PHYTOCHEMICAL SCREENING AND EVALUATION OF ANTIDIARRHOEAL ACTIVITY OF FICUS HISPIDA LEAVES
HTML Full TextPHYTOCHEMICAL SCREENING AND EVALUATION OF ANTIDIARRHOEAL ACTIVITY OF FICUS HISPIDA LEAVES
S. M. Mushiur Rahman *, Sadiur Rahman Sajon, Arman Ahamed, Asraful Islam, Md. Shafiul Islam and Md. Imran Hossain
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore - 7408, Bangladesh.
ABSTRACT: As a source of remedies, medicinal plants are widely used as alternative medicines for the treatment or prevention of many diseases. Ficus hispida is traditionally used for treating diarrhoea, wounds, pain, inflammation, diabetes, fever, and neurological disorders. To evaluate the qualitative phytochemical constituents and antidiarrhoeal activity of methanolic extract of Ficus hispida leaves the present study was designed. Phytochemical constituents and anti-diarrhoeal activities were determined and assessed by various tests such as Molisch’s test, Fehling test, Mayer’s test, frothing test, FeCl3 test, alkali test, Salkowski’s test, Keller-killiani test and CuSO4 test, castor oil, and MgSO4 induced diarrheal test. This extract figured the presence of carbohydrates, flavonoids, tannins, glycosides, triterpenoids, fat and fixed oils. Moreover, both doses of (200 mg/kg and 400 mg/kg) methanolic extract of Ficus hispida leaves significantly (p<0.05, vs. control) reduced the gastrointestinal motility and inhibited the percentage of diarrhoea in antidiarrhoeal models. But 400 mg/kg dose showed better antidiarrhoeal activity than 200 mg/kg dose compared to control in both antidiarrhoeal tests. The results indicate that Ficus hispida leaves may provide a potential source of anti-diarrhoeal activities.
| Keywords: |
Phytochemical screening, Ficus hispida, Anti-diarrhoeal, Castor oil induced diarrhoea
INTRODUCTION: Ficus hispida is a member of the Moraceae family. It is a medium but well-distributed species of tropical fig tree or shrub that is coarsely hairy and dioeciously. It is generally known as Dumoor in Bangladesh. Ficus hispida [Family: Moraceae; English nme: Hairy fig; Botanical name: Ficus hispida; Local name: Dumor, kack dumur] is a medicinal tree, which can attain a height up to 10 m. It is commonly a popular plant which is widely distributed throughout subcontinent from Bangladesh to India and Malaysia and is also found in Australia 1.
Usually, the leaves are opposite, leaf blade ovate, oblong or obovate-oblong. They measure 10-25 cm × 5-10 cm, thickly papery. Secondary veins are 6-9 on each side of the midvein. The petiole measure 1-4 cm long with short thick hairs. The fig appears axillary on normal leafy shoots, measuring 1.2-3 cm diameter with short scattered hairs. The male flowers are numerous near the apical pore; calyx lobes 3, thinly membranous; stamen single. The gall flowers are without calyx, style subapical, short and thick. The female flowers are also without calyx; the style is lateral and with hairs 2.
The plant generally contains ficushispimines A and B, ficushispidine, hispiloscine, 𝛽-amyrin acetate, N-triacontanyl acetate, ficusin A, lupeol acetate, and 10-keto- tetracosyl arachidate 3, 4, 5 which are revealed from ra ecent publication. Astringent, antidysenteric, antipsoriasis, antianemic, and antihemorrhagic properties of the whole plant (bark, fruit, root, and leaves) has already been demonstrated and reported elaborately 6, 7. The roots and leaves are comprehended for their anti-diarrhoeal 8, anti-diabetic 9, anti-bacterial 10, hepatoprotective 11, anti-oxidant 12, and cardio-protective 13 properties. The fruit is edible and acts as a coolant and tonic. A mixture of honey and its juice is a good antihemorrhagic 14. Diarrhoea has long been recognized as one of the most important health problems in developing countries. In Bangladesh diarrhoea is a principal cause of infant mortality and morbidity. Treatment of diarrhoea is generally nonspecific and is usually aimed at reducing the discomfort and inconvenience of frequent bowel movements. To overcome the menace of diarrheal disease in developing countries the World health organization (WHO) has included a programme for the control of diarrhoea, which involves the use of traditional herbal medicine 15.
As a regular folklore medicine, Ficus hispida leaves is practiced in some regions of Bangladesh and used against diarrhoea, neurological disorders, pain, diabetes, fever, and inflammation. So, medicinal compounds that derived from plant sources such as tannins, flavonoids, terpenoids, glycosides, and coumarins could provide an excellent fountainhead to develop new anti-diarrhoeal agent, which could be more efficacious, affordable, safer and accessible for patients. Therefore, the present study was designed to identify the phytoconstituents and justify the antidiarrhoeal activity of Ficus hispida leaves and evaluate the traditional usage scientifically.
MATERIALS AND METHODS:
Collection and Identification of the Plant: For performed this study, green and freshness leaves of Ficus hispida plant was collected from Jessore University of Science and Technology Campus, Jessore, Bangladesh, in September 2017. The collected leaves were identified and confirmed by National Herbarium, Bangladesh.
Extraction of Leaves: Around 250 gm of powdered leaves were taken for methanol extraction. First, the leaves of Ficus hispida were thoroughly washed with fresh water to remove all dirt and contaminants and dried in the shade at room temperature (25 ± 2 °C) for two weeks. The materials were ground into coarse powder, and cold extraction method was used to extract the active components. The ground leaves (250 gm) were soaked in a sufficient amount of methanol for 14 days at room temperature with periodical shaking and stirring. The whole mixture was primarily filtered through cotton and then through Whatman no. 1 filters. The solvent was evaporated with a rotary evaporator under reduced pressure at 40 °C temperature to yield semisolid crude extract. The percentage yield of the extract was 3.73% (w/w). The extract was then preserved in a refrigerator until further use.
Experimental Animals: For conducted the antidiarrhoeal study, fifty Swiss albino mice of either sex, aged 4 - 5 weeks, weighing about 25 - 30 gm were collected from the Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh. Before initiating the experiment, the animals were exposed to alternative 12:12 h light and dark cycle at an ambient temperature of 26 ± 2ºC. Proper supplies of foods and water ad libitum were ensured. Institutional Animal Ethical Committee of Jessore University of Science and Technology, Jessore, Bangladesh was approved all protocols for this animal experiment. Mice were acclimatized for 7 days in the laboratory environment before the study and maintained the constant environmental and adequate nutritional conditions throughout the experiment.
Standard Drug: Loperamide HCl (purchased from ACME Laboratories Ltd., Bangladesh) was used as a standard drug and was administered orally.
Phytochemical Screening: Freshly prepared Ficus hispida leaves extract was subjected to different qualitative tests.
Molisch’s Test for Carbohydrates: Approximately 500 mg of crude extract was dissolved in 5 ml of distilled water and later filtered. A few drops of Molisch’s reagent (α-naphthol 10% (w/v) in 90% ethanol) were added to the filtrate. Then 1 ml of concentrated H2SO4 was poured carefully along the side of the test tube. Two minutes later, 5 ml of distilled water was added. A positive test, indicating the presence of carbohydrates, was confirmed with the formation of dull violet or red color at the interphase of the two layers 16.
Fehling’s Test for Reducing Sugars: 2 mg plant extract was dissolved in 1 ml of distilled water and filtered. Then, 1 ml mixture of Fehling’s solutions A and B (a ratio of 1:1) was added to the filtrate, which was heated in a water bath for a few minutes. Formation of brick-red precipitate confirmed the presence of reducing sugars 17.
Mayer’s Test for Alkaloids: In Mayer’s test, one or two drops of 0.35 mol/l Mayer’s reagent (potassium - mercuric iodide solution, 1.36 g mercuric chloride and 5 g of potassium iodide, dissolved in 100 ml distilled H2O) was added to 2 ml (50 mg extract dissolved in 5 ml of 1% aqueous HCl) filtrate along the side of the test tube. A positive test, demonstrating the presence of alkaloids, was indicated by a white creamy precipitate 18.
Frothing Test for Saponins: 100 mg plant extract was dissolved in 10 ml of methanol for making stock solutions. These stock solutions were diluted to 0.5 mg/ml by the additions of 20 ml of distilled water. Test tube containing the dilution was then shaken for 15 min. Formation of foam on the top of the test tubes indicated the presence of saponin 17.
FeCl3 Test for Tannins: 50 mg plant extract was dissolved in 5 ml distilled water, followed by the addition of a few drops of 5% FeCl3. Tannin was confirmed by the development of a bluish- black color 19.
Alkali Test for Flavonoids: For this test, a few drops of 5% NaOH solution were added to 1 ml of filtered stock solution (100 mg of extract dissolved in 10 ml of methanol), which produced a deep-yellow color. The color was lost in the presence of dilute HCl and confirmed flavonoids 19.
Salkowski’s Test for Triterpenoids: 2 mg plant extract was shaken in 1 ml of CHCl3. Then, a few drops of concentrated H2SO4 were added to the solution along the side of the test tube.
Development of a red-brown color at the interface indicated the presence of triterpenoids 17.
Keller-killiani Test for Glycosides: For this screening, 1 ml of extract, 1 ml of glacial acetic acid and few drops of 2% FeCl3 were added and then 1 ml of conc. H2SO4 is also added in the mixture. The appearance of Brown ring shows the presence of glycosides 20.
CuSO4 Test for Fat and Fixed Oils: 5 drops of extract solution (0.25 g extract dissolved in 25 ml mother solvent) mixed with 1 ml of 1% CuSO4 and then few drops of 10% NaOH was added. The appearance of clean blue solution shows the presence of fat and fixed oils.
Anti-diarrhoeal Study:
Castor Oil Induced Antidiarrhoeal Test: Diarrhea was induced in mice using a slightly modified method of Shoba and Thomas 21. By administering 0.5 ml of castor oil orally the preliminary screening of animals was performed, and those animals that started diarrhea were selected finally for the test. Twenty diarrheal screened mice were divided into control group (distilled water), positive control or standard group (loperamide HCl, 3 mg/kg b.w.), and test groups (MFHL 200 mg/kg and 400 mg/kg b.w.), containing five mice in each group.
Experimented animals were fasted for around 16 h with water ad libitum. Mice in the control group, standard group, and test groups orally received one dose of distilled water, loperamide HCl, MFHL 200 mg/kg and 400 mg/kg respectively. Then, each animal received 0.5 ml of castor oil orally for initiating diarrhea after 30 min of the above treatments. Observation for defecation continued up to 4 h on blotting paper lined individual cage was used for placing every animal. These papers were replaced every hour. The number of diarrheal feces was count and recorded for a period of 4 h, and the percentage of inhibition of defecation was calculated for every group of animals.
MgSO4 Induced Anti-diarrhoeal Test: The method described by Doherty 22 was applied for this study with slight modification. Here, a similar procedure as for castor oil induced diarrhea test was maintained for magnesium sulfate induced diarrheal model. The animals all were screened for diarrhea was done by administering magnesium sulfate at a dose of 2 g/kg orally. Experimented animals fasted for 16 h with water ad libitum. Then, mice were grouped and treated as described before.
Then, each animal received 2 g/kg of magnesium sulfate orally for initiating diarrhea after 30 min of the above treatments. Observation for defecation is same as for castor oil induced diarrhea test, and the anti-diarrhoeal activity was expressed by comparing the percent of inhibition of defecation of different groups with the control group.
Statistical Analysis: The experimental results were expressed as mean ± SEM (standard error of the mean). Statistical analyses for anti-diarrhoeal studies were evaluated by one-way ANOVA following Dunnett’s test through the SPSS software (version 16; IBM Corporation, New York, USA). The obtained results were compared with the vehicle control group. The p<0.05 was considered to be statistically significant.
RESULTS:
Phytochemical Screening: After evaluation of the pharmacological activities of plant extract, it is important to depict the chemical nature of plant materials. Phytochemical screening of the Ficus hispida leaf showed the presence of several primary and secondary metabolites, or phyto-constituents, which are summarized in Table 1. In the phytochemical screening, MFHL showed the presence of almost all of the phytoconstituents like carbohydrates, flavonoids, tannins, phenols, glycosides, triterpenoids, fat and fixed oils that were tested here. However, some tests did not show consistent results such as carbohydrate content in MFHL was indicated by Molisch’s test, but not by Fehling’s test.
TABLE 1: PHYTOCHEMICAL SCREENING OF METHANOLIC EXTRACT OF FICUS HISPIDA LEAVES
| Phytoconstituents | Test name | Observation |
| Carbohydrates | Molisch’s test | + |
| Fehling’s test | - | |
| Alkaloids | Mayer’s test | - |
| Saponins | Frothing test | - |
| Tannins | FeCl3 test | + |
| Flavonoids | Alkali test | + |
| Triterpenoids | Salkowski’s test | + |
| Glycosides | Keller-killiani test | + |
| Fat and Fixed oils | CuSO4 test | + |
‘+’ mean the presence of specific phytoconstituents and ‘-’ means the absence of specific phytoconstituents
Anti-diarrhoeal Study:
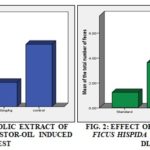
Castor Oil Induced Anti-diarrhoeal Test: In the castor oil induced diarrheal mice, loperamide HCl (3 mg/kg) and methanolic extract of Ficus hispida leaves at the doses of 200 mg/kg and 400 mg/kg significantly (p<0.05, vs. control) reduced the total number diarrheal feces. Here, the decrease in the total number of diarrheal feces is dose dependent in manner. Highest and significant (p<0.05, vs. control) a percentage of inhibition of diarrhea (61.11%) was revealed by MFHL 400 mg/kg. Castor oil induced anti-diarrhoeal results are shown in Table 2.
TABLE 2: EFFECT OF METHANOLIC EXTRACT OF FICUS HISPIDA LEAVES IN CASTOR-OIL INDUCED DIARRHEAL TEST
| Group | Dose | Number of diarrheal feces | % of inhibition of diarrhea |
| Control | 10 mL/kg | 7.20 ± 0.86 | - |
| Loperamide HCl | 3 mg/kg | 1.60 ± 0.24 | 77.78 |
| MFHL | 200 mg/kg | 4.20 ± 0.58 | 41.67 |
| MFHL | 400 mg/kg | 2.80 ± 0.37 | 61.11 |
Numbers of feces are presented as mean ± SEM (standard error of the mean). P<0.05, vs. control (Dennett’s t-test)
MgSO4 Induced Anti-diarrhoeal Test: In the magnesium sulfate induced diarrheal mice, loperamide HCl (3 mg/kg) and methanolic extract of Ficus hispida leaves at the doses of 200 mg/kg and 400 mg/kg significantly (p<0.05, vs. control) reduced the total number diarrheal feces.
TABLE 3: EFFECT OF METHANOLIC EXTRACT OF FICUS HISPIDA LEAVES IN MgSO4 INDUCED DIARRHEAL TEST
| Group | Dose | Number of diarrheal feces | % of inhibition of diarrhea |
| Control | 10 mL/kg | 6.20±0.58 | - |
| Loperamide HCl | 3 mg/kg | 1.20±0.37 | 80.65 |
| MFHL | 200 mg/kg | 3.60±0.60 | 41.77 |
| MFHL | 400 mg/kg | 2.60±0.40 | 58.06 |
Numbers of feces are presented as mean ± SEM (standard error of the mean). P<0.05, vs. control (Dennett’s t-test)
X-axis – a group of experimented animal; Y-axis – number of diarrheal feces
Level of Significance = p<0.05 compared to control (Dennett’s t-test)
Here, the decrease in the total number of diarrheal feces is dose dependent in manner. Highest and significant (p<0.05, versus control) the percentage of inhibition of diarrhea (58.06%) was revealed by MFHL 400 mg/kg. Magnesium sulfate induced anti-diarrhoeal results are shown in Table 3.
DISCUSSION: The phytochemical analysis of the leaves of Ficus hispida revealed the presence of carbohydrates, tannins, flavonoids, glycosides and triterpenoids which are act as a palliative of pain, inflammation, diarrhea, fever, neuropharmacological disorders, and diabetes. Diarrhoea (loose motions) is a condition in which feces are discharged from the bowels frequently and in a liquid form. It may be characterized as the abnormally frequent expulsion of feces of low consistency which may be due to a disturbance in the transport of water and electrolytes in the intestines 23.
Prostaglandins strongly contribute to the pathophysiological functions in the gastrointestinal tract. The major cause of arachidonic acid-induced diarrhea is the release of prostaglandins 24. It also characterized by an increase in the secretion of water and electrolytes, as well as an increase in intestinal transit and an increase in watery feces (diarrheal feces).
Traditionally, the prevalence of diarrhea can be controlled by using Ficus hispida. Castor oil (diarrheal agent) increases peristaltic activity and produces permeability changes in the intestinal mucosal membrane to electrolytes and water, which is associated with prostaglandin release 25. As a result, absorption of sodium and potassium ions are reduced, which sequentially lessens the function of Na+, K+ -ATPase in colon plus small intestine 26. In the study, both doses of methanolic extract (200 mg/kg and 400 mg/kg) of Ficus hispida leaves showed a significant inhibition (p< 0.05, versus control) of castor oil induced diarrhea in mice and may be due to the inhibition of electrolyte permeability of the intestine and prostaglandin release.
In another experiment, after the oral administration of magnesium sulfate, results in the gathering of fluid in the intestinal lumen and its movement from proximal to the distal intestine occurs. Discharge of cholecystokinin and nitric oxide from duodenal mucosa occurs after its oral administration. Then two recurrently results come about, and one is the inhibition of re-absorption of NaCl and water that occurs from the previous case. Another is the rise of secretion and motility of small intestine 27.
Methanolic extract of Ficus hispida leaves extract (200 mg/kg and 400 mg/kg) was effective in reducing diarrhea, and that was expected due to increasing in electrolyte and water re-absorption from the gastrointestinal tract. Above activities are seems to be due to the presence of tannins in the methanolic extract of Ficus hispida leaves. Tannins are responsible for the denaturation of proteins and form protein tannate, which reduces the intestinal mucosa permeability 28. The methanolic extract of Ficus hispida leaves was administered at the dose of 200 mg/kg, and 400 mg/kg showed 41.67% and 61.11% reduction of diarrhea in castor-oil induced diarrheal test and 41.47% and 58.06% reduction of diarrhea in MgSO4 induced diarrheal test respectively. So, we can conclude that the present study seems to support the claims of a traditional medicine practitioner about the use of Ficus hispida in diarrhea.
CONCLUSION: From the results of the existing study, it can be concluded that methanolic extract of Ficus hispida leaves might possess remarkable anti-diarrhoeal properties. Data obtained from this study showed that all activities were dose-dependent in the manner and statistically significant. It is also reasonable to believe that the methanolic extract of Ficus hispida leaves might be effective in inflammatory diarrhea, secretary diarrhea, and infectious diarrhea.
The presence of tannins, flavonoids, β-amyrin acetate, lupeol acetate, and phenolic compounds might be responsible for these activities. Based on these findings, we hope that a further detailed investigation is needed to confirm to find out the active components of the extracts for discovering the mechanism of actions in the improvement of anti-diarrhoeal agents. Moreover, it could be a potential source for novel ‘lead’ discovery for anti-diarrhoeal drug development.
ACKNOWLEDGEMENT: The authors would like to thank Department of Pharmacy, Jessore University of Science and Technology, Jessore, Bangladesh for providing facilities throughout the work.
CONFLICT OF INTEREST: The authors declare that there is no conflict of interests regarding the publication of this paper.
REFERENCES:
- Ripu M, Kunwar I and Rainer WB: Ficus species in Nepal; a review of diversity and indigenous uses. Journal of Ecology 2006; 11: 85-87.
- Flora of China. Available from http://www.efloras.org/ florataxon.aspx?flora_id=2&taxon_id=200006361.Accessed on 19th February 2013.
- Shi ZF, Lei C, Yu BW, Wang HY and Hou AJ: New alkaloids and 𝛼-glucosidase inhibitory flavonoids from ficus hispida. Chemistry and Biodiversity 2016; 13(4): 445-450.
- Yap VA, Loong BJ and Ting KN: Hispidacine, an unusual 8,4’-oxyneolignan-alkaloid with vasorelaxant activity, and hispiloscine, an antiproliferative phenanthroindolizidine alkaloid, from Ficus hispida Phytochemistry 2015; 109: 96-102.
- Sharma PC, Yelne MB and Dennis TJ: Database on medicinal plants used in Ayurveda, Central Council for Research in Ayurveda and Siddha, New Delhi, India 2002; 5.
- Nadkarni KM: Indian MateriaMedica, Popular Prakashan, Mumbai, India 1976; 1.
- Rastogi R and Mehrotra BN: Compendium Indian Medicinal Plants, CDRI, Lucknow, Publication and Information Directorate, New Delhi, India 1993; 2.
- Mandal SC and Kumar CKA: Studies on the anti-diarrhoeal activity of Ficus hispida. Leaf extract in rats. Fitoterapia 2002; 73(7-8): 663-667.
- Ghosh R, Sharatchandra K, Rita S and Thokchom IS: Hypoglycemic activity of Ficus hispida (bark) in normal and diabetic Albino rats. Indian Journal of Pharmacology 2004; 36(4): 222-225.
- Kon´e WM, Atindehou KK, Terreaux C, Hostettmann K, Traore D and Dosso M: Traditional medicine in North Cˆote-d’Ivoire: screening of 50 medicinal plants for antibacterial activity, Journal of Ethnopharmacology 2004; 93(1): 43-49.
- Mandal SC, Saraswathi B, Kumar CKA, Lakshmi SM and Maiti BC: Protective effect of leaf extract of Ficus hispida against paracetamol-induced hepatotoxicity in rats. Phytotherapy Research 2000; 14(6): 457-459.
- Saha MR, Shill MC, Biswas SK and Faruque A: In-vitro antioxidant and cytotoxic activities of methanolic leaf extract of Ficus hispida Stamford Journal of Pharmaceutical Sciences 2011; 3(2): 29-36.
- Shanmugarajan TS, Arunsundar M, Somasundaram I, Krishnakumar E, Sivaraman D and Ravichandiran V: Cardioprotective effect of Ficus hispida on cyclophosphamide provoked oxidative myocardial injury in a rat model. International Journal of Pharmacology 2008; 4(2): 78-87.
- Peraza-S´anchez SR, Chai HB and Young GS: Constituents of the leaves and twigs of Ficus hispida. Planta Medica 2002; 68(2): 186-188.
- Snyder JD and Merson MH: The magnitude of the global problem of acute diarrheal disease: a review of active surveillance data. Bulletin of the World Health Organization 1982; 60: 604-613.
- Sofowora A: Screening plants for bioactive agents. In: Medicinal plants and traditional medicine in Africa. Sunshine House, Ibadan: Spectrum Books Ltd., 1993; 2: 134-156.
- Harborne JB: Phytochemical methods, a guide to modern techniques of plant analysis. London: Chapman and Hall 1998; 2: 54-84.
- Evans WC: Pharmacology. Singapore: Harcourt Brace and Company 1997; 226.
- Raaman N: Phytochemical techniques. Pitam Pura, New Delhi: New India Publishing Agency 2006; 22.
- Edeoga HO, Okwu DE and Mbaebie BO: Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology 2005; 4(7): 685-688.
- Shoba FG and Thomas M: Study of antidiarrhoeal activity of four medicinal plants in castor-oil induced diarrhea. Journal of Ethnopharmacology 2001; 76(1): 73-76.
- Doherty NS: Inhibition of arachidonic acid release as the mechanism by which glucocorticoids inhibit endotoxin-induced diarrhea. British Journal of Pharmacology 1981; 73(2): 549-554.
- Lutterodt GD: Inhibition of Microlax-induced experimental diarrhea with narcotic-like extracts of Psidium guajava leaf in rats. J Ethnopharmacol 1992; 37: 151-157.
- Bennet A and Sanger GJ: Acidic lipids: Prostaglandins. In Mediators and Drugs in Gastrointestinal Motility Bertaccini G. (ed.) Springer-Verlag: Berlin 1982; 2: 219-238.
- Capasso F, Mascolo N, Autone G and Romano V: Laxatives and the production of autacoids by rat colon. J Pharm Pharmacol 1986; 38: 627-629.
- Tenorio JAB, do Monte DS, da Silva TMG, da Silva TG, and Ramos CS: Solanum paniculatum root extract reduces diarrhea in rats. Revista Brasileira de Farmacognosia 2016; 26(3): 375-378.
- Aziz MA, Uddin N, Chowdhury MMH and Faruque A: Acute toxicity study and evaluation of anti-diarrheal, neuropharmacological, anthelmintic, antidiabetic activity of Microcos paniculata Stamford Journal of Pharmaceutical Sciences 2014; 6(1-2): 9-18.
- Tripathi KD: Essentials of Medical Pharmacology. Jaypee Brothers Medical Publishers (P), New Delhi 1994; 775.
How to cite this article:
Rahman SMM, Sajon SR, Ahamed A, Islam A, Islam MS and Hossain MI: Phytochemical screening and evaluation of antidiarrhoeal activity of Ficus hispida leaves. Int J Pharmacognosy 2018; 5(8): 493-99. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(8).493-99.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
493-499
867
1377
English
IJP
S. M. M. Rahman *, S. R. Sajon, A. Ahamed, A. Islam, M. S. Islam and M. I. Hossain
Department of Pharmacy, Faculty of Biological Science and Technology, Jessore University of Science and Technology, Jessore, Bangladesh.
smushiurjustphar@gmail.com
16 May 2018
05 June 2018
13 June 2018
10.13040/IJPSR.0975-8232.IJP.5(8).493-99
01 August 2018