PHYTOCHEMICAL AND PHARMACOLOGICAL INVESTIGATIONS OF RHIZOME EXTRACTS OF KAEMPFERIA GALANGA
HTML Full TextPHYTOCHEMICAL AND PHARMACOLOGICAL INVESTIGATIONS OF RHIZOME EXTRACTS OF KAEMPFERIA GALANGA
Mohammad Zubair Chowdhury 1, Zobaer Al Mahmud *1, Mohammad Shawkat Ali1 and Sitesh C. Bachar 2
Department of Clinical Pharmacy and Pharmacology 1, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh.
Department of Pharmaceutical Technology 2, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh.
ABSTRACT: Rhizome of Kaempferia galanga (Family: Zingiberaceae) has been investigated for isolation of secondary metabolites and evaluation of bioactivities. Two cinnamic acid derivatives, Ethyl p-methoxycinnamate (KG-2) and 4-methoxy cinnamic acid (KG-9) have been isolated from the chloroform fraction of the methanolic extract of the rhizome of K. galanga using chromatographic analysis. Structures of these compounds were elucidated by extensive spectroscopic analysis and by comparing the data with the published one. In the carrageenan-induced paw edema test, at a dose of 400 mg/kg the methanolic extract, chloroform and n-hexane fraction showed a significant (p<0.05) inhibition of paw edema with 55.08, 48.85 and 71.88 % inhibition after a 2nd hour of the study period. In acetic- acid-induced writhing test, the chloroform and n-hexane extract at a dose of 400 mg/kg showed a significant (p<0.001) reduction in the number of writhes with38.19 and 62.31% writhing inhibition respectively. In hypoglycemic evaluation study by oral glucose tolerance test, all of the extracts at 200 mg/kg body weight inhibited the rise in blood glucose level. After 30 min of a glucose load, the methanolic extract, and chloroform soluble fraction remarkably reduced blood glucose level with 61.2 and 89.63% reduction compared to control which were more prominent than that of glibenclamide (34.78% reduction compared to control. The maximal percentage inhibition of glucose load of n-hexane extract vs. control was 133.33 % which was higher than that of the standard. We reported for the first time that the rhizome of K. galangal showed potential antihyperglycemic activity in mice.
| Keywords: |
Kaempferia galangal, antihyperglycemic, Paw edema, Writhing, Bioactivities, Glucose tolerance test
INTRODUCTION: In many developing nations of the world, large numbers of people still rely heavily on traditional healers and medicinal plants to meet their daily primary healthcare needs. Most of the drugs used presently for the management of pain and inflammatory conditions possess more or less side and toxic effects 1.
On the contrary, many medicines of plant origin had been used since long time reported without any adverse effects. It is therefore essential that efforts should be taken to develop new drugs of plant origin, which will be economically feasible, as well as with lesser side effects. Currently, conventional drugs used for diabetes treatment are associated with drawbacks such as rigid dosing regimens, high cost, inaccessibility and unexpected side effects 2.
Therefore, screening for new antidiabetic compounds from natural plants used in folk medicine is still attractive because of their efficacy, low incidence of side effects and low cost.
Bangladesh owing to its favorable climatic influences have been blessed with immense natural resources including explored and unexplored herbal medicinal plants. Kaempferia galanga (Family: Zingiberaceae) is used as a traditional medicinal plant in Bangladesh as well as its neighboring countries 3. It is well known for its action as a remedy for inflammation. Various sources indicated that the rhizome extract of Kaempferia galanga is used to relieve pain around Southeast Asia 4, 5. In Bangladesh, it is used by Chakma tribe for Headache, paralysis of arms and legs 6. The plant has been extensively used for the treatment of various disorders including hypertension, rheumatism, and asthma 4. In Thailand, the rhizome of the plant is used by people in many regions for relieving toothache, abdominal pain, muscular swelling, and rheumatism 4. Due to its extensive ethnopharmacological use, an attempt has been taken to study the chemical constituents and biological activities of Kaempferia galanga. In this study, we have evaluated the anti-inflammatory, antinociceptive and antihyperglycemic effect of different extracts of Kaempferia galanga to establish scientific evidence in support of the folklore claim and for the first time, we reported the antihyperglycemic activity of the rhizome extract in mice.
MATERIALS AND METHODS:
Plant Material: Fresh rhizomes of Kaempferia galanga was collected from Dhaka, Bangladesh. It was identified in Bangladesh National Herbarium, Dhaka. A voucher specimen has been deposited in the Bangladesh National Herbarium, Dhaka. The rhizomes were at first cut into small pieces and then sun-dried for ten consecutive days. The pieces were then oven dried for 24 h at considerably low temperature to effect grinding. Finally, the dried crispy bark was ground into a coarse powder using a grinding machine.
Preparation of the Extracts: About 580 gm of the powdered material was taken in a clean, round-bottomed flask (5 liters) and soaked in 2.5 liters of methanol. The container with its content was sealed by foil and kept for 15 days accompanying occasional shaking and stirring. The whole mixture was then filtered through filter paper and the filtrate thus obtained was concentrated at 45 °C with rotary evaporation. The above process was repeated three times with fresh methanol (3 × 2.5 Liter) to gather more concentrated extracts. Finally, all the filtrates were mixed to get the crude methanol extract. A portion of the concentrated methanol extract (10.0 gm) was partitioned using n-hexane and chloroform, and subsequent evaporation of solvents yielded n-hexane fraction (1.5 gm); chloroform fraction (2.1 gm), an aqueous fraction (AQF, 1.42 gm). The residues were then stored in a refrigerator for further investigation.
Isolation and Identification of Ethyl p-methoxycinnamate and 4-methoxy cinnamic acid: 500 mg of the chloroform extract was subjected to gel permeation Chromatography using Sephadex (LH-20) for fractionation. Sephadex was soaked in a mixture of solvents with a ratio of n-hexane: Dichloromethane: Methanol= 2:5:1 for at least 12 h for proper swelling. After that, a slurry of Sephadex was made and added into a glass column having the length and diameter of 55 cm and 1.1 cm, respectively. When sufficient height of the adsorbent bed was obtained, a few hundred milliliters of solvent mixture with the same ratio was run through the column for proper packing of the column. The sample was dissolved in this solvent mixture and subsequently applied on top of the adsorbent layer with the help of a pasture pipette. The column was then eluted with the same solvent mixture, and finally, the column was washed with dichloromethane and methanol mixtures of increasing polarity Table 1.
TABLE 1: DIFFERENT SOLVENT SYSTEMS USED FOR THE SEPHADEX COLUMN CHROMATOGRAPHIC ANALYSIS OF CHLOROFORM SOLUBLE FRACTION
| Fraction no. | Solvent systems | Volume collected |
| 1 - 20 | n-hexane : Dichloromethane : Methanol = (2:5:1) | 100 ml |
| 21 - 25 | Dichloromethane : Methanol = (9:1) | 50 ml |
| 26 - 30 | Dichloromethane : Methanol = (1:1) | 50 ml |
| 31-40 | Methanol 100% | 100 ml |
Total 40 fractions were collected Table 1 and marked as 1 to 40, each of which was screened by TLC under UV light and by spraying with the vanillin-sulphuric acid reagent. Column fractions 12-16 of chloroform extract were found to give identical spots. So, these 5 fractions were mixed. The combined was subjected to band TLC and found to be mixed. Then preparative thin layer chromatography (PTLC) (Stationary phase: - Silica gel PF254, Mobile phase:-toluene-ethyl acetate, 92:8, the thickness of plates - 0.5 mm) was undergone to separate KG-2. From the developed plates a single band visible under a UV lamp at 254 nm was scrapped on to aluminum foil and eluted initially using a 50:50 mixture of ethyl acetate and chloroform followed by 100% ethyl acetate. The white amorphous material which was obtained from this fraction was checked for purity and termed as KG-2. Column fraction no. twenty-nine of chloroform extract was screened on TLC plate and was found to give identical spots.
So, the fraction was subjected to band TLC and found to be pure. Then preparative thin layer chromatography (PTLC) (Stationary phase:- Silica gel PF254, Mobile phase:-toluene-ethyl acetate, 50: 50, the thickness of plates - 0.5 mm) was undergone triple times to separate KG-9 to total purification. From the developed plates a single band visible under a UV lamp at 254 nm was scrapped on to aluminum foil and eluted initially using a 50:50 mixture of ethyl acetate and chloroform followed by 100% ethyl acetate. The white amorphous material which was obtained from this fraction was checked for purity and termed as KG-9. Both the compounds were characterized by spectroscopic analysis.
Experimental Animals: Long Evans rats (Rattus norvigicus) weighing 100-150 gm of both sexes, aged 4-5 weeks, and Swiss-albino mice (Mus muscles) weighing 20-25 g of both sexes, aged 4-5 weeks, obtained from the Animal Resource Branch of the International Centre for Diarrheal Diseases and Research, Bangladesh (ICDDR, B) were used for the experiment. The animals were kept and maintained under laboratory conditions of temperature, humidity, and light; and were allowed free access to food (standard pellet diet) and water. All the animals fasted overnight, but still allowed free access to water, before the commencement of the experiments. The Ethical Review Committee has approved the design and performance of research study involving mice and rats, Faculty of Biological Science, the University of Dhaka through the submission of a research protocol before the study.
Carrageenan-Induced Paw Edema Test: The animals were weighed and randomly divided into five groups of 5 rats. According to the method designed by Ibrahim et al., and Amdekar et al., 7, 8 the initial right hind paw volume of each rat is measured using plethysmometer (UGO Basile, Italy) and then 0.1 ml of 1 % (w/v) carrageenan is subcutaneously injected into the sub-plantar region of the right hind paw in order to induce acute inflammation. The volume of right hind paw was measured at 1st, 2nd, 3rd and 4th h after carrageenan injection and the paw edema was determined. Rhizome extract of K. galanga (400 mg/kg) or standard anti-inflammatory drug diclofenac sodium (40 mg/kg) or distilled water was administered orally one hour before the subplantar injection. The inhibitory activity was calculated according to the following formula:
Percentage inhibition = (Ct - Co) control - (Ct - Co) treated × 100 / (Ct - Co) control
Where Ct is the right hind paw thickness volume (in mm3) at time t, Co is the right hind paw thickness volume (in mm3) before carrageenan injection. Ct - Co is paw edema. (Ct-Co) control is edema or paw size after carrageenan injection to control rats at time t. (Ct - Co) treated is edema or paw size after carrageenan injection to treated (reference or sample drug) rats at time t.
Acetic Acid Induced Writhing Inhibition Method: The ‘acetic acid’ analgesic test method used in this study was adopted from those described earlier by Hossain et al., 9 and Ferreira et al., 10 Twenty five (25) experimental animals were randomly selected and divided into five groups denoted as test group(s), standard group and control group consisting of 5 mice in each group. At zero hour test samples, control (0.1% NaCl saline) and Diclofenac Sodium were administered orally using a long needle with a ball-shaped end. After 30 min acetic acid (0.7%) was administered intra-peritoneally to each of the animals of all the groups. The thirty minutes interval between the oral administration of test materials and intraperitoneal administration of acetic acid was given to assure proper absorption of the administered samples.
Five minutes after the administration of acetic acid, some squirms or writhing were counted for each mouse for ten minutes. Percentage inhibition of writhing compared to the control group was taken as an index of analgesia and was calculated using the following formula:
Inhibition (%) = [(Wc-Wt) × 100] / Wc
Where Wc is the average number of writhing reflex in the control group, and Wt is the average number of writhing in the test groups.
Antihyperglycemic Study with Oral Glucose Tolerance Test: The test is performed using a method previously described by Shejuty et al11 and Ahmed et al. 12 The animals were weighed and randomly divided into five groups consisting of 5 mice in each group. Then the samples were administered (0.1% saline for control, glibenclamide for standard and plant extracts) using oral feeding needle. After 30 min of extract administration, the glucose solution 10% was orally treated at 1.5 g/kg. Blood glucose level from each group was measured from tail vein just before glucose administration by using Glucometer (Braun G-423 S from Hongkong) and Glucose oxidase-peroxidase reactive strips. To measure the blood glucose level, the tail tip of mice was cut with a sharp blade, and then Little amount of blood was collected and exposed to the touch of glucose test strips. Within seconds blood glucose level was visualized. Nebanol (Bacitracin)
Ointment was applied on the wound to avoid infection. At 30, 90 and 150 min after glucose loading, blood was collected in the same procedure and blood glucose level measured to see the hypoglycemic effect of the test sample in relative to control and standard groups.
RESULTS AND DISCUSSION:
Characterization of KG-2 as Ethyl para-methoxy Cinnamate: KG-2 was isolated as a white amorphous powder from the chloroform soluble fraction of the methanolic extract of Kaempferia galanga. It was appeared as a violet spot on TLC (Silica gel PF254), after spraying with vanillin-sulfuric acid reagent followed by heating at 110 °C for 5-10 min. The compound was found to be soluble in dichloromethane, chloroform, ethyl acetate, and methanol.
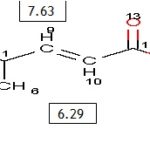
The 1H NMR spectrum (400 MHz, CDCl3) of KG-2 (Fig. 1, Table 2) showed well dispersed resonances between d 1.00 and 8.00, which were in close agreement to that observed for ‘ethyl trans-4- methoxy cinnamate’ reported by Stabile and his co-worker18 which suggest a close structural similarity between these compounds.
The 1H NMR spectrum of KG-2 displayed a characteristic triplet (OCH2CH3, δ 1.35) and quartet (OCH2CH3, δ 4.26) splitting pattern reveals ethyl esterification has taken place. The spectrum also showed resonances at d 3.83 (3H, s, OCH3), deshielded resembling methoxy protons are present. A doublet at δ 6.29 and another at δ 7.63 (1H, d, J=16 HZ) with trans geometry again evident towards the presence of olefinic proton. Two aromatic doublets (δ 6.89 and δ 7.47 (2H, d, J = 8.8 Hz) are indicative of para-disubstitution with an electron donating (shielding) -OCH3 moiety and an electron withdrawing (deshielding) -CH=CH- group attached.
FIG. 1: CHARACTERIZATION OF KG-2 AS ETHYL P-METHOXY CINNAMATE
TABLE 2: SPECTRAL DATA COMPARISON OF KG-2 AND ETHYL TRANS-4-METHOXYCINNAMATE 13
|
Proton |
dH in ppm | |
| KG-2 | Ethyl trans-4- methoxy cinnamate | |
| H-2 | 7.47(d, 2H, J = 8.8 Hz) | 7.49 (d, 2H, J = 8.8 Hz) |
| H-3 | 6.89 (d, 2H, J = 8.8 Hz) | 6.91 (d, 2H, J = 8.6 Hz) |
| H-8 | 3.83 (s, 3H) | 3.84 (s, 3H) |
| H-9 | 7.63 (d, 1H, J = 16.0 Hz) | 7.66 (d, 1H, J = 16.0 Hz) |
| H-10 | 6.29 (d, 1H, J = 16.0 Hz) | 6.31 (d, 1H, J = 16.0 Hz) |
| H-14 | 4.24 (q, 2H, J = 7.2 Hz) | 4.26 (q, 2H, J = 7.2 Hz) |
| H-15 | 1.32 (t, 3H, J = 7.2 Hz) | 1.35 (t, 3H, J = 7.2 Hz) |
These 1H NMR data were in good agreement to those reported for Ethyl trans-4- methoxy cinnamate 13. On this basis compound, KG-2 was identified as Ethyl 4-methoxycinnamate. Several other workers have reported the compound from phytochemical investigation 14, 15.
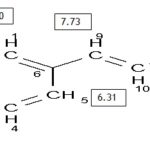
Characterization of KG-9 as para-methoxy Cinnamic Acid: KG-9 was isolated as a white amorphous crystal from the chloroform soluble fraction of the methanolic extract of Kaempferia galanga. It was appeared as a blue spot on TLC (Silica gel PF254), after spraying with vanillin-sulfuric acid reagent followed by heating at 110 °C for 5-10 min. The compound was found to be soluble in dichloromethane, chloroform, ethyl acetate, and methanol. The 1H NMR spectrum (400 MHz, CDCl3) of KG-9 (Fig. 2, Table 3) showed well-dispersed resonances between d 3.00 and 8.00, which were in close resemblance to the 1H NMR spectrum of ‘trans-4-methoxy cinnamic acid’ reported by Victor et al. 16
The 1H NMR spectrum of KG-9 displayed characteristic resonances at d 3.84 (3H, s, OCH3), deshielded resembling methoxy protons are present. A doublet at δ 6.31 and another at δ 7.73 (1H, d, J=16 Hz) with trans geometry again evident towards the presence of olefinic proton. Two aromatic doublets (δ 6.91 and δ 7.50 (2H, d, J = 8.4 Hz) are indicative of para-disubstitution with an electron donating (shielding) -OCH3 moiety and an electron withdrawing (deshielding) -CH=CH- group attached. But both the reported and investigated NMR spectra do not pose for the presence for the carbonyl group. As no other proton is present in methoxy region (d 3.84) the proton at d 7.73 is much deshielded than normal we can conclude that a carboxyl group is attached on another side of the olefinic proton. Again the presence of the carboxyl group in the NMR spectra would be in the higher region (>d 9) and would give a high peak which may not be visible.
FIG. 2: CHARACTERIZATION OF KG-9 AS 4-METHOXY CINNAMIC ACID
TABLE 3: SPECTRAL DATA COMPARISON OF KG-9 AND TRANS-4-METHOXY-CINNAMIC ACID 16
|
Proton |
dH in ppm | |
| KG-9 | 4-methoxy cinnamic acid | |
| H-1 | 7.50(d, 2H, J = 8.4 Hz) | 7.54 (distorted d, 2H, J = 8.8 Hz) |
| H-2 | 6.91 (d, 2H, J = 8.4 Hz) | 6.96 (distorted d, 2H, J = 8.8 Hz) |
| H-8 | 3.84 (s, 3H) | 3.83 (s, 3H) |
| H-9 | 7.73 (d, 1H, J = 16.0 Hz) | 7.63 (d, 1H, J = 16.0 Hz) |
| H-10 | 6.31 (d, 1H, J = 16.0 Hz) | 6.33 (d, 1H, J = 16.0 Hz) |
These 1H NMR data were in good agreement to those reported for trans-4-methoxy cinnamic acid 16. On this basis compound, KG-9 was identified as 4-methoxy cinnamic acid.
Carrageenan-Induced Paw Edema: In Carrageenan-induced rat paw edema model, different fractions of Kaempferia galanga produced significant anti-inflammatory activity. The results are shown in Table 4. All of the fraction showed the highest inhibition of paw edema after a 2nd h of induced inflammation.
Among the fractions, the Hexane extract showed the highest inhibition: 71.88% at 400 mg/kg at 2nd h while the diclofenac sodium showed highest 94.63% after a 3rd h. The methanol and chloroform extract also showed maximal inhibition at 2nd h at 55.08 and 48.85% respectively. The results support the previously reported data 17, 18 that the extract of K. galanga has anti-inflammatory properties. The excessive result rather than normal after 24 h may be due to the extracts’ diuretic and dehydrating effect.
TABLE 4: EFFECT OF RHIZOME EXTRACTS OF KAEMPFERIA GALANGA ON CARRAGEENAN-INDUCED RAT PAW EDEMA
| Treatment | Dose (mg/kg) | Paw volume (mL)a | |||||
| 0 h | 1 h | 2 h | 3 h | 4 h | 24 h | ||
| Control | - | 0.77±0.07 | 1.01±0.09 | 1.24±0.24 | 1.07±0.23 | 0.98±0.07 | 0.83±0.06 |
| Diclofenac | 50 | 0.68±0.04 | 0.78±0.01***
(58.82) |
0.71±0.02*
(92.37) |
0.69±0.03*
(94.63) |
0.69±0.04**
(92.31) |
0.67±0.04
(106.90) |
| MKG | 400 | 0.76±0.02 | 0.93±0.04
(28.44) |
0.97±0.05*
(55.08) |
1.0±0.03
(20.47) |
0.94±0.04
(14.49) |
0.68±0.02
(246.42) |
| CHKG | 400 | 0.76±0.02 | 1.01±0.03
(-4.35) |
1.0±0.02*
(48.85) |
1.03±0.05
(8.44) |
0.95±0.04
(7.21) |
0.77±0.02
(80.89) |
| HKG | 400 | 0.75±0.02 | 0.87±0.04*
(47.07) |
0.88±0.04*
(71.88) |
0.95±0.04
(29.90) |
0.89±0.05
(31.55) |
0.72±0.08
(71.88) |
values are mean±S.E.M. (n=5);
***p<0.001(One-way ANOVA and Dunnett’s t-test), significantly different from control.
**p<0.01, (One-way ANOVA and Dunnett’s t-test), significantly different from control.
*p<0.05 (One-way ANOVA and Dunnett’s t-test), significantly different from control.
Figures in parentheses are the % inhibition of paw edema.
MKG: methanolic extract of rhizomes of Kaempferia galanga; CHKG: chloroform extract of rhizomes of Kaempferia galanga; HKG: hexane extract of rhizomes of Kaempferia galanga
The carrageenan-induced rat paw edema model is believed to be a biphasic process 19. The first phase, which occurs between 0-2 h after injection of the phlogistic agent, has been attributed to the release of histamine or serotonin (5-HT) and the second phase of inflammatory reaction is associated with the production and release of bradykinin, protease, prostaglandins and lysosome 20. Since, the extract showed peak inhibition of paw edema at 2 h after carrageenan injection, the anti-inflammatory activity observed may be due to an inhibitory effect on the release of histamine and serotonin.
Mouse-Writhing Test: Table 5 shows the antinociceptive effect of various extracts of K. galanga assessed using the abdominal constriction test in mice. The Methanolic extract showed the least writhing inhibition which also differs from previously reported results 18. Among all the fractions the n-hexane extract showed the highest analgesic effect (62.31% of inhibition) whiles the chloroform extract moderate analgesic activity with 38.19% inhibition of writhing.
Oral Glucose Tolerance Test: The results found from different extracts of Kaempferia galanga showed significant blood glucose lowering activity Table 6. All of the extracts at 200 mg/kg body weight inhibited glucose level increase after the glucose load. After 30 minutes of a glucose load, the crude methanolic extract, and chloroform soluble fraction remarkably reduced blood glucose level with 61.2 and 89.63% reduction compared to control which are more prominent than that of standard drug glibenclamide (34.78% reduction compared to control, (P<0.05). The maximal percentage inhibition of glucose load of n-hexane extract vs. control was 133.33% which was higher than that of the standard.
TABLE 5: ANALGESIC ACTIVITY OF VARIOUS EXTRACT OF KAEMPFERIA GALANGA ASSESSED USING THE ACETIC ACID-INDUCED ABDOMINAL CONSTRICTION TEST
| Treatment | Dose (mg/kg) | Writhinga | % inhibition |
| Control (Vehicle) | 10 mL/kg | 39.8±2.31 | - |
| Diclofenac sodium | 50 | 14.4±1.63** | 63.82 |
| MKG | 400 | 34.8±2.06* | 12.56 |
| CHKG | 400 | 24.6±1.94** | 38.19 |
| HKG | 400 | 15.0±1.09** | 62.31 |
values represent mean ± SEM (n=5).
**p<0.001 (One-way ANOVA and Dunnett’s t-test, significantly different from control. *p<0.01 (One-way ANOVA and Dunnett’s t-test, significantly different from control.
MKG: methanolic extract of rhizomes of Kaempferia galanga; CHKG: chloroform extract of rhizomes of Kaempferia galanga; HKG: hexane extract of rhizomes of Kaempferia galanga
TABLE 6: EFFECT OF VARIOUS EXTRACTS OF KAEMPFERIA GALANGA ON BLOOD GLUCOSE CONCENTRATION ON FASTING CONDITION OF MICE
| Groups | Dose (mg/kg) | Blood glucose (mmol/l) | |||
| 0 h | 30 min | 90 min | 150 min | ||
| Control | - | 5.92± 0.7 | 11.9±1.26 | 6.52±0.61 | 5.16±0.43 |
| Glibenclamide | 10 | 5.4± 0.30 | 9.3±0.34*
(34.78) |
5.62±0.70*
(63.33) |
4.28±0.45*
(47.36) |
| MKG | 200 | 5.76±0.21 | 8.08±0.36*
(61.2) |
6.12±0.28*
(40) |
6.42±0.08
(13.15)b |
| CHKG | 200 | 6.52±0.76 | 7.14±0.33*
(89.63) |
6.86±0.6*
(43.33) |
5.48±0.69
(36.84) |
| HKG | 200 | 6.76±0.3 | 7.26±0.3*
(91.63) |
5.36±0.55*
(133.33) |
5.52±0.57*
(63.15) |
Each value represents the mean ± SEM., N= 5. *P<0.05 compared to control, Dunnett’s t-test after analysis. Figures in parentheses are the % reduction in blood glucose level compared to control. b percent increase in blood glucose level. MKG: methanolic extract of rhizomes of Kaempferia galanga; CHKG: chloroform extract of rhizomes of Kaempferia galanga; HKG: hexane extract of rhizomes of Kaempferia galangal.
This action could be due to direct stimulation of insulin secretion. Bioactive molecules present in these extracts of K. galanga may probably possess insulin–like effect or stimulate the pancreatic β cells to produce insulin which in turn lowers the blood glucose level. The data of the present study support the idea that the plant has anti-hyperglycemic effects in animal models which yet not been reported.
Further investigations like checking hypoglycemic action in diabetes-induced rats may conclude the hypothesis. Also, further elaborate investigations are essential to establish on a human trial to see the effect of K. galanga on diabetes patients.
CONCLUSION: The overall results of the present study indicated the anti-nociceptive, anti-inflammatory, and antihyperglycemic activities of the rhizome extracts of K. galanga which validate the ethnopharmacological use of the plant. Therefore, the extract will be of potential benefit in the treatment of pain and inflammatory disorders as well as in diabetes.
We reported for the first time that the rhizome of K. galangal showed potential antihyperglycemic activity in mice and further investigations are required to explore the potential antidiabetic actions using different animal models.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Qais N, Mahmud ZA, Karim MR and Bachar SC: Anti-nociceptive, anti-inflammatory and sedating activities of leaf extracts of Premna esculenta (Roxb). Journal of Pharmacy Research 2011; 4: 3463-3465.
- Singh SK, Rai PK, Jaiswal D and Watal G: Evidence-based critical evaluation of the glycemic potential of Cynodon dactylon. Evidence-Based Complementary and Alternative Medicine 2008; 5: 415-420.
- Kanjanapothi D, Panthong A, Lertprasertsuke N, Taesotikul T, Rujjanawate C, Kaewpinit D, Sudthayakorn R, Choochote W, Chaithong U, Jitpakdi A and Pitasawat B: Toxicity of crude rhizome extract of Kaempferia galanga L. (Proh Hom). Journal of Ethnopharmacology 2004; 90: 359-365.
- Wibool R, Chutha S, Wantana R and Malinee W: Antinociceptive activity of the methanolic extract of Kaempferia galangal in experimental animals. Journal of Ethnopharmacology 2008; 118: 225-230.
- Mohammad Y: Encyclopedia of Flora and Fauna of Bangladesh: Angiosperms: Monocotyledons: Orchidaceae-Zingiberaceae. Asiatic Society of Bangladesh, First Edition 2008.
- Mohammed Y, Wahab MA, Yousuf M, Jasim UC and Jaripa B: Some tribal medicinal plants of Chittagong Hill Tracts, Bangladesh. Bangladesh Journal of Plant Taxonomy 2007; 14: 117-128,
- Ibrahim B, Sowemimo A, van Rooyen A and Van de Venter M: Antiinflammatory, analgesic and antioxidant activities of Cyathula prostrata (Linn.) Blume (Amaranthaceae). Journal of Ethnopharmacology 2012; 141: 282-289.
- Amdekar S, Roy P, Singh V, Kumar A, Singh R and Sharma P: Anti-inflammatory activity of Lactobacillus on carrageenan-induced paw edema in male wistar rats. International Journal of Inflammation 2012. doi: 10.1155/2012/752015.
- Hossain H, Karmakar UK, Biswas SK, Shahid-Ud-Daula AF, Jahan IA, Adnan T and Chowdhury A: Antinociceptive and antioxidant potential of the crude ethanol extract of the leaves of Ageratum conyzoides grown in Bangladesh. Pharmaceutical Biology 2013; 51: 893-898.
- Ferreira LC, Grabe-Guimarães A, de Paula CA, Michel MC, Guimarães RG, Rezende SA, de Souza Filho JD and Saúde-Guimarães DA: Anti-inflammatory and anti-nociceptive activities of Campomanesia adamantium. Journal of Ethnopharmacology 2013; 145:100-108.
- Shejuty S, Joyanta B, Abdul H, Shahnaz R, Anahita TZ, Abu S, Majeedul HC and Mohammed R: Anti-hyperglycemic activities of leaves of three edible fruit plants (Averrhoa carambola, Ficus hispida and Syzygium samarangense) of Bangladesh. African Journal of Traditional, Complementary and Alternative Medicine 2012; 9: 287-291.
- Ahmed F, Rahman S, Ahmed N, Hossain M, Biswas A, Sarkar S, Banna H, Khatun MA, Chowdhury MH and Rahmatullah M: Evaluation of Neolamarckia cadamba (Roxb.) Bosser leaf extract on glucose tolerance in glucose-induced hyperglycemic mice. African Journal of Traditional, Complementary and Alternative Medicines 2011; 8: 79-81.
- Stabile RG and Andrew PD: Two-Step Semi-Microscale Preparation of a Cinnamate Ester Sunscreen Analog. Journal of Chemical Education 2004; 81: 1488-1490.
- Tanasorn T, Niran V, Anchalee C and Nijsiri R: Pharmacognostic specification of Kaempferia galanga rhizome in Thailand. Journal of Health Research 2007; 21: 207-214.
- Tewtrakul S, Yuenyongsawad S, Kummee S and Atsawajaruwan L: Chemical components and biological activities of of volatile oil of Kaempferia galanga Songklanakarin Journal of Science and Technology 2005; 27: 503-507.
- Victor SS, Bruce WH, Thomas LP, Stephen TD and James BG: Production of Stilbenoids and phenolic acids by the peanut plant at early stages of growth. Journal of Agricultural and Food Chemistry 2006; 54: 3505-3511.
- Sulaiman MR, Zakaria ZA, Daud IA, Ng FN, Ng YC and Hidayat MT: Antinociceptive and anti-inflammatory activities of the aqueous extract of Kaempferia galanga leaves in animal models. Journal of Natural Medicine 2008; 62: 221-227.
- Reanmongkol W, Wongnawa M and Ridtitid W: Studies on analgesic, antipyretic and anti-inflammatory activities of methanol extract of Kaempferia galanga in experimental animals. Journal of Ethnopharmacology 2008; 118: 225-230.
- Sarada K, Margret RJ and Mohan VR: Anti-inflammatory activity of ethanol extracts of leaf and bark of Naringi crenulata (Roxb.) Nicolson. International Journal of Pharmaceutical Sciences and Research 2012; 3: 4540-4544.
- George will OA, Georgewill UO and Nwankwoala RNP: Antiinflammatory effects of Morninga oleifera lam extract in rats. Asian Pacific Journal of Tropical Medicine 2010; 3: 133-135.
How to cite this article:
Chowdhury MZ, Mahmud ZA, Ali MS and Bachar SC: Phytochemical and pharmacological investigations of rhizome extracts of Kaempferia galanga. Int J Pharmacognosy 2014; 1(3): 185-92. doi: 10.13040/IJPSR.0975-8232.1(3).185-92.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
185-192
557
3565
English
IJP
M. Z. Chowdhury, Z. A. Mahmud *, M. S. Ali and S. C. Bachar
Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh.
zalmahmud@du.ac.bd
07 December 2013
19 February 2014
28 February 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(3).185-92
01 March 2014