PHYTOCHEMICAL AND ANTIBACTERIAL POTENTIAL OF ACETONE LEAF EXTRACT OF ACACIA CONCINNA (WILLD.) DC.
HTML Full TextPHYTOCHEMICAL AND ANTIBACTERIAL POTENTIAL OF ACETONE LEAF EXTRACT OF ACACIA CONCINNA (WILLD.) DC.
Rina Antil 1, Priyanka 1, Pushpa Dahiya * 1 and Nikhil Singh 2
Department of Botany 1, Maharshi Dayanand University, Rohtak - 124001, Haryana, India.
National Institute of Pharmaceutical Education and Research 2, Hajipur - 844102, Bihar, India.
ABSTRACT: The present study was aimed to find out the antimicrobial potential of acetone extract of Acacia concinna leaf against five different bacterial strains at 100 and 200mg/ml concentrations. The disc diffusion assay was performed to access the antimicrobial potential of the extract. The agar plate containing a bacterial culture was divided into 4 zones, and each zone was independently impregnated with four sterile filter paper discs (6 mm in diameter) containing (10 µl of each concentration) distill water (Negative control), ampicillin (Positive control); Acacia concinna leaf extract (100 mg/ml and 200 mg/ml as test). Zone of inhibition and MIC (minimum inhibitory concentration) for test drug was evaluated to access the antimicrobial potency. The phytochemical analysis shows the presence of alkaloids, tannins, and glycosides that might be responsible for the aimed biological activities. Out of five bacterial strains, the test drug exhibit zone of inhibition against Mycobacterium smegmatis and Bacillus subtilis in a concentration-dependent manner. Minimum inhibitory concentration (MIC) of test drug against Mycobacterium smegmatis and Bacillus subtilis, was found to be 2.5 (mg/ml) and 1.25 (mg/ml) respectively. The basis of above findings suggests the need for further future perspective works on the plant extract of Acacia concinna which might be very useful as it seems to be a potential source for arresting the growth and metabolic activities of strains like Mycobacterium smegmatis and Bacillus subtilis.
| Keywords: |
Acacia concinna, Antimicrobial, Ampicillin, Disc Diffusion, MIC (Minimum Inhibitory concentration)
INTRODUCTION: Medicinal plants have been identified used by mankind since time immemorial 1. Plants can synthesize a wide variety of chemical compounds that perform essential biological functions 2. India is a country with a vast reserve of natural resources and a rich history of traditional medicine 3. Medicinal plants contain numerous biologically active compounds which help improve human life 4.
The traditional medical system, especially the use of medicinal plants, still plays a vital role to cover the basic health needs in the developing countries 5. India is rich in the medicinal herbs and therefore, it can be accurately called the “Botanical Garden of the World” 6.
Acacia concinna is widely distributed in Burma, southern China, and Malaysia. It is found in central India commonly in Madhya Pradesh, Maharashtra and some regions of pinnae. The leaf stalks are 1.5cm long with a prominent gland about the middle longer. It is a climbing shrub with thorny branches having smooth brown stripes. Thorns are short, broad-based, flattened surface. The main stem is brown. The stem is strong, woody and armed. The barks present on the stem have longitudinal striations. The bark is generally thick and rough. The stem is dotted with white dots on its bark. Its bark contains thorns on its surface. The girth of the main stem is generally 16-20 cm. Leaves have a characteristic odor and taste.
Flowers are 1.5-2 cm long. They are round to oblong shape. They have a diameter of 1cm. They are pink in color. They are bisexual, mostly funnel-shaped. The flowers are stalked, complete, bracteate, regular, actinomorphic, pentamerous, and hypogynous. The inflorescence is terminal cyme, each bearing 60 to 90 flowers. Calyx is five-lobed and green in color. Corolla is white in color and is inferior with five petals. Stamens are inferior and about 10 in number. The female part is 5 to 6 mm long. Stigma is bright and sticky. Style is short, and ovary is superior. Fruits are known as legumes and these are fleshy, beaked and constricted. There are 6-10 seed in each fruit. The seeds are brown in colour. The pod is about 5-6 cm 7-10 Acacia concinna (Shikakai) grows in the tropical forest of India. Its fruits are very well known for use as natural hair shampoo. Its pods turn dark brown and wrinkled on drying. For medicinal purposes, its leaves and leaves and fruits are used.
Its dried pods are powdered to produce shikakai powder. Shikakai is also used in traditional medicine to treat jaundice, constipation and skin problem, itching, pimples, hyperpigmentation, bad, leprosy, psoriasis and gum infection, dandruff, leprosy, psoriasis 11-12. Nowadays there are many major problems that the world is facing among of them the microbial resistance against the existing antibiotics, which is of great concern and this issue have necessitated the search for new antimicrobials 13. More and more about plants, therefore, are to be screened to find out their therapeutic potential.
Keeping in view these issues, in the present investigation, we have selected Acacia concinna Fig. 1, Table 1 for evaluating its phytochemical and antibacterial properties as it has been traditionally used for the treatment of various human ailments 14. The antibacterial properties have been studied against five pathogenic bacteria using disc diffusion and MIC assay. The plant is known by different names in different part of the country and the world.
The names Acacia is derived from the Greek word "akis" meaning "sharp point" 15 and relates to the sharp thorny shrubs and tree of tropical Africa and Western Asia that were only known acacias at the time that the name was published 16. The Australian acacias are commonly called "wattles" because of their pliable branches that were woven into the structure of early wattle house and fenced 17. Keeping in view the importance of plants for the Benefit of mankind, phytochemical and antimicrobial potential of Acacia concinna was studied in the present investigation.
MATERIALS AND METHODS:
Microbial Assay:
Preparations of Plant Extract: 18 The leaves were collected in January from the herbal garden of the Maharshi Dayanand University (MDU), Rohtak. The leaves were washed and shaded dry at room temperature followed by oven drying at 30-35 °C. The dried leaves were pulverized and were further used for extraction. The powdered plant material was extracted in acetone using Soxhlet apparatus.
Test Organism: The bacterial strains used for assessing the antibacterial potential of the plant material were procured from Institute of Microbial Technology, CSIR, Chandigarh. Bacterial strains used in the present studies were Pseudomonas auregiuosa (2453), Klebsella pneumonia (109), Mycobacterium smegmatis (992), Staphylococcus aureus (96) and Bacillus subtilis (2657).
Phytochemical Screening: Acetone extract of Acacia concinna leaf was subjected to various phytochemical tests for the identification of phyto-constituents present therein 19, 20, 21, 22, 23.
Disc Diffusion Assay: 24 Disc of Whatman filter paper (6 mm diameter) were prepared, and the bacterial strains were revived by inoculating in broth media. Incubation was carried out at 37 ºC for 18 h for bacterial growth. The agar plate containing a bacterial culture was divided into 4 zones, and each zone was independently impregnated with four sterile filter paper discs (6 mm in diameter) containing (10 µl of each concentration) distill water (Negative control), ampicillin (Positive control); Acacia concinna leaf extract (100 mg/ml and 200 mg/ml as Test) and were air dried to eliminate any residual solvent and were placed on their respective zone on the agar plate containing microbial strain. The plates were incubated for 24 h at 37 °C in a B.O.D. incubator after that, the diameter of the inhibition zones obtained was measured.
Minimum Inhibitory Concentrations (MIC) Assay: 25-29 MIC was determined by micro broth dilution technique using serially diluted (2 fold) plant extracts with little modifications. An equal volume of each extract and nutrient broth were mixed well. A specifically equal amount of standardized inoculum (1 × 107 cfu/ml) and resazurin sodium salt indicator were added in each well. The plates were incubated at 37 °C for 24 h. The negative control used was broth media, resazurin sodium salt indicator, and bacterial inoculum. The lowest concentration (highest dilution) of the extract that produced no color change (purple to pink) when compared with the control was regarded as MIC for those particular bacteria.
% Inhibition = 100- [OD of culture with sample (Test)] / OD of culture without sample (Control) × 100]
RESULTS:
Phytochemical Analysis: The results for the phytochemical analysis obtained are depicted below in Table 1. The result showed that acetone extract of the leaf Acacia concinna leaf contains alkaloids, tannins, and glycosides.
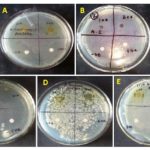
Determination of Zone of Inhibition by Disc Diffusion Assay: Out of five strain, Mycobacterium smegmatis and Bacillus subtilis showed zone of inhibition at the concentration of 200 mg/ml were 12 mm & 10 mm and minimum at 100 mg/ml were 10 mm & 8 mm respectively and control drug (Ampicillin) showed 9.3 mm & 9 mm respectively, but distilled water did not show any zone of inhibition in both bacteria.
When the acetone leaf extract was tested against Klebsiella pneumonia, Pseudomonas auregiuosa, Staphylococcus aureus no zone of inhibition was observed with all the concentration of extracts used, but the positive control exhibit 7.5 mm, 9 mm & 1 cm respective zone of inhibition but against Mycobacterium smegmatis and Bacillus subtilis, zone of inhibition was observed, maximum zone of inhibition was shown at the concentration of 200 mg/ml were 12 mm & 10 mm and minimum at 100mg/ml were 10 mm & 8 mm respectively and control drug (Ampicillin) showed 9.3 mm & 9 mm respectively, but distilled water did not show any zone of inhibition in both bacteria.
TABLE 1: QUALITATIVE ANALYSIS OF PHYTO-CHEMICAL SCREENING OF ACETONE EXTRACT OF ACACIA CONCINNA
| Phytochemical constituents | Result |
| Flavonoids | - |
| Alkaloids | + |
| Tannins | + |
| Saponins | - |
| Glycosides | + |
The + sign indicates the presence of phytochemical constituents and – sign indicates the absence of phyto-chemical constituent.
Determination of Minimal Inhibitory Concentration (MIC) by Micro Broth Dilution Technique: The leaf extract of Acacia concinna was analysed to find out the minimum inhibitory concentration against Mycobacterium smegmatis and Bacillus subtilis which exhibited maximum activity by disc diffusion assay among all the pathogens selected in the study. The results of the MIC are given below in Table 3.
TABLE 2: ANTIMICROBIAL EFFECT OF ACACIA CONCINNA ON ZONE OF INHIBITION AGAINST DIFFERENT BACTERIAL STRAINS
| Pathogens | Treatment | |||
| Acacia concinna
(100 mg/ml) |
Acacia concinna
(200 mg/ml) |
Positive control
Ampicillin (1 µg/ml) |
Negative
control (Broth Media) |
|
| Pseudomonas auregiuosa | Nil | Nil | 9.0 mm | Nil |
| klebsilla pneumonia | Nil | Nil | 7.5 mm | Nil |
| Staphylococcus aureus | Nil | Nil | 10 mm | Nil |
| Bacillus subtilis | 8 mm | 10 mm | 9 mm | Nil |
| Mycobacterium smegmatis | 10 mm | 12 mm | 9.3 mm | Nil |
FIG. 1: ZONE OF INHIBITION WHEN PLANT EXTRACT WAS TESTED AGAINST (A) KLEBSIELLA PNEUMONIAE (B) PSEUDOMONASAUREGIUOSA (C) STAPHYLOCOCCUS AUREUS (D) MYCOBACTERIUM SMEGMATIS (E) BACILLUS SUBTALIS
TABLE 3: MIC RESULTS AGAINST MYCOBACTERIUM SMEGMATIS
| S. no. | Microorganism | MIC (mg/ml) |
| 1 | Mycobacterium smegmatis | 2.5 |
| 2 | Bacillus subtilis | 1.25 |
DISCUSSION AND CONCLUSION: The present study was carried out to find out the phytochemical and the antibacterial potential of the leaves of Acacia concinna extracted with acetone solvent. The extract was analyzed qualitatively for the presence of various phytochemical compounds and alkaloids, tannins and glycosides were found to be present in the extract. The screening of secondary Metabolites has shown that higher plants represent a potential source of new anti-infective agents 30-33.
Earlier studies on phytochemical investigations on the leaf extract of Acacia concinna reported the presence of terpenoids 34-35 saponins 36, tannins 37. Johnson et al., 2005 and Raja, et al., (2011) also analyzed the leaf extract phytochemically and reported the presence of alkaloids and flavonoids, respectively 38-39. However, in the present study, flavonoids and saponins were found to absent in the leaf extract. This is probably due to the collection of plant material from a different geographical zone. In addition, the solvent used might also restrict the release of some phytocompounds during extraction.
In this Study, maximum activity was observed against Mycobacterium smegmatis, followed by Bacillus subtilis. The present study thus supports that the plants possess antibacterial potential. Similar to my results, earlier research also reported the antibacterial activity of leaf extract 37. Some of the research has also reported the antifungal activity of the Leaf extract. Ethanolic leaf extract of Acacia concinna has been shown to exhibit maximum activity against Aspergillus niger 37 followed by Penicillium species 39 and Candida albicans 37.
Plant extracts have assumed increased importance In the healthcare industry due to their biological properties. In this study, we used only the crude leaf extract of Acacia concinna. The survey can be extended further by identifying the active compound and compare the activity with known antimicrobial agents. The study should be carried out with other pathogens also like pathogenic fungi. In addition to acetone, more solvents can be used for investigating the biological properties of the plant. Thus, further future perspective works on the plant extract of Acacia concinna can be very useful as the plant seems to be a potential source for arresting the growth and metabolic activities of bacterial and fungal strains.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Carlini EA: Plants and the central nervous system. Pharmacology Biochemistry and Behavior 2003; 75(3): 501-12
- Tapsell, Linda C, Hemphill I, Cobiac L, Sullivan DR, Fenech M, Patch CS and Roodenrys S: Health benefits of herbs and spices: the past, the present, the future 2006
- Handral HK, Pandith A and Shruthi SD: A review on Murrayakoenigii: multipotential medicinal plant. Asian Journal of Pharmaceutical and Clinical Research 2012; 5(4): 5-14
- JJi HF, Li XJ and Zhang HY: Natural products and drug discovery. EMBO Reports 2009; 10(3): 194-200
- Alavijeh PK, Alavijeh PK and Sharma D: A study of antimicrobial activity of few medicinal herbs. Asian J Plant Sci Res 2012; 2(4): 496-502
- Kumar SR, Loveleena D and Godwin S: Medicinal property of murrayakoenigii-a review. Int Res J Biological Sci 2013; 2: 80-3.
- Meena K: Some rare, endemic & threatened angiosperms of Southern Rajasthan, India.
- Taxon: Acacia concinna (Wild.) DC. (n.d.). Retrieved December 30, 2015, from https://npgsweb.ars-grin.gov/gringlobal/ taxonomydetail.aspx?809
- Pantoporia (n.d.). Retrieved December 30, 2015, from http://www.nic.funet.fi/pub/sci/bio/life/insecta/lepidoptera/ditrysia/papilionoidea/nymphalidae/limenitidinae/pantoporia/index.html#hordonia
- Acacia concinna- Shikakai (n.d.). Retrieved December 30,2015, from http://www.flowersofindia.net/ catalog/ slides/Shikakai.html
- Sharma L, Agarwal G and Kumar A: Medicinal plants for skin and hair care. Indian Journal of Traditional Knowledge 2003; 2(1): 62-8.
- Prajapati ND, Purohit SS, Sharma AK and Kumar T: Medicinal plants. Agrobios published company, 3rd edition, India 2003; 353.
- Antimicrobial resistance. (n.d.). Retrieved December 30, 2015, from http://www.who.int/mediacentre/factsheets /fs194/en/
- Wuthi-udomlert M and Vallisuta O: In-vitro effectiveness of Acacia concinna extract against dermatomycotic pathogens. Pharmacognosy Journal 2011; 3(19): 69-73.
- Starr F, Starr K and Loope L: Acacia mangium.
- Smith AW: A gardener's handbook of plant names: their meanings and origins. Courier Corporation; 2013 Jun 10.
- Links, C. A. M. "Related Terms."
- Lech K and Brent R: Techniques for bacterial cell culture: media preparation and bacteriological tools. Current Protocols in Cytometry 2001: A-3E.
- Czeczot H, Tudek B, Kusztelak J, Szymczyk T, Dobrowolska B, Glinkowska G, Malinowski J and Strzelecka H: Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutation Research/Genetic Toxicology. 1990; 240(3): 209-16.
- Wu Y and Guo Y. [Determination of tannin in cotton plant]. Ying yong sheng taixuebao= The journal of applied ecology/Zhongguo sheng tai xuexuehui, Zhongguo-kexueyuan Shenyang yingyong sheng tai yanjiusuozhu ban 2000; 11(2):243-5.
- Sermakkani M and Thangapandian V: Phytochemical screening for active compounds in Pedalium murex Recent Research in Science and Technology 2010; 2(1).
- Edeoga HO, Okwu DE and Mbaebie BO: Phytochemical constituents of some Nigerian medicinal plants. African Journal of Biotechnology 2005; 4(7): 685-8.
- Ayoola GA, Coker HA, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia EC and Atangbayila TO: Phyto-chemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research 2008; 7(3): 1019-24.
- McFarland J: Standardization of bacterial culture for the disc diffusion assay. J Am Med Assoc 1987; 49: 1176-8.
- Whithear KG, Bowtell DD, Ghiocas E and Hughes KL: Evaluation and use of a micro-broth dilution procedure for testing sensitivity of fermentative avian mycoplasmas to antibiotics. Avian Diseases 1983: 937-49.
- Zulfiker AH, Siddiqua M, Nahar L, Habib MR, Uddin N, Hasan N and Rana MS: In-vitro antibacterial, antifungal & cytotoxic activity of Scoparia dulcis Int J Pharm Pharm Sci 2011; 3(2): 198-203.
- Wiegand I, Hilpert K and Hancock RE: Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols 2008; 3(2): 163-75.
- National Committee for Clinical Laboratory Standards 5th ed. Wayne, PA, USA: NCCLS; 2000. Methods for Dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standards. NCCLS document M7-A5. NCCLS.
- Bébéar C and Robertson JA: Determination of minimal inhibitory concentration. Molecular and diagnostic procedures in mycoplasmology 1996; 2: 189-99.
- Cowan RS: Maslin. Acacia miscellany: 17. Miscellaneous new taxa and lectotypifications in Western Australian Acacia, mostly section Plurinerves (Leguminosae: Mimosoideae) 1999; Nuytsia 12: 413-452.
- Johns SR, Lamberton JA and Sioumis AA: Alkaloids of the Australian Leguminosae. VII. Nb-Methyltetrahydro-harman from Acacia complanata A. Cunn. exBenth. Australian Journal of Chemistry 1966; 19(8): 1539-40.
- Matamala G, Smeltzer W and Droguett G: Comparison of steel anticorrosive protection formulated with natural tannins extracted from Acacia and from pine bark. Corrosion Science 2000; 42(8): 1351-62.
- Sahai R, Agarwal SK and Rastogi RP: Auriculoside, a new flavan glycoside from Acacia auriculiformis. Phytochemistry 1980; 19(7): 1560-2.
- Raja AX and Sama K: Phytochemical and biochemical analysis of the plant extract of Acacia concinna (Wild). Int J Pharm Res Develop 2012; 12: 136.
- Anjaneyulu AS, Bapuji M, Row LR and Sree A: Structure of acacigenin-B, a novel triterpene ester isolated from Acacia concinna. Phytochemistry 1979; 18(3): 463-6.
- Kiuchi F, Gafur MA, Obata T, Tachibana A and Tsuda Y: Acacia concinna II. Structures of monoterpenoid glycosides in the alkaline hydrolysate of the saponin fraction. Chemical and Pharmaceutical Bulletin 1997; 45(5): 807-12.
- Todkar SS, Chavan VV and Kulkarni AS: Screening of secondary metabolites and antibacterial activity of Acacia concinna. Research Journal of Microbiology 2010; 5(10): 974-9.
- Johnson W: Final report of the safety assessment of Acacia catechu gum, Acacia concinna fruit extract, Acacia dealbata leaf extract, Acacia dealbata leaf wax, Acacia decurrens extract, Acacia farnesiana extract, Acacia farnesiana flower wax, Acacia farnesiana gum, Acacia senegal extract, Acacia senegal gum, and Acacia senegal gum extract. International Journal of Toxicology 2005; 24: 75-118.
- Vashist H and Jindal A: Antimicrobial activities of medicinal plants-Review. Int J Res Pharm Biomed Sci 2012; 3(1): 222-30.
How to cite this article:
Antil R, Priyanka, Dahiya P and Singh N: Phytochemical and antibacterial potential of acetone leaf extract of Acacia concinna (Willd.) DC. Int J Pharmacognosy 2016; 3(3): 161-66. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.3(3).161-66.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
7
161-166
539
1851
English
IJP
R. Antil, Priyanka, P. Dahiya* and N. Singh
Department of Botany, Maharshi Dayanand University, Rohtak, Haryana, India
pushpa.dahiya@hotmail.com
30 January 2016
04 March 2016
27 March 2016
10.13040/IJPSR.0975-8232.IJP.3(3).161-166
31 March 2016