PHYTOCHEMICAL ANALYSIS AND EFFECTS OF AQUEOUS EXTRACT OF WITHANIA SOMNIFERA ON ISOLATED SMOOTH MUSCLE, PERFUSED HEART, BLOOD PRESSURE AND DIURESIS IN RATS
HTML Full TextPHYTOCHEMICAL ANALYSIS AND EFFECTS OF AQUEOUS EXTRACT OF WITHANIA SOMNIFERA ON ISOLATED SMOOTH MUSCLE, PERFUSED HEART, BLOOD PRESSURE AND DIURESIS IN RATS
S. Masoud 1, M. Abu-Zarga 2, A. Harb 1 and S. S. Abdalla * 1
Department of Biological Sciences 1, Department of Chemistry 2, School of Science, The University of Jordan, Amman, 11942, Jordan.
ABSTRACT: The plant Withania somnifera has many acclaimed medicinal uses. This work aims to explore the efficacy of the aqueous extract (AE) of W. somnifera on the isolated trachea and aorta rings, perfused heart, blood pressure and diuresis in rats. Phytochemical analysis of the whole plant of W. somnifera was carried out using standard extraction procedures followed by column chromatography. An aqueous extract (AE) was also prepared by boiling the whole plant material in water followed by evaporation under reduced pressure. The smooth muscle relaxant effect of AE of W. somnifera (10-4 - 3×10-1 mg/mL) was tested on isolated rat tracheal and aortic rings. The effect of AE on heart rate and contractility of the isolated perfused heart were recorded. The hypotensive effect of AE (0.4-120 mg/kg) was recorded from the carotid artery in anesthetized normotensive rats. The diuretic activity of AE was evaluated in rats at two different doses (800 and 1600 mg/kg), and urine volume was measured throughout 24 h. Phytochemical analysis of the Jordanian locality of W. somnifera resulted in the isolation of the four known compounds: withanone, withaferin A, β-sitosteryl glucoside, and 5, 6, 17, 27-tetrahydroxy withanolide. AE (10-4 - 3×10-1 mg/mL) caused modest relaxation of the carbachol-precontracted trachea but significant concentration-dependent relaxation of the phenylephrine precontracted aorta. Although AE doses (10-3 - 3 mg) had a negligible effect on the rate or the contractility of the isolated perfused heart, intravenous doses of AE (0.4-120 mg/kg) caused dose-dependent fall of systolic and diastolic blood pressure of anesthetized rats. When AE was administered orally at a dose of 1600 mg/kg, it significantly increased the rate of urine excretion measured in conscious rats. These observations provide scientific support for using W. somnifera in traditional medicine as a hypotensive, anti-asthmatic, and diuretic agent.
| Keywords: |
Withania somnifera, Phytochemistry, Hypotensive, Diuretic, Vasodilator
INTRODUCTION: Withania somnifera (Linn.) belongs to the Solanaceae family and grows in India, Africa, and the Mediterranean region. It is commonly known as “Ashwagandha” in Sanskrit and as "Indian ginseng" in Ayurveda 1.
Several common names have been given to this plant. For example, since it bears red cherry-like fruits, it has been called winter cherry in many places. In Jordan, it is popularly known as Samm Al-far ‘mouse poison’. The plant material is widely used for impotence and erectile dysfunction in Jordanian folk medicine 2.
W. somnifera has been reputed in the Ayurvedic medicinal system as an herbal tonic, and to increase longevity and vitality and to protect against neurotoxicity 3. It was traditionally prescribed as an aphrodisiac, for nervous exhaustion, bronchial asthma, fatigue, geriatric debility, memory-related conditions and insomnia 3, 4. The species name "somnifera" may be attributed to this latter use, indicating that it has a sedative effect 4. Clinical and experimental research has confirmed that W. somnifera improves male fertility by increasing testosterone level, semen volume, and sperm count and motility 2, 5, 6. The roots and leaves of this plant were regarded as a potential diuretic, hypoglycemic and hypocholesterolemic agent 7, 8, 9, 10, 11, 12. Other researchers reported anti-inflammatory, analgesic, antioxidant, anticancer, and immunomodulatory effects for W. somnifera 1, 13.
Clinical studies have shown that consumption of W. somnifera leaf and root extract causes a reduction in pulse rate, blood pressure, serum cortisol and C reactive protein in chronically stressed people with no side effects 14. Another clinical trial has shown that supplementation of W. somnifera with milk decreases both systolic and diastolic blood pressure in stress-oriented hypertensive subjects 15. Moreover, it has been documented that the administration of W. somnifera root powder protects against pulmonary hypertension in rats 16. The pharmacological activities of W. somnifera have been attributed to the presence of a large number of alkaloids, withanolides, flavonoids and saponins like sitoindosides VII and VIII. 17
Among withanolides, withaferin A is considered as the key metabolite of this plant. Withaferin A has great potential against multiple targets associated with cardiovascular disease including HMG-CoA reductase, angiotensinogen - converting enzyme, beta-adrenergic receptors and C-reactive protein 18. A withanolide isolated from Withania coagulans has a moderate hypotensive effect due to autonomic ganglion blocking action, a myocardial depressant effect as well as mild positive inotropic and chronotropic effects 19. The alkaloid content of the plant had hypotensive, bradycardia and pulmostimultory but variable effects on heart contractility 20.
Currently, W. somnifera is available for human use as a dietary supplement in different formulations. It is found either as a single herb or as a part of polyherbal or herbomineral formulations. The recommended dose of W. somnifera for human use is generally in the range of 4-6 g/day 15. Many formulations containing Withania somnifera are prescribed for a variety of musculoskeletal conditions, as a general tonic to increase energy, to improve overall health, and to prevent diseases in athletes and elderly 21.
Numerous ethnopharmacological studies have been done on this plant due to its nutritional and medicinal value. However, very limited scientific studies assessing its smooth muscle relaxant, hypotensive, diuretic and cardiac contractile potential are currently available 7, 15, 16, 20. Therefore, the present study aimed to explore the potential effects of the aqueous extract of W. somnifera on the rat isolated trachea and aorta by exploring its smooth muscle relaxant properties. The isolated perfused rat heart was also used as a model to evaluate the efficacy of this plant on cardiac contractility. Additional in-vivo experiments were performed for assessment of the hypotensive and diuretic potentials.
MATERIALS AND METHODS:
Chemicals: All chemicals used in the present study were purchased from Sigma Aldrich (Germany) unless stated otherwise. For extraction and chromatography, the chemicals used were petroleum ether, ethanol (Fluka), chloroform (GCC), hexane and methanol (BDH). For the in-vitro and in-vivo studies, the chemicals used were carbachol, phenylephrine, papaverine (Acros Organics, New Jersey), and furosemide. For the preparation of physiological salt solution (PSS), the used chemicals were (mM): NaCl 118, KCl 4.7; CaCl2 2.5; MgCl2 0.5; NaH2PO4 1.0; NaHCO3 24.0; and glucose 11.1. PSS was daily prepared while other stock solutions were prepared by dissolving them in distilled water or 0.9% NaCl, kept refrigerated until shortly before use where they were warmed to 37 °C.
Extraction and Chromatography: The whole plant of W. somnifera was collected from Jordan Valley, 20 km north of the Dead Sea. A voucher specimen was deposited at the Herbarium of the Department of Biological Sciences, The University of Jordan under the number S-File 10. The air-dried, ground plant material (11 kg) was defatted with petroleum ether; the remaining plant material was repeatedly extracted with 95% ethanol. Ethanol was evaporated under reduced pressure, resulting in a gummy material (1.5 kg) which was subjected to further fractionation with 1:1 H2O-CHCl3. The chloroform layer was separated and chloroform was evaporated. The chloroform extract was then treated with hexane-10% aqueous methanol, and the aqueous methanol layer was separated. Methanol was then evaporated under reduced pressure, giving 500 g extract, which was adsorbed onto 100 g silica gel and loaded onto a glass column containing 900g silica gel, eluted with chloroform then increasing the polarity with methanol resulting in 68 fractions (500 ml each). Fractions 2-16, 22-32, 39-40, and 48-51 were treated with methanol, yielding a white solid of withanone, withaferin A, 5,6,17,27-tetrahydroxy withanolide, & β-sitosteryl glucoside, respectively.
Preparation of the Aqueous Extract of W. somnifera: AE was prepared by boiling 50 g of the ground plant material in one liter of distilled water for 15 min with continuous stirring. The solution was filtered, and the filtrate was evaporated under reduced pressure at 60 °C. The extract was wrapped with aluminum foil as a precaution against photo-oxidation. A stock solution of AE was prepared by dissolving the powdered extract in 0.9% NaCl solution, and dilutions thereof were prepared with 0.9% NaCl solution.
Animals: The experiments were conducted on male and female Wistar albino rats (Rattus norvegicus) weighing 300 ± 50 g. The experimental procedures involving animals were carried out by the guidelines of the Committee for Control and Supervision on Experiments on Animals (CPCSEA) and with guidelines and regulations of the University. The study approved by the Ethical Committee of the Institution.
In-vitro Preparations: Male and female rats were lightly anesthetized with ether, and then sacrificed by a blow to the head. The chest cavity was opened to obtain the whole heart, trachea, and aorta. The trachea was cleaned of excess tissues, and two transversely-cut rings (4-5 mm each) were obtained from the middle of the trachea and prepared for the recording of isometric contractions. The main trunk of the aorta was cleaned of excess tissue and cut into two rings (3-4 mm each) which were prepared for the recording of isometric contractions.
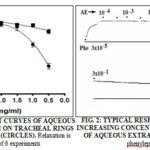
Preparations were mounted individually in water-jacketed 10 ml glass tissue baths and connected from one end to a thread connected to a force transducer (Grass FT 03) and from the other end to a glass hook fixed to the bottom of the tissue bath. The transducer was connected to a physiograph (Graphtec Thermal Arraycorder, WR 5000). Tissues were left to equilibrate in the tissue baths for 90 min under a tension of 2 g and at a temperature of 37 ± 0.5 °C. Tissue baths were aerated with 95% O2 - 5% CO2 gas mixture all through the experiment. After equilibration, tracheal rings were precontracted with 5×10-5 M carbachol and the aorta rings were precontracted with 3×10-5 M phenylephrine. After the contractions in these two preparations reached a stable plateau, cumulative concentration-response curves of AE were established by increasing the concentration 3 times after the effect to the previous concentration reached a stable plateau. After the last response had plateaued, papaverine (10-3 M) was added to cause a maximum relaxation of the tissue. Responses of the trachea and aorta were expressed as a percent of the maximum relaxation to papaverine.
In isolated perfused heart experiments, the heart with a piece of the aorta was excised and placed in ice-cold PSS to stop the contractions (5-10 sec). The aorta was mounted to a cannula, and the heart was perfused retrogradely through the aorta with aerated PSS from a reservoir located 70 cm above the heart and left to equilibrate to reach a stable plateau (10-15 min). The reservoir contained PSS gassed continuously with a mixture of 95% O2 and 5% CO2. A small, light stainless steel hook was inserted into the apex of the heart and connected by a thread to a force transducer connected to a physiograph (Gilson Medical Electronics). Isometric concentrations were recorded under a tension of 1 gm 22. Different concentrations of AE were individually injected through a needle located immediately above the aorta. The heart was then perfused with PSS again before the injection of the next higher concentration of AE. Each response to AE was calculated as the percent of the control response obtained with PSS immediately before AE administration.
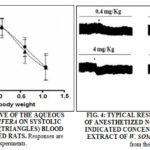
Blood Pressure Measurement: Male rats were anesthetized with thiopental (50 mg/kg body weight; i.p.). The right common carotid artery was catheterized for the recording of blood pressure using P23AA Statham pressure transducer situated at the level of the heart and connected to a Gilson polygraph. The right femoral vein was also catheterized for the intravenous injection of AE 22. AE was injected in doses of 0.04, 0.12, 0.4, 1.2, 4, and 12 mg/kg body weight. The changes in systolic and diastolic blood pressure were recorded and expressed as a percent of their respective control values obtained before AE administration.
Diuresis: Male rats were deprived of food for 24 hrs and water for 30 min before the beginning of the experiment. Animals were divided into 4 groups (each of 6 animals), and were administered orally with 10 ml/kg of the following treatments: Group 1: received 0.9% NaCl solution as a control group; Group 2: received AE at a concentration of 800 mg/kg body weight; Group 3: received AE at a concentration of 1600 mg/kg body weight; Group 4: received 40 mg/kg of furosemide, a known diuretic agent, as a positive control group. Animals were then individually housed into metabolic cages (North Kent Plastic Cages LTD), and urine was collected continuously in graduated cylinders where the volume was recorded every two hours for a total of 24 h. W. somnifera is considered safe since the acute oral LD50 of this plant in Wister rats was greater than 2000 mg/kg 23.
Statistical Analysis: All data are presented as means ± SEM. One-way analysis of variance (ANOVA) and Student’s t-test for independent samples was used to detect differences between the means. Differences were declared significant when P<0.05. Experimental data were analyzed by a computer fitting procedure using GraphPad Prism 5 software.
RESULTS:
Chemical Analysis of W. somnifera: Three withanolides and one sitosterol were isolated and identified from W. somnifera in this study. These isolated compounds and their quantities are listed in Table 1.
TABLE 1: COMPOUNDS ISOLATED FROM W. SOMNIFERA AND THEIR QUANTITIES
| Compound | Quantity (mg) | % of dry weight |
| Withanone | 5,900 | 0.054 |
| Withaferin A | 710 | 0.006 |
| 5,6,17,27-tetrahydroxywithanolide | 37 | 0.0003 |
| β- sitosteryl glucoside | 1,000 | 0.009 |
Effect of Aqueous Extract of W. somnifera on the Relaxation of Isolated Smooth Muscle and Cardiac Contractility: AE of W. somnifera (10-4 – 3×10-1 mg/mL) caused a modest relaxation of the carbachol-precontracted trachea and a larger relaxation of the phenylephrine-precontracted aorta Fig. 1. The maximum relaxation was 12.7 ± 1.7% and 39.0 ± 2.2% of papaverine maximum for the two preparations, respectively, Fig. 1. A typical response of the aorta to increasing concentrations of AE is shown in Fig. 2.
Doses ranging between 0.001 to 3 mg had an insignificant inhibitory effect on the contractility or the rate of the isolated perfused heart (data not shown).
Effect of Aqueous Extract of W. somnifera on Blood Pressure: Intravenous doses of AE (0.4 – 12 mg/kg b.w.) caused dose-dependent fall of systolic and diastolic blood pressure Fig. 3. The largest dose caused a transient decrease in blood pressure followed by a sustained reduction of blood pressure. A typical experiment demonstrating the effect of AE on blood pressure is shown in Fig. 4.
Effect of Aqueous Extract of W. somnifera on Diuresis: Treatment of animals with 800 mg/kg of AE caused an insignificant increase in the urine volume excreted by rats whereas animals treated with 1600 mg/kg of AE showed a significant increase in urine volume when compared to the control animals treated with 0.9% NaCl. Animals treated with a known diuretic agent, furosemide, in a concentration known to cause diuresis excreted significantly increased volume of urine when compared to the control animals Table 2.
TABLE 2: URINE VOLUME (ml) IN RATS COLLECTED AFTER ADMINISTRATION OF 0.9% NaCl, AE AND FUROSEMIDE OVER A PERIOD OF 24 ha
| Treatment
|
Time after administration | |||
| 2 h | 6 h | 12 h | 24 h | |
| Control (0.9% NaCl) | 0.7 ± 0.34 | 1.5 ± 0.5 | 2.6 ± 0.6 | 4.5 ± 0.7 |
| AE (800 mg/kg) | 0.5 ± 0.2 | 1.9 ± 0.5 | 3.4 ± 0.4 | 5.7 ± 0.6 |
| AE (1600 mg/kg) | 1.2 ± 0.5 | 2.7 ± 0.4 | 4.4 ± 0.2* | 6.9 ± 0.2** |
| Furosemide (4 mg/kg) | 11.5 ± 1.0*** | 14.5 ± 0.9*** | 16.0 ± 1.0*** | 18.8 ± 1.4*** |
aValues are expressed as means ± SEM (n=6/group). *p< 0.05, **p< 0.01, and ***p< 0.001 compared with control group during the same time course.
DISCUSSION: Withania somnifera is a well-known and valued medicinal plant that has been used for centuries for its nutritional and remedial potentials. It is enriched with phytochemical compounds that possess many health benefits. Phytochemical analysis of Withania somnifera revealed that over 35 withanolides have been isolated and identified, including steroidal lactones (withanolides and withaferins), and 12 alkaloids (somniferine, tropine, withananine and anaferine) as well as sitoindosides (saponins) and glycosides 17, 24. The biologically most active compounds are the alkaloids, withanolides, and sitoindosides.17 In the current study, partial chemical analysis of the methanol extract of the Jordanian locality of W. somnifera (whole plant including roots) yielded four known compounds; withanone, withaferin A, b-sitosteryl glucoside, and 5, 6, 17, 27-tetrahydroxy withanolide 24, 25.
In the present study, the aqueous extract of W. somnifera was found to cause a reasonable relaxation of tracheal segments, and it was also found to cause a decrease in the tone of aortic segments. The smooth muscle relaxant effect of W. somnifera AE may be attributed to the presence of several active ingredients which are known to cause relaxation of many smooth muscles including vascular muscles. These compounds include ashwagandholine, withanolides (withaferin A and withanone), β-sitosterol and flavonoids (quercetin, catechin, hesperetin, and naringenin) 26. The vasodilator and bronchodilator effects of these compounds are mediated by several suggested mechanisms in which they ultimately decrease intracellular Ca2+. For example, ashwagandholine, the total alkaloids extracted from the roots of W. somnifera, has relaxant and antispasmodic effects against various agents that produce smooth muscle contractions in intestinal, uterine, tracheal, and vascular muscles 20, whereas withanolides isolated from W. somnifera displayed dose-dependent (0.005–1.0 mg/mL) spasmolytic and Ca2+ antagonistic potential in isolated rabbit jejunum which lead to smooth muscle relaxation 27.
Also, withanone extracted from W. somnifera, in our laboratory, caused dose-dependent (0.001-3 µg/mL) smooth muscle relaxation in isolated rat aorta and tracheal preparations (unpublished observations), whereas β-sitosterol had a potent spasmolytic effect via Ca2+ antagonistic mechanism in isolated rabbit jejunum28. On the other hand, the flavone catechin has antispasmodic, bronchodilator and vasodilator activities by blockade of Ca2+ influx, and it induces vasodilation by activation of muscarinic receptors on the endothelium, thereby stimulating endothelium-dependent production of nitric oxide 29. Quercetin may be responsible for the bronchodilator effect of W. somnifera because it potentiates beta-agonist action through inhibition of both PLCβ and PDE4 which help relieve bronchospasm in asthma 30. Also, it inhibits rat tracheal tone via presynaptic and postsynaptic mechanisms in which the presynaptic mechanism is NO-mediated 31.
Furthermore, quercetin acts as a vasodilator through inhibition of L-type voltage-gated Ca2+ channels current and activation of protein kinase C 32. Hesperetin selectively inhibits the activity of phosphodiesterase (PDE4) and causes an increase in cAMP, and resulting in bronchodilation 33, whereas the flavone naringenin has enzymatic inhibitory activity against phosphodiesterase-1 (PDE1), a well-known enzyme involved in airway smooth muscle activity and airway inflammation, leading to bronchodilation and reduced airway inflammation 34. It is also worth noting that W. somnifera inhibited the antigen-induced bronchospasm in Balb/c mice through an anti-inflammatory activity which is manifested by a decrease in white blood cells in both bronchial lavage and blood smear 35.
On the other hand, the present experiments on the isolated perfused heart failed to show any marked effects on the contractility or the rate of the heart. This seems to be in contradiction with other works that showed cardiotropic and cardioprotective effects for W. somnifera on the frog and rat hearts 36, 37. This controversy may be due to species differences, differences in the plant part used or due to a difference in the experimental protocols. The previous studies used W. somnifera in-vivo in relatively high concentrations for several days whereas, in our study, W. somnifera was used in-vitro in small concentrations. In the whole organism, it is reasonable to assume the interference of many reflexes which could shape the final response.
The hypotensive effect of the aqueous extract of W. somnifera in unconscious rats observed in the present experiments is partly consistent with other works that showed that W. somnifera extract decreased arterial systolic and diastolic blood pressure in normotensive dogs 38 and in stress-oriented hypertensive subjects 14, 15. Furthermore, it protected against pulmonary hypertension in rats 16. Therefore, the in-vitro vasodilator effect of W. somnifera and the effect on blood pressure observed in anesthetized rats in this work support the reported hypotensive action. It has been documented that W. somnifera can produce NO which is known to dilate blood vessels and may, therefore, lead to hypotension 39.
The high concentration of the aqueous extract (1600 mg/kg) increased urine volume excreted throughout 24 h, but this increase was not as marked as that caused by a reference diuretic drug in this experiment. Our observations support a significant diuretic effect for AE of W. somnifera which is consistent with previous reports that showed that roots of W. somnifera caused an increase in urine sodium and urine volume 7. It is likely that the observed diuretic action of W. somnifera could be due to the presence of some active constituents that have a diuretic effects such as gallic acid 40, quercetin 41, and withaferin A 42. The diuretic activity of the extract may also be attributed to the mineral contents such as potassium, sodium, and magnesium 43. The mineral analysis of different parts of W. somnifera showed that this plant contains Na, K, Ca, Mg, N, P, Mn, Zn, Fe and Cu 44. These compounds (both organic and inorganic) might be acting synergistically to stimulate diuresis. Moreover, W. somnifera extract is one of the components of a polyherbal formulation known as "NR-ANX-C' that has diuretic activity. It has been reported that the possible mechanism of the diuretic property of the NR-ANX-C formulation is strong saluretic action which increases urinary Na+ level thereby inhibiting the Na+ re-absorption in the nephron 45.
CONCLUSION: In conclusion, the plant Withania somnifera contains many active ingredients and most of these are water soluble and can theoretically be found in the aqueous extract as we prepared it. The present experiments support the therapeutic importance of W. somnifera aqueous extract in ameliorating asthma by acting as a bronchodilator and in hypertension by acting as a vasodilator and as a diuretic. The diuretic effect may be useful in treating heart failure, pulmonary edema, and hypertension, as suggested by ethnopharmacological studies.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: No conflict of interest.
REFERENCES:
- Kumar V, Dey A, Hadimani MB, Marcović T and Emerald M: Chemistry and pharmacology of Withania somnifera: An update. TANG 2015; 5(1): e1.
- Abbas MA: Is the use of plants in Jordanian folk medicine for the treatment of male sexual dysfunction scientifically based? Review of in-vitro and in-vivo human and animal studies. Andrologia 2017; 49(3): e12619.
- Elhadidy ME, Sawie HG, Meguid NA and Khadrawy YA: Protective effect of ashwagandha (Withania somnifera) against neurotoxicity induced by aluminum chloride in rats. Asian Pacific J Trop Biomed 2018; 8(1): 59-66.
- Verma SK and Kumar A: Therapeutic uses of Withania somnifera (ashwagandha) with a note on withanolides and its pharmacological actions. Asian J Pharm Clin Res 2011; 4(1): 1-4.
- Ahmad MK, Mahdi AA, Shukla KK, Islam N, Rajender S and Madhukar D: Withania somnifera improves semen quality by regulating reproductive hormone levels and oxidative stress in seminal plasma of infertile males. Fertil Steril 2010; 94(3): 989-96.
- Ambiye R, Langade D, Dongre S, Aptikar P, Kulkarni M and Dongre A: Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: A pilot study. Evid Based Complement Altern Med 2013; 2013: 571420.
- Andallu B and Radhika B: Hypoglycemic, diuretic and hypocholesterolemic effect of Winter cherry (Withania somnifera, Dunal) root. Ind J Exp Biol 2000; 38: 607-09.
- Gajjar AV and Deshpande SS: Evaluation of anti-atherosclerotic and anti-hyperlipidemic effect of cyclosporine and ashwagandha in high-fat-diet-induced atherosclerosis in rats. J Pharm Res 2016; 10(5): 334-42.
- Laylani LAS and Saleh AH: Alcoholic extract effect of Withania somnifera roots on cholesterol diet-induced hyperlipidemia in male rabbits. Iraqi J Sci 2018; 59(1B): 267-70.
- Sarangi A, Jena S, Sarangi AK and Swain B: Anti-diabetic effects of Withania somnifera root and leaf extracts on streptozotocin-induced diabetic rats. J Cell Tissue Res 2013; 13: 3597-01.
- Udayakumar R, Kasthurirengan S, Mariashibu TS, Rajesh M, Anbazhagan VR, Kim SC: Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int J Mol Sci 2009; 10: 2367-82.
- Visavadiya NP and Narasimhacharya AV: Hypo-cholesteremic and antioxidant effects of somnifera (Dunal) in hypercholesteremic rats. Phytomedicine 2007; 14: 136-42.
- Grunz-Borgmann E, Mossine V, Fritsche K and Parrish AR: Ashwagandha attenuates TNF-α-and LPS-induced NF-κB activation and CCL2 and CCL5 gene expression in NRK-52E cells. BMC Complement Altern Med 2015; 15(1): 434.
- Auddy B, Hazra J, Mitra A, Abedon B and Ghosal S: A standardized Withania somnifera extract significantly reduces stress-related parameters in chronically stressed humans: a double-blind, randomized, placebo-controlled study. J Am Nutraceutical Assoc 2008; 11(1): 50-56.
- Kushwaha S, Betsy A and Chawla P: Effect of Ashwagandha (Withania somnifera) root powder supplementation in treatment of hypertension. Ethno-Medicine 2012; 6(2): 111-15.
- Kaur G, Singh N, Samuel SS, Bora HK, Sharma S and Pachauri SD: Withania somnifera shows a protective effect in monocrotaline-induced pulmonary hypertension. Pharm Biol 2015; 53(1): 147-57.
- Pratibha C, Madhumati B and Akarsh P: Therapeutic properties and significance of different parts of ashwagandha-A medicinal plant. Int J Pure App Biosci 2013; 1(6): 94-01.
- Ravindran R, Sharma N, Roy S, Thakur AR, Ganesh S and Kumar S: Interaction studies of Withania somnifera’s key metabolite withaferin A with different receptors associated with cardiovascular disease. Curr Comput Aided Drug Des 2015; 11(3): 212-21.
- Ojha SK and Arya DS: Withania somnifera Dunal (Ashwagandha): A promising remedy for cardiovascular diseases. World J Med Sci 2009; 4(2): 156-58.
- Malhotra CL, Das PK, Dhalla NS and Prasad K: Studies on Withania ashwagandha, Kaul. IV. The effect of total alkaloids on the cardiovascular system and respiration. Indian J Med Res 1981; 49: 448-60.
- Geetha M and Rajasekar S: Quality assessment of phytochemical analysis of Withania somnifera. Int J Curr Res Life Sci 2018; 7(4): 1435-37.
- Abdalla S, Zarga MA and Sabri S: Effects of the flavone luteolin, isolated from Colchicum richii, on guinea-pig isolated smooth muscle and heart and on blood pressure and blood flow. Phytother Res 1994; 8(5): 265-70.
- Patel SB, Rao NJ and Hingorani LL: Safety assessment of Withania somnifera extract standardized for Withaferin A: Acute and sub-acute toxicity study. J Ayurveda Integr Med 2016; 7(1): 30-37.
- Chatterjee S, Srivastava S, Khalid A, Singh N, Sangwan RS and Sidhu OP: Comprehensive metabolic finger-printing of Withania somnifera leaf and root extracts. Phytochemistry 2010; 71(10): 1085-94.
- Madina BR, Sharma LK, Chaturvedi P, Sangwan RS and Tuli R: Purification and characterization of a novel glucosyltransferase specific to 27β-hydroxy steroidal lactones from Withania somnifera and its role in stress responses. Biochim Biophys Acta- Proteins Proteom 2007; 1774(9): 1199-07.
- Sivamani S, Joseph B and Kar B: Anti-inflammatory activity of Withania somnifera leaf extract in stainless steel implant induced inflammation in adult zebrafish. J Genet Eng Biotechnol 2014; 12(1): 1-6.
- Choudhary MI, Nawaz SA, Ul-Haq Z, Lodhi MA, Ghayur MN and Jalil S: Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Bioche Bioph Res Com 2005; 334(1): 276-87.
- Gilani AH, Khan AU, Raoof M, Ghayur MN, Siddiqui BS and Vohra W: Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ Fundam Clin Pharmacol 2008; 22(1): 87-99.
- Ghayur MN, Khan H and Gilani AH: Antispasmodic, bronchodilator and vasodilator activities of (+)-catechin, a naturally occurring flavonoid. Arch Pharmacal Res 2007; 30(8): 970-75.
- Townsend EA and Emala SCW: Quercetin acutely relaxes airway smooth muscle and potentiates βagonist-induced relaxation via dual phosphodiesterase inhibition of PLCβ and PDE4. Am J Physiol Lung Cell Mol Physiol 2013; 305: L396-L403.
- Capasso R, Aviello G, Romano B, Atorino G and Pagano E, Borrelli F: Inhibitory effect of quercetin on rat trachea contractility in-vitro. J Pharm Pharma 2009; 61(1): 115-19.
- Hou XM, Zhang MS and Qin XJ: Vasodilation of quercetin on rat renal artery and the relationship with L-type voltage-gated Ca2+ channels and protein kinase C. Acta Physiologica Sinica 2017; 69(6): 775-80.
- Shih CH, Lin LH, Hsu HT, Wang KH, Lai CY and Chen CM: Hesperetin, a selective phosphodiesterase 4 inhibitor, effectively suppresses ovalbumin-induced airway hyper-responsiveness without influencing xylazine/ketamine-induced anesthesia. Evid Based Complement Altern Med 2012; 2012: 472897.
- Rauf A, Saleem M, Uddin G, Siddiqui BS, Khan H and Raza M: Phosphodiesterase-1 inhibitory activity of two flavonoids isolated from Pistacia integerrima JL stewart galls. Evid Based Complement Altern Med 2015; 2015: 506564.
- Oberholzer HM, Pretorius E, Smit E, Ekpo OE, Humphries P and Auer RE, et al: Investigating the effect of Withania somnifera, selenium and hydrocortisone on blood count and bronchial lavage of experimental asthmatic BALB/c mice. Scand J Lab Anim Sci 2008; 35(4): 239-28.
- Dhuley JN: Adaptogenic and cardioprotective action of ashwagandha on rats and frogs. J Ethnopharmacol 2000; 70(1): 57-63.
- Mohanty I, Arya DS, Dinda A, Talwar KK, Joshi S and Gupta SK: Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol 2004; 94(4): 184-90.
- Ahumada F, Aspee F, Wikman G and Hancke J: Withania somnifera Its effect on arterial blood pressure in anaesthetized dogs. Phytother Res 1991; 5: 111-14.
- Iuvone T, Esposito G, Capasso F and Izzo AA: Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci 2003; 72(14): 1617-25.
- Schlickmann F, Boeing T, Mariano LNB, da Silva R, da Silva LM and de Andrade SF: Gallic acid, a phenolic compound isolated from Mimosa bimucronata (DC.) Kuntze leaves, induces diuresis and saluresis in rats. Naunyn-Schmiedeberg's Arch Pharmacol 2018; 391(6): 649-55.
- Mounnissamy VM: Diuretic activity of quercetin isolated from Cansjera rheedii Gmelin (Opiliaceae). Int J Pharm Arch 2015; 4(11): 50-53.
- Benjumea D, Martín-Herrera D, Abdala S, Gutiérrez-Luis J, Quiñones W and Cardona D: Withanolides from Whitania aristata and their diuretic activity. Journal Ethnopharmacol 2009; 123(2): 351-55.
- Szentmihályi K, Kery A, Then M, Lakatos B, Sándor Z and Vinkler P: Potassium-sodium ratio for the characterization of medicinal plant extracts with diuretic activity. Phytother Res 1998; 12(3): 163-66.
- Krishnamurthy SR and Sarala P: Proximate nutritive values and mineral components of Withania somnifera (Linn.) Dunal. J Chem 2010; 7(3): 985-96.
- Chatterjee D, Pai PG, Nair V, Gopalakrishna HN, Ullal S and Pai MRSM: Diuretic activity of NR-ANX-C (a polyherbal formulation) in normal rats. J Pharm Res 2010; 3(5): 956-59.
How to cite this article:
Masoud S, Abu-Zarga M, Harb A and Abdalla SS: Phytochemical analysis and effects of aqueous extract of Withania somnifera on isolated smooth muscle, perfused heart, blood pressure and diuresis in rats. Int J Pharmacognosy 2019; 6(4): 133-40. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.6(4).133-40.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
133-140
899
1408
English
IJP
S. Masoud, M. Abu-Zarga, A. Harb and S. S. Abdalla *
Department of Biological Sciences, School of Science, The University of Jordan, Amman, Jordan.
shtaywy@yahoo.com
20 March 2019
22 April 2019
27 April 2019
10.13040/IJPSR.0975-8232.IJP.6(4).133-40
30 April 2019