PHYTOCHEMICAL AND ANTI-CONVULSANT STUDY OF NYCTANTHES ARBOR-TRISTIS EXTRACT
HTML Full TextPHYTOCHEMICAL AND ANTI-CONVULSANT STUDY OF NYCTANTHES ARBOR-TRISTIS EXTRACT
Sweta Pundir * and Arvind Kumar
Department of Pharmaceutical Sciences, S. D. College of Pharmacy and Vocational Studies, Muzaffarnagar - 251001, Uttar Pradesh, India.
ABSTRACT: The drug is obtained from the dried fruit of Nyctanthes arbor-tristis of family Oleaceae. It is also called Coral Jasmine, Parijat, and Harsingar, etc. To confirm the veracity of the claim above, the anticonvulsant effect of the ethanolic extract has been evaluated. In this investigation, the rats were pretreated with different dose of Nyctanthes arbor-tristis ethanolic extract (100, 200 mg/kg) for 14th days and then, they were subjected to maximal electroshock-seizures, the dose of 100 and 200 mg/kg significantly reduced the duration of hind limb extension, and both the doses were statistically found to be equipotent. The reference, standard, Phenytoin (30 mg/kg) provided complete protection. Thus, the present study revealed the anticonvulsant effect of Nyctanthes arbor-tristis against maximal electroshock-induced convulsion in the rat.
| Keywords: |
Anticonvulsant, Nyctanthes arbor-tristis extract, Phenytoin, Maximal electroshock
INTRODUCTION: Nyctanthes arbour - tristis commonly called as coral jasmine. It is a tree growing to 10 m tall, with flaky grey bark. The leaves are simple, opposite, 6 - 12 cm long and 2 - 6.5 cm broad, with an entire margin. The flowers are fragrant, with a five to eight-lobed white corolla with an orange-red center. The calyx is 6 - 8 cm long. The fruit is a flat brown heart-shaped to round capsule 2 cm diameter, with two sections each containing a single seed 1. The plant is found in different parts of India, including Himalayan region, Western Uttar Pradesh, Punjab. It is also distributed in Bhutan, Nepal, Burma, and Bangladesh 2.
Different parts of Nyctanthes arbor-tristis are known to possess various ailments by rural mainly tribal people of India along with its use in Ayurveda, Siddha and Unani systems of medicines. Juice of the plant leaves is used as antimicrobial activity 3, anxiolytic activity 4, and hepatoprotective activity 5. In the traditional system of medicine, it is also used in nervous disorders so Nyctanthes arbour - tristis is recommended for the treatment of epilepsy 6.
Epilepsy is a common neurological disorder which is manifested by recurrent unprovoked seizures. It is affecting about 5% of the world population. A seizure is an abnormal function of ion channels and neural networks which results in rapid, synchronous, and uncontrolled spread of electrical activity in brain 7. This revival of interest in plant-derived drugs is mainly due to the current widespread belief that “Green Medicine” is safe and more dependable than the costly synthetic drugs, many of which have adverse effects 8.
Nyctanthes arbor-tristis belongs to the family Oleaceae and has chemicals constituents such as flavonoids 9, polyphenolic compounds, etc. It is used for the treatment of convulsions 10, aggression 11, diabetes 12 and inflammation 13 in Indian indigenous medical system. However, there is no scientific report available in support of anticonvulsant activity of fruit of Nyctanthes arbor-tristis in mice. Therefore, to justify the traditional claims we have assessed the anticonvulsant activity of fruit of Nyctanthes arbor-tristis in the rat.
MATERIAL AND METHOD:
Preparation of the Extract: The dried fruit of the plant were collected and powdered mechanically to a coarse powder. This powered drug was kept in airtight container until the time of use. About 1kg of the powder material was subjected to Soxhlet extraction using 70% ethanol for 48 h. The solvent was distilled off at low temperature under reduced pressure and evaporate at 40 ºC. The yield of the product was approximately 5% w/w. The ethanolic extract of Nyctanthes arbor-tristis (NAE) was stored in a refrigerator and reconstituted in water for injection just before use.
Phytochemical Screening: Qualitative test for the presence of secondary plant metabolites such as alkaloids, carbohydrates, saponins, glycosides, flavonoids, protein and amino acid, fixed oils, gum, and mucilage were carried out on the ethanolic extract using standard procedure.
Experimental Animals: Wister rat (8 - 10 weeks) of both sexes were fed with standard diet before and after during the experiment. After randomization into various groups and before initiation of experiment, the rats were acclimatized for 7 days under standard environmental conditions of temperature, relative humidity, and dark/light cycle. Animals described as fasting were deprived of food and water for 16 h ad libitum. The present study was conducted after getting experimental protocol approval from the Institutional animal ethics committee (SDCOP& VS/CPCSEA/IAEC/ 014/M.PHARM/2015).
Standard Drug: Phenytoin injection (Sun Pharmaceuticals Pvt. Ltd.,) was used as a standard drug and was administered intraperitoneally.
Acute Toxicity Studies: Acute oral toxicity study of ethanolic extract of Nyctanthes arbor-tristis was carried out according to OECD guidelines 423. Acute toxicity studies were conducted by using Wister rat of either sex weighing 150 - 200 gm. The animals fasted overnight before the experimental procedure. The method of Up and down or staircase was used to determine the dose. The median lethal dose of the extracts having anticonvulsant activity was determined by administering 100, 200, 2000 mg/kg dose and percent mortality was observed 24 h later.
Assessment of Anticonvulsant Activity:
Maximum Electroshock Induced Seizures (MES): The electrical shock applied through ear-clip electrodes separately to each mouse. The stimulus duration was 0.2 s and the current frequency 150 mA. Each group containing five rats which was administered with extract and standard drug (phenytoin 30 mg/kg I.P.) for seven days and on an experimental day, the test was started 30 min after drug administration. The animals were observed for the occurrence of tonic hind limb extension and mortality for a duration of 15 min. The NAE was administered to Group III, and IV (100 and 200 mg/kg body P.O) whereas group I and II received 1% of Tween 80 and Phenytoin 30 mg/ kg I.P. respectively.
Statistical Analysis: All the data were expressed in mean ± SEM. The significance of the difference in means between control and treated animals was determined by One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. Significance of difference between normal and control group was considered P<0.05.
RESULT AND DISCUSSION:
Preliminary Phytochemical Studies:
TABLE 1: PRELIMINARY PHYTOCHEMICAL SCREENING OF THE ETHANOLIC EXTRACT OF NYCTANTHES ARBOR-TRISTIS SHOWS THE PRESENCE OF VARIOUS FUNCTIONAL GROUPS
| Category | Name of the test | Pet. Ether | Chloroform | Ethanol | Distilled water |
| Alkaloids | Mayer’s test | - | + | - | - |
| Wagner’s test | + | + | + | + | |
| Hager’s test | - | + | + | + | |
| Dragendorff’s Test | - | - | + | - | |
| Carbohydrates and Glycosides | Molisch test | + | + | + | + |
| Fehling test | - | - | + | - | |
| Barfoed’s test | - | - | - | - | |
| Benedict's test | + | + | + | + | |
| Borntrager’s test | - | - | + | + | |
| Legal test | + | + | + | + | |
| Saponins | Foam test | + | + | + | + |
| Protein and
Amino acid |
Millon’s reagent | - | + | + | - |
| Biuret test | - | + | + | - | |
| Fixed oils and Fats | Spot test | - | - | - | - |
| Saponification test | + | + | - | - | |
| Phenolic compound and Flavonoids | FeCl3 test | + | + | + | + |
| Gelatin test | + | + | - | - | |
| Lead acetate | + | + | + | + | |
| Alkaline reagent | + | + | + | + | |
| Gum and Mucilage | Test for gum and mucilage | - | - | - | - |
Acute Toxicity Studies: There was neither change in the behavioral pattern or any sign of toxicity during the observations up to 24 h for mortality. The extracts were safe up to a maximum dose of 2000 mg/kg. The biological evaluation was carried out at doses of 100 and 200 mg/kg.
Assessment of Anticonvulsant Activity:
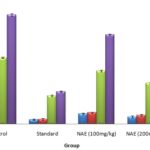
Effect on Maximum Electroshock Induced Seizures: NAE showed significant anticonvulsant activity by significantly lowering the duration of hind limb tonic extension (HLTE) induced by maximal electroshock. NAE at a dose of 100 mg/kg P.O. not showed significant reduction HLTE and reduced the mortality to 40%. NAE dose of 200 mg/kg P.O. significantly reduced the duration of HLTE (p<0.05) and no mortality was found. Phenytoin (30 mg/kg I.P.) significantly reduced the duration of MES-induced HLTE (p<0.01) and completely prevented the various phases of convulsion induced by MES.
TABLE 2: EFFECT OF ETHANOLIC EXTRACT OF NYCTANTHES ARBOR-TRISTIS AGAINST MES INDUCED SEIZURES IN RATS
| Group | Time taken (sec) | ||||
| Flexion | Extensor | Stupor | Recovery | % protection | |
| Control 1% Tween 80 (2 mL/kg, P.O) | 8.80 ± 0.37 | 13.20 ± 0.37 | 39.60 ± 0.50 | 65.20 ± 0.86 | 1/5 (20%) |
| Standard Phenytoin sodium (30 mg/kg, I.P.) | 2.60 ± 0.40** | 2.8 ± 0.52** | 16.80 ± 0.58** | 19.20 ± 0.58** | 5/5 (100%) |
| NAE (100 mg/kg, P.O.) | 6.0 ± 0.54 * | 6.8 ± 0.37* | 31.60 ± 0.50* | 53.20 ± 0.58* | 3/5 (60%) |
| NAE (200 mg/kg, P.O.) | 4.20 ± 0.66* | 5.20 ± 0.58* | 24.40 ± 0.50** | 47.0 ± 0.44** | 5/5 (100%) |
All the results were expressed as mean ± SEM (n = 5), for each experimental group. The statistical analysis was carried out using one way ANOVA method. Significant after analysis of variance (ANOVA) followed by Dunnett’s test. *P ≤ 0.05, **P≤ 0.01 and ***P≤ 0.001; when compared to control group.
FIG. 1: EFFECT OF ETHANOLIC EXTRACT OF NYCTANTHES ARBOR-TRISTIS ON MAXIMAL ELECTRO CONVULSION SHOCK (MES) INDUCED SEIZURE IN RATS
CONCLUSION: The present study revealed that Nyctanthes arbor-tristis ethanolic extract possesses significant anticonvulsant activity against MES induced convulsions. In conclusion, the ethanolic fruit extract of Nyctanthes arbour-tristis demonstrated possess anticonvulsant properties and less toxicity in experimental animals at the doses used. However, further studies still needed to be carried on the exposure of the extract to human and its use in folk medicine for seizures control.
ACKNOWLEDGEMENT: I express my sincere thanks to S. D. College of Pharmacy and vocational studies, Muzaffarnagar for their support in providing the facilities to complete the work.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Sandhar HK, Kaur M, Kumar B and Prasher S: An update on Nyctanthes arbor-tristis International Pharmaceutical Science 2011; 1(1): 77-86.
- Sharma S, Gupta S and Ahmad A: Evaluation of protective potential of Nyctanthes arbor-tristis against chloroform-induced Hepatotoxicity in rats. International Journal of Current Trends in Pharmaceutical Research 2013; 1(3): 194-04.
- Vyas A and Sarin A: Analysis of the Phytochemical Content and antimicrobial activity of Nyctanthes arbor tristis. Int J Pharm Bio Sci 2013; 4(1): 201-06.
- Tripathi S, Tripathi PK, Vijayakumar M, Rao ChV and Singh PN: Anxiolytic activity of leaf extract of Nyctanthes arbor-tristis in experimental rats. Pharmacology Online 2010; 2: 186-193.
- Mayee R, Thosar A and Kondapure A: Evaluation of hepatoprotective activity of the leaves of Nyctanthes arbor-tristis International Journal of Pharmaceutical and Clinical Research 2010; 2(3): 109-11.
- Cuddapah R, Reddy RK, Sekhar CKB and Das RC: Anticonvulsant activity of ethanolic extract of Nyctanthes arbor - tristis in albino mice. American Journal of Advanced Drug Delivery 2014; 2(6): 711-718.
- David EG, Armen HT, Ehrin JA and April WA: Principles of pharmacology. Lippincott Williams and Wilkins, New Delhi, Third Edition, 2012: 225-27.
- Suneetha D and Lakshmana RA: Stability-indicating hplc method for the determination of donepezil hydrochloride in bulk drug and pharmaceutical dosage form. Int J Pharma Sci and Tech 2011; 6: 34-43.
- Jain R and Mittal M: Naringenin, a flavonone from the stem of Nyctanthes arbor tristis International Journal of Biology Pharmacy Allied Sciences 2012; 1(7): 964-72.
- Singh D, Maurya VB, Prajapati SK, Kumar H, Pankaj S and Niranjan S: Evaluation of anticonvulsant activity of the leaves ethanolic and aqueous extracts of Nyctanthes Arbor tristis against seizures induced by pentylenetetrazole and electroconvulsive shock in mice. IJPSR 2010; 1(2): 63-71.
- Tripathi S and Tripathi PK: A study on anti-aggressive activity in fruits of Nyctanthes arbor-tristis Der Pharmacia Lettre 2013; 5(4): 7-12.
- Suresh V, Jaikumar S and Arunachalam G: Anti-diabetic activity of ethanol extract of stem bark of Nyctanthes arbor-tristis Research Journal of Pharmaceutical. Biological and Chemical Sciences 2010; 1(4): 311-17.
- Tripathi S and Tripathi PK: Anti-inflammatory activity of Nyctanthes arbor- tristis of fruit in rodents. Pharmacology online 2011; 3: 275-80.
How to cite this article:
Pundir S and Kumar A: Phytochemical and anti-convulsant study of Nyctanthes arbor-tristis extract. Int J Pharmacognosy 2018; 5(5): 298-01. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(5).298-01.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
7
298-301
533
1627
English
IJP
S. Pundir * and A. Kumar
Department of Pharmaceutical Sciences, S. D. College of Pharmacy and Vocational Studies, Muzaffarnagar, Uttar Pradesh, India.
swetapundir2011@gmail.com
21 December 2017
20 January 2018
13 February 2018
10.13040/IJPSR.0975-8232.IJP.5(5).298-01
01 May 2018