PHYTOCHEMICAL ANALYSIS, ANTIOXIDANT ACTIVITY AND GROWTH INHIBITORY EFFECTS OF EXTRACTS FROM ANNONA SENEGALENSIS, WALTHLERIA INDICA AND ANTHOCLEISTA VOGELII
HTML Full TextPHYTOCHEMICAL ANALYSIS, ANTIOXIDANT ACTIVITY AND GROWTH INHIBITORY EFFECTS OF EXTRACTS FROM ANNONA SENEGALENSIS, WALTHLERIA INDICA AND ANTHOCLEISTA VOGELII
Isaac L. Fayum 1, 2, Chukkol Ismail Buba 3, John Atogwe 1, Daniel Chinedu Okeoma 1 and Samuel Ehiabhi Okhale * 1
Department of Medicinal Plant Research and Traditional Medicine 1, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, P.M.B. 21 Garki, Abuja, Nigeria.
Department of Chemistry 2, Benue State University, P.O. Box 161, Makurdi, Nigeria.
Department of Chemistry 3, Abubakar Tafawa Balewa University, Bauchi, P.M.B. 0248, Bauchi, Nigeria.
ABSTRACT: Antioxidant potential, phytochemical and growth inhibitory characteristics of Anthocleista vogelii stem bark (AVSB) and root (AVRT), Annona senegalensis stem bark (ANSB) and Waltheria indica leaf (WILV) aqueous extracts were evaluated. Phytochemical analysis showed the presence of flavonoids, tannins, saponins, sterols, alkaloids, and terpenes. Fourier Transform Infra-Red (FT-IR) analyses indicated the presence of O-H, C-H, C=O, C=C, N-H and C-O bonds. AVSB, AVRT, ANSB, and WILV significantly (P<0.001) and dose-dependently (1-32 mg/mL) inhibited Sorghum bicolor seed radicle growth throughout 24-72 h compared with the control seeds. This result indicated the propensity of Anthocleista vogelii (stem bark and root), Annona senegalensis (stem bark) and Waltheria indica (leaf) extracts to inhibit the proliferation of fast-growing cells. The presence of the phytochemicals and the inhibitory activity of the extracts provided a preliminary indication of their cytotoxicity and potential for the management of cancer.
| Keywords: |
Anthocleista vogelii, Annona senegalensis, Waltheria indica, Antioxidant, Antiproliferative
INTRODUCTION: Natural products are regarded as major and important sources of molecules used in chemotherapy 1. Cancer is one of the leading causes of death worldwide, with an estimated 6.7 million deaths and 24.6 million people living with cancer in 2002. Presently, there is a global increase in prevalence, mortality, and health burden of various malignancies. World Health Organization (WHO) report projected that cancer prevalence rates could further increase by 50% to 15 million new cases in the year 2020. 2
Cancer is characterized by an uncontrolled proliferation of abnormal cells that can affect other organs of the body. This leads to the phenomenon of metastasis, which is the leading cause of death by cancer. Low or middle-income countries are the most affected, with nearly two-thirds of all cancer diagnoses 3. One of the prerequisites for the success of primary health care is the availability and use of suitable drugs. Traditional medicine is still the most affordable and easily accessible source of treatment in the primary healthcare system 4.
For the discovery of new anticancer agents from plants, the extracts are obtained from selected plant species and subsequently evaluated using several anticancer screening methods 5. To exploit medicinal plants as potential agents against different medical exigencies, it is imperative that the active principles are identified and the pharmacological properties are well documented considering that this will serve as an ethnobotanical database 6. Cancer can affect people of all ages, but risk tends to increase with age because DNA damage becomes more apparent in aging DNA. Statistics indicate that men are largely plagued by lung, colon, rectum, and prostate cancer, whilst women increasingly suffer from breast, colon, rectal, and stomach cancer 7. Antioxidants have become synonymous with good health; they are class of compounds that prevent certain types of chemical damage caused by an excess of free radicals generated by a variety of sources including, smoking, pesticide, and fumes from the exhaust. Destroying free radicals may help fight cancer, heart diseases, stroke, and other immune compromising diseases 8.
Medicinal plants represent a vast potential resource for anticancer compounds. Various medicinal plants reported having anti-proliferative activity. Cancer is the second leading cause of death worldwide. World Health Organization estimates that 80% of the world population depends mainly on traditional medicines for their basic health care. During the last decades of the 20th century, medical researchers have developed new methods for cancer treatment by combining surgery with chemotherapy, radiations, and various photo-chemical obtained from different plant species. It is important to note that chemotherapy does not only kills the cancer cells, but it also has some side effects on normal cells. Medicines obtained from plants have less or no side-effects 9. Over 200 types of cancer have been reported. Most cancers are classified according to symptoms presented by cellular morphology.
However, histological classification or grouping of cancers depends on identifying the basic cell type present and on the tissue of origin, not according to tissue in which they may have spread. The factors which cause cancer development are vaguely known. Exposure to particular substances has been linked to specific types of cancer; these substances are called carcinogens. Treatment modalities are always changing and developing. Options include surgery, radiation therapy, chemotherapy, hormone therapy, targeted therapy, and traditional herbal medicine 10. These agents have been used in the management of both benign and malignant tumors. In cancer chemotherapy, an active ingredient extracted from Podophyllum peltatum L. has been used by Native Americans in the treatment of cancer. However, the limitations of orthodox drugs, such as cost, accessibility, effectiveness, and side effects necessitate the search for cheap, safe, and effective phytomedicine in cancer management 11.
The genus Anthocleista is a medium-size tropical Africa genus composed usually of small trees or scrambling shrubs with soft white wood. It belongs to the family Loganiaceae. There are about 50 species in the genus Anthocleista, native mainly to tropical Africa, Madagascar, and Mascarene Island. Among the 50 species, six are found to be of economic importance in various parts of Nigeria. Of the six species in Nigeria, a phytogeographical study has revealed four species of common occurrence in parts of Niger Delta.
The species are completely glabrous and very large with rather leathering leaves; some of them being over 1-5 ft long even in mature trees and saplings. The base of the petiole is dilated (and sometimes more or less winged) running around the branch-let and joining the base of the opposite petiole, and leaving a conspicuous scar after falling. The stems of young trees of some species are unbranched or branched only at the top with huge leaves clustered at the end of the shoot giving rise to the popular name of cabbage tree. Some species have high characteristics 2-pronged spines close together or diverging. The inflorescences are terminal and very stout, completely hairless branching in trees with each lateral pairs of branches at right angles to the pair above and below 11.
Anthocleista of the Gentianaceae family contains 14 species of trees and shrub-like plants distributed in tropical Africa, in Madagascar, and on Comoros. Traditionally, they are commonly used in the treatment of diabetes, hypertension, malaria, typhoid fever, obesity, diarrhea, dysentery, hyperprolactinemia, abdominal pain, ulcer, jaundice, asthma, hemorrhoids, hernia, cancer, wounds, chest pains, inflammations, rheumatism, STDs, infertility and skin diseases. They serve as an anthelmintic, laxative, diuretic, and contraceptive. Chemical constituents and pharmacological activities of the Anthocleista species are so used to unveil opportunities for future research 12. Anthocleista vogelii is commonly known as ‘cabbage tree’ in English, ‘Sapo’ or ‘Apaoro’ in Yoruba, ‘Kwari’ In Hausa, ‘Orimi’ in Benin and ‘Odogwu.’ The leaves and stem bark are used for the treatment of leprosy, jaundice, bronchitis, and venereal diseases. The stem bark and seeds are also used in Nigeria as an antipyretic, tonic, and as purgative 13. A tree 6 to 20m high, trunk 15-55 cm diameter, twigs with spines, usually leaves to 40 cm long, even to 150cm, by 24 to 45 cm wide, inflorescence terminal with white sweet-scented flowers; of the closed-forest or mature regenerated jungle.
Annona senegalensis, commonly known as African custard-apple, wild custard apple, and wild soursop, is a species of flowering plant in the custard apple family, Annonaceae. The specific epithet, senegalensis, translates to mean “of Senegal,” the country where the type specimen was collected 14. It is a multi-purpose tree providing food, medicine, and a range of commodities for the local people. The plant is not generally cultivated but is often gathered from the wild. The fruit is sometimes sold in local markets. The plant is said to have the potential for domestication. Fruit- raw the edible white pulp of the ripe fruit has a pleasant, pineapple-like aroma with the flavor of apricots. The yellow to orange fruit is around 5 cm in diameter. The leaves are sometimes used as vegetables. The dried leaves contain 8.2% protein. The bark is used for treating guinea worms and other worms, diarrhea, gastroenteritis, snakebite, toothache, and respiratory infections.
Essential oil was found to be present in the leaves, among which car-3-ene and linalool being their major constituent. Perfume is made from boiled leaves. Ash from the wood is added to chewing or snuff tobacco and also is a solvent in soap production. This last probably means that the wood ashes are used as a source of potash for mixing with oil to make soap. The leaves are sometimes used as a stuffing when filling mattresses and pillows. Fiber from young sucker shoots is used in binding 15. Waltheria indica is commonly known as the sleepy morning, monkey bush, velvet-leaf, marshmallow, “hankufah” in Hausa, “kanakaloa” in Hawaii and “nallabenda” in India and is a plant growing in many regions of the world 15. Waltheria indica is a short-lived, perennial plant producing several erect or ascending stems that can be branched from the base. The stems become more or less woody and persist. The plant can grow from 0.5 - 2 m tall. The plant is harvested from the wild for local medicinal use and is also the source of fibre.
Existing reports revealed that W. indica had been reported to have the following activities: analgesic, anti-inflammatory, anti-bacterial, anti-fungal, anti-diarrheal, anti-malarial, antiviral, anti-convulsant, anti-anemic, and anti-oxidant activities. The pharmacological studies performed on W. indica revealed the therapeutic potential in the treatment of infectious diseases 16. The plant is antisyphilitic and febrifuge. A decoction of various plant parts is taken as a treatment for fever and syphilis. It is applied externally on skin eruptions and wounds. A decoction of the leafy stems is taken to relieve fevers, coughs, colds, bladder ailments, vaginal infections, hypertension, ulcer and as a remedy for hemoptysis. A decoction of the root is given as an antidiarrhoeal and general tonic to children.
It is also used as cough medicine and for healing wounds. General screening of the plant revealed the presence of some general flavonoids and caffeic acid. Three peptide alkaloids have been isolated: adouétine X, Y, and Z. Adouétine Z (in the form of its amidosulphonate) acts as a sedative of the central nervous system and as a stimulant of the medulla. In dogs, it produces hypertension, slows down the heartbeat, and has a relaxing action on the smooth muscle fibers of the intestine. Two antifungal flavonoids have been isolated from the chloroform extract of the plant. The first showed high antifungal activity against Candida albicans and low activity against T. mentagrophytes, while the second showed moderate antifungal activity against Aspergillus niger and Trichophyton mentagrophytes. A total aqueous extract of the plant showed in vitro antibacterial activity against 3 enterobacteria: Escherichia coli, Salmonella typhi, and Shigella dysenteriae at a minimum inhibitory concentration of 5, 2.5 and 2.5 mg/ml, respectively.
The medial lethal dose of the pet ether extract of A. vogelii (leaves) that would kill 50% of the animals in a population (LD50) was determined intra-peritoneally. Albino mice were divided into five groups of six (6) animals, each weighing between (18-20 g). The mice were subjected to 24 h fasting (with only water) before administering the extract. The extract was suspended in vehicle (tween 80 in distilled water, 3% v/v) was administered in dose of 1,000, 2000, 4,000, 6,000 and 8,000 mg/kg i.p. The sixth group served as control received only 3% tween 80. All animals were kept at room temperature in cross ventilated rooms without illumination at night. The mice were then observed for toxicity and fatalities over 48 h. The result of behavioral and toxicity studies on the extract showed that it was safe for further biological studies as no lethality was observed at 2000 mg/kg i.p. in mice. The behavioral signs of toxicity observed in mice given above 2000 mg/kg body weight include paw licking, salivation, stretching, and reduced activity 15. The aim of the present study is to evaluate the phytochemical, FTIR and antiproliferative properties of the leaves of Waltheria indica, stem bark of Annona senegalensis, stem bark and root of Anthocleista vogelii hot water extracts.
MATERIALS AND METHODS:
Plant Materials: The stem bark and root of Anthocleista vogelii, the leaves of Waltheria indica and the stem bark of Annona senegalensis were collected from the National Institute for Pharmaceutical Research and Development, Abuja (NIPRD) medicinal plant garden by Mallam Muazam Ibrahim and were identified by Mr. Akeem Lateefata Taxonomist at the herbarium of NIPRD, Abuja where voucher specimen of samples were deposited. The stem was cut off with the leaves and flower; air dried for two weeks and powdered using mortar and pestle.
Extraction of Plant Materials: Anthoclesta vogelii root and the stem bark, leaves of Waltheria indica and the stem bark of Annona senegalensis were grounded. Powdered plant materials (100 g) were measured and dissolved in 2000 ml quantity of boiling water for 24 h with stirring 17. At the end of the 24 h, the mixture was filtered with filter paper (Whatman no. 1). The extract was concentrated by heating over a water bath to obtain a solvent free extract.
Experimental Plant: Guinea corn (Sorghum bicolor) seeds were obtained from Karmo market, Nigeria in February 2017 and the seeds were subjected to viability test by submerging in water and observing their ability to remain submerged. The seeds that floated were discarded while the submerged seeds were allowed to dry before use.
Phytochemical Analysis: The phytochemical screening of the water extracts from the stem bark, root of Anthocleista vogelii, leaves of Waltheria indica and stem bark of Annona senegalensis were carried out using the method and procedures described by 18.
Fourier Transform Infrared Spectroscopy Analysis: Dried powder of the plant extracts were used for FT-IR analysis. Dried extract powder 10mg was encapsulated in 100 mg of KBr pellet, to prepare translucent sample discs. The powdered sample of each extract was loaded in FT-IR spectrophotometer (Shimadzu, Japan), with a scan range from 400 to 4000 cm-1 with a resolution of 4 cm-1.
Antioxidant Assay: The method of Muraina et al., 24 was used with some modifications. A two-fold dilution of 20 mg/mL of an extract with 50 µl of distilled water in a 96-well micro-dilution plate was performed. After that, 50 µl of 0.2 mg/ml of 3-(4, 5-dimethylimidazole-2-yl) -2, 5-diphenyltetrazolium bromide (MTT) was added to every well, and the plate was incubated for one week at 37 ºC after which the result was read. Distilled water was used as the negative control, and ascorbic acid was used as standard drug. Formation of bluish coloration/ precipitate was indicative of antioxidant activity. The lowest concentration of the sample at which the presence of antioxidant activity is detected, the highest dilution at which the formation of bluish coloration disappears was recorded as the minimum inhibitory concentration. The experiment was done in triplicate. To evaluate the DPPH radical scavenging activity of the extract, 10 µl of 10 mg/mL of extract in methanol was spotted on TLC plate and eluted in a polar solvent comprising the mixture of ethyl acetate, methanol in the ratio of 3:2. The developed plate was dried and immediately sprayed with 0.2% DPPH reagent in methanol and left at room temperature for 30 min. Yellow spots formed from bleaching of the purple color of DPPH reagent were evaluated as positive antioxidant activity 25.
Determination of Growth Inhibitory Effects: The modified method of Ayinde and Agbakwuru 19 was used for this study. From each extract, 3.2 g was weighed and dissolved in 100 ml of distilled water to obtained 32 mg/mL stock solution. Then, various concentrations (1 mg/mL, 2 mg/mL, 4 mg/mL, 8 mg/mL and 16 mg/mL) of stem bark and root of Anthocleista vogelii, leaves of Waltheria indica and Annona senegalensis were prepared from the stock solution by dilution. The standard drug for the work, methotrexate (2.5 mg) was made to a concentration of 0.05 mg/mL as the positive control. Petri dishes were layered with cotton wool and filter paper (Whatman no. 1). Twenty four (24) seeds of S. bicolor were placed in each of the Petri dishes.
The control seeds were treated with 15 ml of distilled water. The test seeds were treated with the different preparations of the four extracts as the seeds in each specific petri dish received 15 ml of a particular concentration. The seeds were incubated in a dark room and observed for growth after 24 h, 48 h and 72 h 19. The mean lengths (mm) of radicle emerging from the seeds were measured after each 24 h using divider and meter rule. The percentage of inhibition was calculated as:
Mean radicle length control ‒ Mean radicle length treated / Mean radicle length control ×100
Percentage growth was calculated as 100 ‒ % inhibition 22.
Statistical Analysis: The data obtained were expressed as mean ± standard error mean and analyzed using Graph pad prism (version 7). Two-way analysis of variance was used to test for significance at P<0.0001.
RESULTS AND DISCUSSION:
Phytochemical Analysis: The qualitative analysis of the aqueous extracts of Anthocleista vogelii, Annona senegalensis and Waltheria indica Table 1 showed the presence of major phytoconstituents like saponins, tannins, flavonoids, terpenes, alkaloids, and steroids. This can be compared with the study carried out by 21. Also, sample ANSB showed the presence of cardiac glycoside.
TABLE 1: PHYTOCHEMICAL COMPOSITION OF THE AQUEOUS EXTRACTS
| Test | AVSB | AVRT | ANSB |
| Saponins | + | + | + |
| Tannins | + | + | + |
| Flavonoids | + | + | + |
| Terpenes | + | + | - |
| Steroids | + | + | - |
| Alkaloids | + | + | + |
| Cardiac glycosides | - | - | + |
Keys: + = present; - = negative; Anthocleista vogelii stem bark (AVSB) and root (AVRT), Annona senegalensis stem bark (ANSB) and Waltheria indica leaf (WILV).
Fourier Transform Infrared Spectroscopy Analysis: FT-IR spectrophotometer was used to identify different chemical bonds and functional groups present in the chemical substance of the samples. The spectrum of ANSB sample extract showed the presence of following functional groups; 3426.88 cm-1 (N-H), 2936.94 cm-1 (C-H), 1617.30 cm-1 (C=O), 1410.29 cm-1 (C=C), 1050.00 cm-1 (C-O) and 778 cm-1. Sample AVSB showed the presence of following functional groups; 3417.45 cm-1 (N-H), 2927.89 cm-1 (C-H), 1611.68 cm-1 (C=O), 1420.89 cm-1 (C=C), 1067.03 cm-1 (C-O) and 705.93 cm-1. Sample AVRT showed the following functional groups; 3418.87 cm-1 (N-H), 2930.47 cm-1 (C-H), 2405.30 cm-1 (O-H), 1615.05 cm-1 (C=O), 1410.47 cm-1 (C=C), 1065.56 cm-1 (C-O) and 704.47 cm-1. WILV sample showed the following functional groups 3438.92 cm-1 (N-H), 2948.48 cm-1 (C-H), 1584.71, 1413.74 cm-1 (C=C), 1285.71 cm-1 (C-O), 1080.87 cm-1 (C-O), 777.59 and 648.60 cm-1.
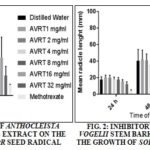
TABLE 2: GROWTH INHIBITORY ACTIVITY OF ANTHOCLEISTA VOGELII ROOT (AVRT) AQUEOUS EXTRACT
| Treatment | Mean radial length (mm) | % Inhibition* | % Growth† | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Control H2O | 17.6 ± 0.53 | 40.45 ± 2.14 | 50.98 ± 2.51 | 0 | 0 | 0 | 100 | 100 | 100 |
| Methotrexate | 4.45 ± 0.21 | 6.75 ± 0.15 | 10.08 ± 0.09 | 76.58 | 78.94 | 74.29 | 23.42 | 21.06 | 25.71 |
| AVRT 1 mg/mL | 17.15 ± 0.33 | 39.7 ± 1.30 | 54.9 ± 1.74 | 72.16 | 81.45 | 87.92 | 27.84 | 18.55 | 72.16 |
| AVRT 2 mg/mL | 16.8 ± 0.4 | 39.23 ± 1.45 | 50.18 ± 2.33 | 70.17 | 80.30 | 78.66 | 13 | 19.70 | 21.34 |
| AVRT 4 mg/mL | 16.25 ± 0.45 | 38.4 ± 1.44 | 49.35 ± 1.85 | 67.05 | 78.24 | 77.03 | 32.95 | 21.76 | 22.97 |
| AVRT 8 mg/mL | 14. 4 ± 0.52 | 34.03 ± 1.18 | 41.33 ± 1.43 | 56.53 | 47.52 | 61.30 | 43.47 | 52.48 | 38.70 |
| AVRT 16 mg/mL | 10.8 ± 0.73 | 27.88 ± 2.51 | 36.45 ± 3.09 | 36.08 | 52.24 | 51.73 | 63.92 | 47.76 | 36.08 |
| AVRT 32 mg/mL | 7.78 ± 0.57 | 15.33 ± 0.96 | 18.85 ± 1.39 | 18.92 | 21.21 | 17.29 | 81.08 | 78.79 | 82.80 |
Anthocleista vogelii root (AVRT). *Percentage inhibition was calculated as [(mean radicle length control-mean radicle length treated)/mean radicle length control] ×100. †Percentage growth was calculated as 100 - % inhibition. Methotrexate (0.05 mg/ml) was used as positive control; n=20.
TABLE 3: GROWTH INHIBITION ACTIVITY OF ANTHOCLEISTA VOGELII STEM BARK (AVSB) AQUEOUS EXTRACT
| Treatment | Mean radial length (mm) | % Inhibition* | % Growth† | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Control H2O | 17.6 ± 0.53 | 40.45 ± 2.14 | 50.98 ± 2.51 | 0 | 0 | 0 | 100 | 100 | 100 |
| Methotrexate | 4.45 ± 0.21 | 6.75 ± 0.15 | 10.08 ± 0.09 | 76.58 | 78.94 | 74.29 | 23.42 | 21.06 | 25.71 |
| AVSB growth inhibition 1 mg/mL | 20.45 ± 0.40 | 42.1 ± 1.77 | 56.08 ± 3.42 | 90.91 | 87.39 | 90.23 | 9.09 | 12.61 | 9.77 |
| AVSB 2 mg/mL | 19.88 ± 0.53 | 40.85 ± 1.22 | 49.1 ± 1.8 | 87.67 | 84.30 | 76.54 | 12.33 | 15.7 | 23.46 |
| AVSB 4 mg/mL | 17.8 ± 0.45 | 35.68 ± 1.38 | 40.18 ± 1.71 | 75.85 | 71.52 | 59.04 | 24.15 | 28.48 | 40.96 |
| AVSB 8 mg/mL | 17.05 ± 0.42 | 30.03 ± 1.43 | 35.8 ± 1.85 | 71.59 | 57.55 | 50.45 | 28.41 | 42.45 | 49.55 |
| AVSB 16 mg/mL | 15.08 ± 0.39 | 27.1 ± 1.02 | 37.28 ± 1.27 | 60.23 | 50.06 | 53.35 | 39.77 | 49.65 | 46.65 |
| AVSB 32 mg/mL | 11.93 ± 0.49 | 21.33 ± 1.18 | 30.18 ± 1.71 | 42.50 | 36.04 | 39.43 | 57.50 | 60.57 | 39.43 |
Anthocleista vogelii stem bark (AVSB) aqueous extract. *Percentage inhibition was calculated as [(mean radicle length control-mean radicle length treated)/mean radicle length control] × 100. †Percentage growth was calculated as 100 - % inhibition. Methotrexate (0.05 mg/ml) was used as positive control; n=20.
TABLE 4: GROWTH INHIBITION OF ANNONA SENEGALENSIS STEM BARK (ANSB) AQUEOUS EXTRACT
| Treatment | Mean radial length (mm) | % Inhibition* | % Growth† | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Control H2O | 17.6 ± 0.53 | 40.45 ± 2.14 | 50.98 ± 2.51 | 0 | 0 | 0 | 100 | 100 | 100 |
| Methotrexate | 4.45 ± 0.21 | 6.75 ± 0.15 | 10.08 ± 0.09 | 76.58 | 78.94 | 74.29 | 23.42 | 21.06 | 25.71 |
| ANSB 1 mg/mL | 22.58 ± 0.94 | 50.13 ± 1.98 | 63.48 ± 2.80 | 103.01 | 107.24 | 106.71 | -3.01 | -7.24 | -6.71 |
| ANSB 2 mg/mL | 20.38 ± 0.90 | 37.23 ± 1.35 | 47.58 ± 1.98 | 90.51 | 75.53 | 73.74 | 9.49 | 24.47 | 26.26 |
| ANSB 4 mg/mL | 18.6 ± 0.67 | 31.28 ± 1.52 | 40.28 ± 2.03 | 80.40 | 60.64 | 59.24 | 19.6 | 39.36 | 40.76 |
| ANSB 8 mg/mL | 15.65 ± 0.89 | 26.23 ± 1.45 | 36.13 ± 1.86 | 63.64 | 48.16 | 51.10 | 36.36 | 51.84 | 48.16 |
| ANSB 16 mg/mL | 14.03 ± 0.83 | 23.63 ± 1.09 | 31.7 ± 1.5 | 54.43 | 41.73 | 42.41 | 45.57 | 58.27 | 57.59 |
| ANSB 32 mg/mL | 4.17 ± 0.60 | 8.03 ± 1.17 | 10.33 ± 1.22 | -1.59 | 3.16 | 0.49 | 101.59 | 96.84 | 99.51 |
Annona senegalensis stem bark (ANSB) aqueous extract.*Percentage inhibition was calculated as [(mean radicle length control-mean radicle length treated)/mean radicle length control] × 100. †Percentage growth was calculated as 100 - % inhibition. Methotrexate (0.05 mg/ml) was used as positive control; n=20.
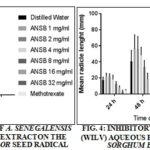
TABLE 5: GROWTH INHIBITION OF WALTHERIA INDICA LEAF (WILV) AQUEOUS EXTRACT
| Treatment | Mean radical length (mm) | % Inhibition* | % Growth† | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| Control H2O | 17.6 ± 0.53 | 40.45 ± 2.14 | 50.98 ± 2.51 | 0 | 0 | 0 | 100 | 100 | 100 |
| Methotrexate | 4.45 ± 0.21 | 6.75 ± 0.15 | 10.08 ± 0.09 | 76.58 | 78.94 | 74.29 | 23.42 | 21.06 | 25.71 |
| W. indica 1 mg/mL | 21.93 ± 0.61 | 56.15 ± 2.72 | 66.78 ± 2.50 | 99.32 | 122.12 | 111.22 | 0.68 | -12.12 | -11.22 |
| W. indica 2 mg/mL | 21.65 ± 0.54 | 55.7 ± 2.47 | 64.68 ± 2.53 | 97.95 | 121.01 | 107.10 | 2.05 | -21.01 | -7.10 |
| W. indica 4 mg/mL | 18.03 ± 0.83 | 47.33 ± 1.52 | 54.48 ± 2.25 | 77.16 | 100.32 | 87.1 | 22.84 | -0.32 | 12.90 |
| W. indica 8 mg/mL | 12.43 ± 0.47 | 33.7 ± 1.23 | 41.52 ± 1.78 | 45.34 | 66.63 | 61.67 | 54.66 | 33.37 | 38.33 |
| W. indica 16 mg/mL | 7.58 ± 0.39 | 18.3 ± 1.17 | 23.9 ± 1.25 | 17.78 | 28.55 | 27.11 | 82.21 | 71.45 | 72.89 |
| W. indica 32 mg/mL | 3.2 ± 0.26 | 5.1 ± 0.4 | 6.18 ± 0.42 | -7.10 | -4.08 | -7.65 | -107.1 | -104.08 | -107.65 |
*Percentage inhibition was calculated as [(mean radicle length control-mean radicle length treated)/mean radicle length control] × 100.†Percentage growth was calculated as 100 - % inhibition. Methotrexate (0.05 mg/ml) was used as positive control; n=20.
These functional groups identified corresponded with the phytochemical screening 21. From the results shown above, all the four samples showed similarities in functional groups.
Antioxidant Activity and Inhibitory Activities: Aqueous extracts (P<0.0001) inhibited S. bicolor seed growth at 24 h, 48 h and 72 h for all concentrations studied compared with the negative control (H2O). The growth inhibitory activity of the samples were observed at different concentrations starting from lowest concentration to highest concentration i.e. 1 mg/mL, 2 mg/mL, 4 mg/mL, 8mg/ml, 16 mg/mL, 32 mg/mL at different time interval of 24 h, 48 h and 72 h.
AVRT Extract: The negative control (distilled water) has no growth of inhibitory activity and was observed to be producing 100% growth as expected. A single concentration of 0.05 mg/mL Methotrexate was used throughout as the positive control. After 24 h, the percentage inhibitory growth activity of AVRT extract was observed to be 76.58% hence, producing an expected % growth of 23.42%. The activities of higher concentration extracts of AVRT was observed to correspond with that of methotrexate. But this is more prominent at 24 h interval Table 2.
AVSB Extract: The negative control (distilled water) has no growth of inhibitory activity and was observed to be producing 100% growth as expected. A single concentration of 0.05 mg/mL methotrexate was used throughout as the positive control. The growth inhibitory activity of AVSB extract at 4 mg/mL can be compared with the positive methotrexate control at 0.05 mg/mL at 24h interval Table 3.
ANSB Extract: Distilled water and methotrexate of 0.05 mg/mL were used as negative and positive control, respectively. The extract exhibited the same activity when compared with the positive control Table 4. WILV extract showed marginal growth inhibitory activities Table 5 when compared with methotrexate.
CONCLUSION: The aqueous extracts of Anthocleista vogelii, Annona senegalensis, and Walteria indica all exhibited growth inhibitory effects. Hence, these aqueous extracts contain some vital phytochemical constituents that helped in suppressing the free radicals, which subsequently lead to the inhibition of cancerous cells. This study provided preliminary evidence that supports the ethnomedicinal use of Anthocleista vogelii, Annona senegalensis and Walteria indicated in the treatment of cancer and other diseases.
ACKNOWLEDGEMENT: The authors wish to thank the management of National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Abuja, Nigeria, for providing facilities for this study.
CONFLICT OF INTEREST: There is no conflict of interest
REFERENCES:
- Françaa HS, Rochab L, Fernandeb CP, Ruizd ALTG and de Carvalhod JE: Antiproliferative activity of the hexanic extract and phloroglucinols from Hypericum brasilienseS. Rev Bras Farmacogn 2013; 23: 844-847.
- Okoye TC, Akah PA, Nworu CS and Ezik AC: Kaurenoic Acid isolated from the root bark of Annona senegalensis induces cytotoxic and antiproliferative effects against PANC-1 and HeLa cells. European Journal of Medicinal Plants 2014; 4(5): 579-589.
- Sawadogo WR, Schumacher M, ´le `ne Teiten MH, Dicato M and Diederich M: Traditional West African Pharma-copeia, plants and derived compounds for cancer therapy. Biochemical Pharmacology 2012; 84: 1225-1240
- Kumar V and Staden JV: A review of Swertia chirayita (Gentianaceae) as a traditional medicinal plant. Front Pharmacol 2016; 6: 308.
- de Melo Gomes J, a Araújo DSTA, de Almeida e Castro TNV, de Vasconcelos Cabral LD, Rodrigues DDM and do Nascimento CS: Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid Northeastern Brazil. Molecules 2010; 15: 8534-8542.
- Ilani P: GC-MS and NMR analysis of the bioactive compounds from the crude extracts of Waltheria indica and the histopathological changes induced in albino rats challenged with Naja nigricollis Journal of Coastal Life Medicine 2016; 4(5): 395-402.
- Shital CS, Manoj DG, Prashant SB and Pawar DP: Traditional medicinal plants for anticancer activity. International Journal of Current Pharmaceutical Research 2013; 5(4): 50-54.
- Odeja O, Obi G, Ogwuche CE, Elemike EE and Oderinlo Y: Phytochemical screening, antioxidant and antimicrobial activities of Senna occidentalis (L.) leaves extract. Clinical Phytoscience 2015; 1: 6.
- Kour A: Plants exhibiting potential for cancer treatment. Int J Pharm Sci Rev Res 2014; 27(2): 23-53.
- Titilayo GI and Morenikeji ES: A review of twenty ethnobotanicals used in the management of breast cancer in Abeokuta, Ogun State, Nigeria. African Journal of Pharmacy and Pharmacology 2016; 10(27): 546-564.
- Anyanwu GO, Nisar-ur-Rehman, Onyeneke CE and Rauf K: Medicinal plants of the genus Anthocleista-A review of their ethnobotany, phytochemistry and pharmacology. Journal of Ethnopharmacology 2015; 175: 648-667.
- Sunday RM, Ilesanmiand OR and Obuotor EM: Acute and sub-chronic oral toxicity of Anthocleista vogelii (Cabbage Tree) root hydroethanolic extract in Albino tats. British Journal of Pharmaceutical Research 2016; 12(1): 1-9.
- Okon OE, Gboeloh LB and Udoh SE: Antimalarial effect of combined extracts of the leaf of Ficusexasperata and stem bark of Anthocleista vogelii on mice experimentally infected with Plasmodium bergheiberghei (Nk 65). Research Journal of Medicinal Plants 2014; 8(3): 99-111.
- Zongo F, Ribuot C, Boumendjel A and Guissou I: Botany: Traditional uses, phytochemistry and pharmacology of Waltheria indica (syn. Waltheria americana): A review. J Ethnopharmacol 2013; 148(1): 14-26.
- Ilani P, Ajodo N, Adewusi F, Yakubu S, Cosmos VY and Eunice A: GC-MS and NMR analysis of the bioactive compounds from the crude extracts of Waltheria indica and the histopathological changes induced in albino rats challenged with Naja nigricollis Journal of Coastal Life Medicine 2016; 4(5): 395-402(8).
- Alaribe CSA, Coker HAB, Shode FO, Ayoola G, Adesegun SA, Bamiro J, Anyim EI and Anyakora C: Antiplasmodial and phytochemical investigations of leaf extract of Anthocleista vogelii (Planch). Journal of Natural Products 2012; 5: 60-67.
- Okhale SE, Ode SS, Oladosu P and Ugbabe GE: Evaluation of the antioxidant and anti-proliferative chemical constituents of pectinata (L.) aerial infusion. International Journal of Pharmacognosy 2018; 5(1): 8-14.
- Harborne JB: Phytochemical Methods. Chapman and Hill Ltd, London, Edition 3rd, 1973: 135-203.
- Ayinde BA and Agbakwuru U: Cytotoxic and growth inhibitory effects of the methanol extract Struchium sparganophora Ktze (Asteraceae) leaves. Pharmacognosy Magazine 2010; 6(24): 293-297.
- Chinedu E, Arome D, Ameh SF and Ameh GE: Evaluation of the antiproliferative and cytostatic effect of Citrus sinensis (orange) fruit juice. Int J App Basic Med Res 2014; 4(3): 20-22.
- Ehiabhi OS, Tijani AY and Ezugwu BO: Assessment of Antiproliferative Potential of Hexalobus crispiflorus (Annonaceae). British Biotechnology Journal 2016; 15(3): 1-7.
- Fakhroo A and Sreerama L: Qualitative analysis of phytochemical compounds in Ocimum basilicum grown in Qatar. International Journal of Applied Pharmaceutical and Biological Research 2016; 1(4): 11-17.
- Chinedu E, David A, Ameh SF and Ilomuanya U: Assessment of the antiproliferative potential of Citrullus lanatus (watermelon) fruit juice. Mintage Journal of Pharmaceuticals & Medical Sciences 2014; 3 (S-2): 1-3.
- Muraina IA, Suleiman MM and Eloff JN: Can MTT be used to quantify the antioxidant activity of plant extracts? Phytomedicine 2009; 16: 665-668.
- Fernandes AC, Cromarty A, Albrecht C, Constance E and Rensburg JV: The antioxidant potential of Sutherlandia frutescens. Journal of Ethnopharmacology 2004; 95: 1-5.
How to cite this article:
Fayum IL, Buba CI, Atogwe J, Okeoma DC and Okhale SE: Phytochemical analysis, antioxidant activity and growth inhibitory effects of extracts from Annona senegalensis, Walthleria indica and Anthocleista vogelii. Int J Pharmacognosy 2018; 5(11): 692-99. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(11).692-99.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
692-699
772
1260
English
IJP
I. L. Fayum, C. I. Buba, J. Atogwe, D. C. Okeoma and S. E. Okhale *
Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, P.M.B. 21 Garki, Abuja, Nigeria.
samuelokhale@gmail.com
11 September 2018
07 October 2018
20 October 2018
10.13040/IJPSR.0975-8232.IJP.5(11).692-99
01 November 2018