Essential Oil Research 2011; 8(5): 471- 485.
- de Morais SR, Oliveira TLS and Bara MTF: Chemical constituents of essential oil from Lippia sidoides (Verbenaceae) leaves cultivated in Hidrolândia, Goiás, Brazil. International Journal of Analytical Chemistry 2012; 2012: 1-4.
- Gudaityte O and Venskutonis PR: Chemotypes of Achillea millefolium transferred from 14 different locations in Lithuania to the Controlled environment. Biochemical Systematics and Ecology 2007; 35: 582-59.
- Van Vuuren SF, Viljoen AM, Őzek T, Demirci B and Başer KHC: Seasonal and geographical variation of Heteropyxis natalensis essential oil and the effect thereof on the antimicrobial activity. South African Journal of Botany 2007; 73: 441-448.
- Oladimeji FA, Orafidiya OO, Ogunniyi TAB and Adewunmi TA: Pediculicidal and scabicidal properties of Lippia multiflora essential oil. Journal of Ethnopharmacology 2000; 72(1): 305-311.
- Owolabi MS, Akintayo O, Labunmi L, Oladimeji MO, William NS and Maria CP: Chemical composition and antibacterial activity of the essential oil of Lippa multiflora Moldenke from Nigeria. Rec Nat Pro 2009; 3(4): 170-177.
- Kunle OF and Egharevba HO: Essential oil of Lippia multiflora Moldenke: A review. Journal of Applied Pharmaceutical Science 2012; 62(01): 15-23.
- Agnaniet H, Makani T, Akagah A, Menut C and Bessière JM: Volatile constituents and antioxidant activity of essential oils from Lippia multiflora Moldenke growing in Gabon. Flavor Fragrance Journal 2004; 20(1): 34-38.
- Juliani HR, Simon JE, Quansah C, Asare E, Akromah R, Acquaye D, Asante-Dartey J, Mensah MLK, Fleischer TC and Dickson R: Chemical diversity of Lippia multiflora essential oils from West Africa. J Ess Oil Res 2008; 20: 49-54.
- Matos FJ, Machado MI, Craveiro AA and Alencar JW: The Essential Oil composition of two chemotypes of Lippia grown in Northeast Brazil. Journal of Essential Oil Research 1996; 8: 695-698.
- Viljoen AM, Subramoney S, Van Vuuren SF, Baser KHC and Demirci B: The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. Journal of Ethnopharmacology 2005; 96: 271-277.
- Rimpler H and Sauerbier H: Iridoid glycosides as chemotaxonomic markers in the genera Lantana, Lippia, Aloysia and Biochemical Systematics and Ecology 1986; 14: 307-310.
- Argyropoulou, Daferera D, Petros A, Tarantilis CF and Polissiou M: Chemical composition of the essential oil from leaves of Lippia citriodoraB.K. (Verbenaceae) at two developmental stages. Biochemical Systematics and Ecology 2007; 35(12): 831-837.
- Kassahun BM, Yosef WB and Mekonnen SA: Performance of Lemon Verbena (Aloysia triphylla) for morphological, economic and chemical traits in Ethiopia. American-Eurasian J Agric & Environ Sci 2013; 13(11): 1576-1581.
- Lou CA, Daferera D, Tarantilis PA, Fasseas C and Polissiou M: Chemical composition of the essential oils from leaves of Lippia citriodoraB.K. (Verbenaceae) at two developmental stages. Biochemical Systematics and Ecology 2007; 35(12): 831-837
- Alavi L, Jabbari A, Barzegar M and Naghdibadi HA: Chemical composition and antioxidant properties of essential oils (Lippia citriodora, Thymus daenensis) 18th National Congress on Food Technology. Mashhad-Iran, 2008.
- Linde JH, Combrinck S, Regnier TJC and Virijevic S: Chemical composition and antifungal activity of the essential oils of Lippia rehmannii from South Africa. South African Journal of Botany 2010; 76(1): 37-42.
- Lageot VGC, Gaydou EM, Parkanyi C: Analysis of essential oil of Lippia graveolens HBK from El Salvador. Flavour Fragance J 2001; 16: 219-226.
- Bassole IHN, Nebie R, Savadogo A, Ouattara CT, Barro N and Traore SA: Composition and antimicrobial activities of the leaf and flower essential oils of Lippia chevalieri and Ocimum canum from Burkina Faso. African Journal of Biotechnology 2005; 4(10): 1156-1160.
- Simões ERB, Santos EA, de Abreu MC, Silva JDN and Nunes NMF: Biomedical properties and potentiality of Lippia microphylla and its essential oils. J Intercult Ethnopharmacol 2015; 4(3): 256-263.
- Toma´s-Barbera´n FA, Harborne JB and Self R: Twelve 6-oxygenated flavone sulphates from Lippia nodiflora and Lippia canescens. Phytochemistry 1987; 26: 2281-2284.
- De Vincenzi M, Maialetti F and Dessi MR: Monographs on botanical flavouring substances used in foods. Part IV. Fitoterapia 1995; 66: 203-210.
- Nakamura T, Okuyama E, Tsukada A, Yamazaki M, Satake M, Nishibe S, Deyama T, Moriya A, Maruno M and Nishimura H: Acteoside as the analgesic principle of Cedron (Lippia triphylla), a Peruvian medicinal plant. Chemical and Pharmaceutical Bulletin 1997; 45: 499-504.
- Oliviera DR, Gilda GL and Daniela AS: Chemical and antimicrobial analyses of essential oil of Lippia OriganoidesB.K. Food Chemistry 2007; 101: 236-240.
- Akhila A: Essential Oil-Bearing Grasses, The genus Cymbopogon; Medicinal and Aromatic Plants-Industrial Profiles, CRC Press, New York 2006.
- Halm MA: Essential oils for management of symptoms in Critically Ill Patients. Am J Crit Care 2008; 17: 160-163.
- Hunter M: Essential oils: Art, Agriculture, Science, Industry and Nova Science Publishers, Inc., New York 2009.
- Margaris N, Koedam A and Vokou D: Aromatic Plants: basic and applied aspects. The Hague, London, Boston, Martinus Nijhoff Publishers 1982.
- Pourmortazavi SM and Hajimirsadeghi SS: Supercritical fluid extraction in plant essential and volatile oil analysis. Journal of Chromatography A 2007; 1163: 2-24.
- Shibamoto K, Mochizuki M and Kusuhara M: Aroma Therapy in Anti-Aging Medicine. Anti-Aging Medicine 2010; 7: 55-59.
- Mori K and Kato M: Synthesis and absolute configuration of (+)-hernandulcin, a new sesquiterpene with intensely sweet taste. Tetrahedron Letters 1986; 27: 981-982.
- Deans SG, Svoboda KP, Gundidza M and Brechany EY: Essential oil profiles of several temperate and tropical aromatic plants: their antimicrobial and antioxidant activities. Acta Hortic 1992; 306: 229-232.
- Ombito JO, Salano EN, Yegon PK, Ngetich WK and Mwangi EM: A review on the chemistry of some species of genus Lippia (Verbenaceae family). Journal of Scientific and Innovative Research 2014; 3(4): 460-466.
- Henbelle T, Sabpaz S, Joseph H and Bailleul F: Ethnopharmacology of Lippia alba. Journal of Ethnopharmacology 2008; 116: 211-222.
- Hennebelle T, Sahpaz S, Dermont C, Joseph H and Bailleul F: The essential oil of Lippia alba: analysis of samples from French overseas departments and review of previous works. Chem Biodivers 2006; 3: 1116-1125.
- Stashenko EE, Martínez JR, Ruíz CA, Arias G, Durán C, Salgar W and Cala M: Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J Sep Sci 2010; 33: 93-103.
- Santos FJB, Lopes JAD, Cito AMGL, Oliveira EH, Lima SG and Reis FAM: Composition and biological activity of essential oil from Lippia origanoides J Essent Oil Res 2004; 16: 504–506
- Rojas J, Morales A, Pascuale S, Márquez A, Rondon R, Mathé I and Verés K: Comparative study of the chemical composition of the essential oil of Lippia oreganoides collected in two different seasons of the year in Venezuela. Nat Prod Commun 2006; 1: 205-207.
- Silva NA, da Silva JKR, Andrade EHA, Carreira LMM, Sousa PJC and Maia JGS: Essential oil composition and antioxidant capacity of Lippia schomburgkiana. Nat Prod Commun 2009; 4, 1281-1286.
- Ribeiro AF, Andrade EHA, Salimena FRG and Maia JGS: Circadian and seasonal study of the cynnamate chemotype from Lippia origanoides Biochem Syst Ecol 2014; 55: 249-259.
- Atal CK: Cultivation and Utilization of Aromatic Plants, 1st ed, Council of Scientific and Industrial Research, New Delhi 1982; 15-21.
How to cite this article:
Okhale SE, Nwanosike EM, Fatokun OT and Kunle OF: Phytochemistry and Ethnopharmacology of Lippia Genus with a Statement on Chemotaxonomy and Essential Oil Chemotypes. Int J Pharmacognosy 2016; 3(5): 201-11. doi: 10.13040/IJPSR.0975-8232.3(5).201-11.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
201-211
787
2547
English
IJP
S. E. Okhale *, E. M. Nwanosike, O. T. Fatokun and O. F. Kunle
Department of Medicinal Plant Research and Tradidtional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Garki, Abuja, Nigeria.
samuelokhale@gmail.com
05 April 2016
19 May 2016
26 May 2016
10.13040/IJPSR.0975-8232.IJP.3(5).201-211
31 May 2016