PHYTOCHEMICAL ANALYSIS AND ANTIOXIDANT PROPERTY OF AERVA LANATA
HTML Full TextPHYTOCHEMICAL ANALYSIS AND ANTIOXIDANT PROPERTY OF AERVA LANATA
R. Krishnamoorthi
Research Department of Zoology, Presidency College (Autonomous), Chennai - 600005, Tamil Nadu, India.
ABSTRACT: The present study was investigated to determine the possible phytochemical components from the solvents such as hexane, ethyl acetate and ethanolic extract of Aerva lanata. Among the phytochemical screening of these extracts, ethanolic extract showed that the whole plant was rich in carbohydrate, tannin, flavonoids, quinones, cardiac glycosides, phenols, and coumarins. Further, the study was extended by analyzing the antioxidant potential using DPPH method. Among all the extracts ethanolic extract showed better antioxidant activity at the concentration of 75 µg/ml when compared to other solvents.
| Keywords: |
Aerva lanata, DPPH, Antioxidant, Phytochemicals
INTRODUCTION: Medicinal plants are the richest bio-resources for the discovery of modern drugs. These medicinal plants possess certain phytochemical compounds which act as an ailment for the infective diseases like bacterial, fungal, viral and cancer disorders. Aerva lanata Linn. belongs to the family Amaranthaceae, is a herbaceous perennial weed growing in the hot region of India at the altitude of 3000 m. Commonly it is called as ‘Chaya’ in Hindi, ‘Bhadram’ in Sanskrit and ‘Pulai’ in Tamil. Traditionally the plant is used for the treatment of diuretic, antiparasitic, anti-helminthic 1, antidiabetic, expectorant 2, anti-microbial, cytotoxicity 3, urolithiasis, anti-inflammatory activity 4, nephroprotective 5, anti-hyperglycemic 6. A partially purified fraction of Aerva lanata was found to be protected against liver damage by acting as an antioxidant agent 7.
Canthin-6-one and beta-carboline alkaloids were isolated from Aerva lanata leave 8. The leaves of Aerva lanata are used as a sap for eye complaints, an infusion is given to cure diarrhea and kidney stone, and the root is used in the snake bite. This study aimed to investigate the phytochemical compounds and antioxidant activity.
Phytochemicals are the bioactive compounds which are responsible for the physiological action of the human body. The most significant phytochemicals include alkaloids, flavonoids, tannins, phenols, quinines, terpenoids, coumarins, etc. These phyto-chemicals act as anti-infective agents 9. Further, the extracts were analyzed for antioxidant activity. Antioxidants are the compounds which help to delay or inhibit the oxidation of lipids and other molecules through the inhibition of either initiation or propagation of oxidative chain reactions 10.
MATERIALS AND METHODS:
Collection of Plants: Plants for this study were collected from Chinnapaliyampattu, Tiruvannamalai district and was authenticated by Dr. Rathna Kumar, Department of plant biotechnology, Presidency College, Chennai-05.
Preparation of Extracts: Collected plants were dried at room temperature and ground to make a fine powder. 20 gm of plant powder was well dissolved in 100ml of solvents (Hexane, Ethyl acetate, and Ethanol) (ratio 1:5). The suspension was filtered by using Filter paper of pore size 0.2µm. The filtrate was then air dried, and extracts were collected in sterile vials for further use.
Phytochemical Tests: The phytochemical test of these extracts was performed using the method adopted by Harborne 11 and Sofowora 12.
Test for Carbohydrates (Molisch’s Test): To 2 ml of plant extract, 1 ml of Molisch’s reagent and a few drops of concentrated sulfuric acid were added. Presence of purple or reddish color indicates the presence of carbohydrates.
Test for Tannins (Ferric Chloride Test): To 1 ml of plant extract, 2 ml of 5% ferric chloride was added. Formation of dark blue or greenish black indicates the presence of tannins.
Test for Saponins (Frothe’s Test): To 2 ml of plant extract, 2 ml of distilled water was added and shaken in a graduated cylinder for 15minutes lengthwise. Formation of a 1 cm layer of foam indicates the presence of saponins.
Test for Flavonoids (Shinoda Test): To 2 ml of plant extract, 1 ml of 2N sodium hydroxide was added. Presence of yellow color indicates the presence of flavonoids.
Test for Alkaloids (Mayer’s Test): To 2 ml of plant extract, 2 ml of concentrated hydrochloric acid was added. Then a few drops of Mayer’s reagent were added. The presence of green color or white precipitate indicates the presence of alkaloids.
Test for Quinines: To 1 ml of extract, 1 ml of concentrated sulfuric acid was added. Formation of red color indicates the presence of Quinones.
Test for Glycosides (Molisch’s Test): To 2 ml of plant extract, 3 ml of chloroforms and 10% ammonia solution was added. Formation of pink color indicates the presence of glycosides.
Test for cardiac Glycosides (Keller – Kiliani Test): To 0.5 ml of extract, 2 ml of glacial acetic acid and a few drops of 5% ferric chloride were added. This was under layered with 1 ml of concentrated sulfuric acid. The formation of a brown ring at the interface indicates the presence of cardiac glycosides.
Test for Terpenoids (Salkowski Test): To 0.5ml of extract, 2 ml of chloroform was added and concentrated sulfuric acid is added carefully. Formation of red-brown color at the interface indicates the presence of terpenoids.
Test for Triterpenoids: To 1.5 ml of extract, 1ml of Liebmann –Buchard Reagent (aectic anhydride + concentrated sulfuric acid) was added. Formation of blue-green color indicates the presence of triterpenoids.
Test for Phenols (Ferric Chloride Test): To 1 ml of the extract, 2 ml of distilled water followed by a few drops of 10% ferric chloride was added. Formation of blue or green color indicates the presence of phenols.
Test for Coumarins: To 1 ml of extract, 1 ml of 10% NaOH was added. Formation of yellow color indicates the presence of coumarins.
Steroids and Phytosteroids (Libermann-Burchard Test): To 1 ml of plant extract equal volume of chloroform is added and subjected with a few drops of the concentrated sulfuric acid appearance of brown ring indicates the presence of steroids and appearance of the bluish-brown ring indicates the presence of phytosterols.
Phlobatannins: To 1 ml of plant extract a few drops of 2% HCl was added the appearance of red color precipitate indicates the presence of phlobatannins.
Anthraquinones (Borntrager’s Test): To 1 ml of plant extract, a few drops of 10% ammonia solution were added, appearance pink color precipitate indicates the presence of anthraquinones.
Antioxidant Activity: The antioxidant activity of Plant extracts was determined by, the DPPH (1,1-diphenyl-2-picryl-hydroxyl) in-vitro method.
DPPH free Radical Scavenging Activity: The antioxidant activity of hexane, ethyl acetate and ethanolic extracts of Aerva lanata and the standard compound BHT was measured in terms of hydrogen donating radical scavenging ability using the stable DPPH method 13. 1 ml of the extract was added to 3.7 ml of methanol solution. After centrifugation, the supernatant is collected 200 μml of DPPH solution is added. Kept in the dark for 45 min and the resulting decrease in absorbance at 517 nm were recorded against blank using a UV-Vis Spectrophotometer.
The radical scavenging activity on DPPH was expressed as, % DPPH radical-scavenging = [(Absorbance of control - Absorbance of test Sample) / (Absorbance of control)] × 100.
RESULTS: The preliminary phytochemical screening of Aerva lanata showed the presence of plant components such as carbohydrates, tannins, flavonoids, cardiac glycosides, terpenoids, phenols and coumarins in hexane extract, carbohydrates, tannins, flavonoids, cardiac glycosides and phenols in ethyl acetate extract and carbohydrates, tannins, flavonoids, quinones, cardiac glycosides, phenols and coumarins in ethanol extract Table 1.
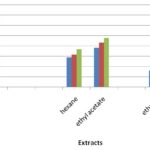
Free Radical Scavenging Activity: The stable free radical scavenging activity by the DPPH method is an easy, rapid and sensitive way to survey the antioxidant activity of specific plant extracts. Fig. 1 indicates the percentage of free radicals scavenging activity in various extractions with different concentrations 25 µg, 50 µg, and 75 µg of Aerva lanata.
TABLE 1: PHYTOCHEMICAL ANALYSIS OF AERVA LANATA
| S.
no. |
Phytochemical
Tests |
Test
Performed |
Hexane Extract | Ethyl Acetate Extract | Ethanol Extract |
| 1 | Carbohydrates | Molisch’stest | + | + | + |
| 2 | Tannin | Ferric chloride test | - | + | + |
| 3 | Saponin | Frothe’s test | - | - | - |
| 4 | Flavonoids | Shinoda test | + | + | + |
| 5 | Alkaloids | Mayer’s test | - | - | - |
| 6 | Quinones | - | - | - | + |
| 7 | Glycosides | Molisch’s test | - | - | - |
| 8 | Cardiac glycosides | Keller – Kiliani test | + | + | + |
| 9 | Terpenoids | Salkowski test | + | - | - |
| 10 | Phenols | Ferric chloride test | + | + | + |
| 11 | Coumarins | - | + | - | - |
| 12 | Steroids | Libermann – Buchard test | - | + | - |
| 13 | Phlobotanins | - | - | - | - |
| 14 | Anthraquinones | Borntrager’s test | - | - | - |
TABLE 2: DPPH ASSAY OF AERVA LANATA AGAINST DIFFERENT EXTRACTS
| Concentrations (µg) | Control | % of Inhibition | |||
| Hexane | Ethyl acetate | Ethanol | BHT | ||
| 25 | 0.9593 | 28.83 ± 0.93 | 38.03 ± 1.44 | 16.12 ± 0.52 | 72.36 ± 1.64 |
| 50 | 0.9593 | 31.48 ± 0.75 | 43.26 ± 0.53 | 21.51 ± 0.45 | 76.23 ± 0.85 |
| 75 | 0.9593 | 36.64 ± 0.56 | 47.57 ± 0.40 | 59.40 ± 0.93 | 81.68 ± 0.61 |
FIG. 1: ANTIOXIDANT ACTIVITY OF AERVA LANATA BY DPPH ASSAY
In this study percentage inhibition of free radicals was carried out with different extractions of selected plants. Ethanol extract with 75μg concentration gives a higher percentage (59.40%), Ethyl acetate extract showed the moderate activity of 47.57% and Hexane extract showed the least activity of 36.64% of free radical scavenging activity. The free radical scavenging activity increases with increase in concentration Table 2, Fig. 1. The percentage inhibition of control was found to be 81.68% which showed higher activity than the extract.
DISCUSSION: The results of the present study reveals that the ethanolic extract of Aerva lanata showed the maximum number of components such as Carbohydrate, tannin is used for the treatment of skin eruption and bowel condition, flavonoids possess wound healing activity due to the astringent, antioxidant and antimicrobial properties which appear to be responsible for wound contraction and elevated rate of epithelisation 14, Quinones, Cardiac glycosides, phenols was found to be toxic to the growth and development of pathogens and Coumarins in the ethanolic extract when compared to other solvents.
The previous study reported, that the presence of flavonoids, tannins, anthraquinones from the stem extract of Aerva lanata 15, 16. The present work also correlates with the aforesaid studies. The ethanolic extract showed maximum antioxidant activity when compared to other solvents. Hence, the ethanolic extract can act as a maximum scavenging activity and can protect the cells from the free radicals.
CONCLUSION: The present study suggests that the Aerva lanata have maximum number of bioactive components and higher amount of antioxidant potential in the ethanolic extract; therefore the ethanolic extract may act as a significant activity and can be further analyzed for many pathogenic disorders as well as may be helpful in future for preventing or slowing the progress of diseases involved. However, it is obvious that fewer information was available further to explore this plant; more researchers should be carried out.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Anantha D, Israiel Kumar T, Santhoshkumar M, Manohar Reddy A, Mukharjee MSV and Lakshmana Rao A: In the vitro antihelminthic activity of aqueous and alcoholic extract of Aerva lanata seeds and leaves. J Pharm Sci and Res 2010; 2(5): 317-321.
- Kirtikar KR and Basu MD: Indian Medicinal Plants. Periodical express book agency. New Delhi, Edition 2nd, 1991; 3: 1926.
- Dulay C: Antimicrobial activity and cytotoxicity of Aerva lanata. Fitoterapia 2002; 73: 93-94.
- Vetrichelvan T, Jagadeesan M, Senthil Palaniappan S, Murali MP and Sasikumar K: Diuretic and anti-inflammatory activities of lanata in rats. Ind J Pharm 2000; 62: 300-02.
- Manokaran S, Jaswanth A, Sengotuvelu S, Nandhakumar J, Duraisami R, Karthikeyan D and Mallegaswari R: Hepatoprotective activity of Aerva lanata against paracetamol-induced hepatotoxicity in rats. Research J Pharm and Tech 2008; 1(4); 398-400.
- Shirwaikar A, Issac D and Malini S: effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethonopharmacol 2004; 90: 81-86.
- Nevin KG and Vijayammal PL: Effect of Aerva lanata against hepatotoxicity of carbon tetrachloride in rats. Environmental Toxicol Pharmacol 2005; 20: 471-477.
- Zapesochnaya G: Canthin-6-one and beta-carboline alkaloids from lanata. Planta Medica 1992; 58: 192-96.
- Duraipandiyan V, Ayyanar M and Ignacimuthu S: Anti-microbial activity of some ethnomedical plants used by paliyar tribe from Tamil Nadu, India. BMC Complementary and Alternative Medicine 2006; 635.
- Jaleel CA, Gopi R, Manivannan P and Paneerselvam R: Antioxidant as protective mechanism in Catharanthus roseus (L).G. Don plants under salinity stress. Turk J Bot 2007; 31: 245-251.
- Harborne JB: Phytochemical methods: A guide to modern techniques of plant analysis. Chapman and Hall, New York, Edition 3rd, 1973: 279.
- Sofowora A: Medicinal plants and Traditional medicinal in Africa. Sunshine house, Ibadan, Nigeria: Spectrum books Ltd; Screening plants for bioactive agents; Edition 2nd, 1993: 134-156.
- Eberhardt MV, Lee CY and Liu RH: Antioxidant activity of fresh apple. Nature 2000; 405: 903-904.
- Shenoy C, Patil MB, Kumar R and Patil S: Preliminary phytochemical investigation and wound healing activity of cepa L. (Liliaceae). Int J Pharm Pharm Sci 2009; 2: 167-175.
- Alwadie HM: Morphology and Distribution of three genera of Amaranthaceae in the South-western area of Saudi Arabia. J King Saud Univ 2005; 18(1): 51-62.
- Soundarajan P: Effect of Aerva lanata on Calcium oxalate urolithiasis in rats. Ind J of Exper Biology 2006; 44: 981-986.
How to cite this article:
Krishnamoorthi R: Phytochemical Analysis and antioxidant property of Aerva lanata. Int J Pharmacognosy 2015; 2(8): 426-29. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.2(8).426-29.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.