PHYTO CHEMICAL INVESTIGATION AND WOUND HEALING ACTIVITY OF JASMINUM GRANDIFLORUM
HTML Full TextPHYTOCHEMICAL INVESTIGATION AND WOUND HEALING ACTIVITY OF JASMINUM GRANDIFLORUM
B. S. Hunasagi * 1, M. Somashekhar 2, N. V. Kalyane 2 and E. N Gaviraj 3
Department of Phytochemistry 1, Department of Pharmaceutical Chemistry 2, Department of Pharmacognosy and Phytochemistry 3, BLDEA’s SSM College of Pharmacy and Research Centre, Vijaypur - 586101, Karnataka, India.
ABSTRACT: The influence of roots and leaf extracts of Jasminum grandiflorum was studied for its wound healing activity using the excision wound model. The roots and leaves of Jasminum grandiflorum were extracted with alcohol 90%, were subjected to phytochemical investigation. The root and leaf extracts were screened for wound healing activity. The animals were divided into four groups in excision wound model; controls were treated with normal saline, standard were treated with betadine and the experimental groups were treated with root and leaf extracts of Jasminum grandiflorum till complete epithelisation. The leaf extract treated wounds were found to epithelise faster as compared to the control group. Leaf extract treated rats exhibited 61.346% reduction in the wound area when compared to the control of 55.72%. The demonstration of an increased rate of wound contraction findings suggests the use of Jasminum grandiflorum leaf extract in the management of wound healing.
| Keywords: |
Jasminum grandiflorum, Wound healing, Phytochemical investigation
INTRODUCTION: A search for medicinal plants during the last several centuries has given an innumerable number of plants which are of great use in the treatment of diseases, promoting health 1. Every disease has a drug in the plant growing in nature. About 80% of individuals from developed countries use traditional medicines. Jati is one of the plant origin drugs which had been mentioned for its various benefits in the literature of Ayurveda 2. It has been claimed that leaf, flower, and roots of Jasminum grandiflorum are being used in many diseases. In the present study, Jasminum grandiflorum was subjected for different studies to know its chemical constituents in the different parts of the plant and an attempt has been made to find out the wound healing efficacy 3.
MATERIALS AND METHODS: The roots and leaves of Jasminum grandiflorum are collected locally from the wild source at Bijapur and identified 4. 250 gm of air-dried roots and leaves of Jasminum graniflorum are powdered and extracted with ethanol 90% separately. The individual extracts were subjected to preliminary phyto-chemical investigation. Healthy adult albino rats of 200-250 gm are used. Each group consists of six Albino rats divided between sexes 5.
Wound Healing Activity: The animals were starved for 12 h before the Wounding. A circular wound of about 2 cm diameter was made on depilated dorsal thoracic region of rats 6.
Animals were divided into 4 groups, control treated with normal saline, test groups treated with 250 mg leaf and root extracts once in a day to the full area of the wound and the standard group treated with the same dose of betadine ointment 7. The parameters studied were wound closure, epithelisation, size and shape of scar area 8.
FIG. 1: PHOTO PLATE SHOWING NATURAL HABITAT OF JATI. (A) The natural habitat of Jati; (Jasminum grandiflorum Linn.); (B and C) Showing inflorescences of jati; (D) Dried leaf of Jati; (E) Coarse powder of Jati Patra.
FIG. 2: WOUND HEALING TREATMENT GROUPS (I, II, III)
RESULTS AND DISCUSSION: TS of Jasminum grandiflorum leaf shows single layered epidermal cells, vascular bundles at midrib and covering trichomes 9.
Preliminary Phytochemical Investigation:
TABLE 1: SHOWING PRELIMINARY PHYTO-CHEMICAL TEST
| Tests: | Leaf extract | Root extract |
| Test for sterols | ||
| Salkowski’s test | + ve | + ve |
| Liberman-Burchardt’s test | - ve | + ve |
| Sulphar test | + ve | + ve |
| Test for proteins | ||
| Biuret test | + ve | - ve |
| Million’s Test | + ve | - ve |
| Xanthoprotein Test | + ve | - ve |
| Test for Triterpenoids | ||
| Liebermann’s Test | -ve | - ve |
| Tschugajew Test | + ve | - ve |
| Test for Alkaloids | ||
| Mayer’s Test | + ve | + ve |
| Wagner’s Test | + ve | + ve |
| Hager’s Test | + ve | + ve |
| Dragendorff’s Test | + ve | + ve |
| Test for carbohydrates | ||
| Molish’s Test | + ve | + ve |
| Barfoed’s Test | - ve | + ve |
| Benedict’s Test | + ve | + ve |
| Test for Saponin’s | ||
| Foam Test | + ve | - ve |
| Hemolytic Test | + ve | - ve |
| Test for Tannin’s | ||
| Ferric chloride test | + ve | + ve |
| Lead acetate test | + ve | + ve |
| Bromine water test | - ve | + ve |
| Test for Flavonoid’s | ||
| Shinoda Test | + ve | + ve |
| Lead acetate | + ve | + ve |
| Alkaline reagent test | + ve | + ve |
| Ferric chloride test | + ve | + ve |
| Bromine water test | - ve | - ve |
| Zinc HCl reduction test | + ve | + ve |
TABLE 2: WOUND HEALING ACTIVITY OF JASMINUM GRANDIFLORUM LEAF AND ROOT EXTRACTS IN THE EXCISION WOUND MODEL
| Wound Area% | Control | Leaf Extract | Root Extract | Standard |
| Day 4 | 10.50
±0.63 |
16.75
±1.33 |
14.58
±1.70 |
21.84
±1.75 |
| Day 8 | 23.06
±1.02 |
28.83
±1.27 |
26.25
±1.27 |
41.84
±2.22 |
| Day 12 | 64.50
±0.69 |
72.66
±2.3 |
67.91
±1.42 |
75.66
±2.45 |
| Day 16 | 79.68
±0.18 |
89.58
±1.33 |
86.66
±3.30 |
94.91
±1.78 |
| Day 18 | 91.43
±0.70 |
100 | 93.58
±0.76 |
100 |
| Epithelisation in Days | 21
±0.365 |
15.66
±0.21 |
17.83
±0.30 |
15.16
±0.47 |
TABLE 3: SHOWING % CLOSURE OF ORIGINAL WOUND AREA ON 4th DAY
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 11.84 | 19 | 13 | 22 |
| 2 | 12.02 | 13.5 | 16 | 27 |
| 3 | 8.48 | 18 | 9.5 | 19 |
| 4 | 10.0 | 21 | 17.5 | 26 |
| 5 | 11.67 | 16.5 | 20.5 | 21.5 |
| 6 | 9.0 | 12.5 | 11 | 15.5 |
| Mean | 10.50 | 16.75 | 14.58 | 21.84 |
| SD | 1.55 | 3.26 | 4.16 | 4.29 |
| SEM | 0.6339 | 1.33 | 1.70 | 1.75 |
| T-value | - | 4.231 | 2.250 | 6.075 |
| P-value | - | 0.0017*** | 0.0482* | 0.0001*** |
TABLE 4: SHOWING % CLOSURE OF ORIGINAL WOUND AREA ON 8th DAY
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 26.50 | 27.5 | 23 | 34.5 |
| 2 | 23.00 | 24 | 27 | 39.5 |
| 3 | 21.00 | 28.5 | 19.5 | 44.5 |
| 4 | 19.55 | 29.5 | 24 | 50 |
| 5 | 24.72 | 33.5 | 34.5 | 44 |
| 6 | 23.64 | 30 | 29.5 | 38.5 |
| Mean | 23.06 | 28.83 | 26.25 | 41.84 |
| SD | 2.51 | 3.12 | 5.29 | 5.45 |
| SEM | 1.025 | 1.27 | 2.13 | 2.22 |
| T-value | - | 3.523 | 1.329 | 7.654 |
| P-value | - | 0.0055*** | 0.2133** | 0.0001*** |
TABLE 5: SHOWING % CLOSURE OF ORIGINAL WOUND AREA ON 12 th DAY
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 63.15 | 78.5 | 64 | 77.5 |
| 2 | 64.80 | 74.5 | 68.5 | 79.5 |
| 3 | 62.19 | 79 | 59.5 | 83.5 |
| 4 | 65.71 | 69.5 | 74 | 71.5 |
| 5 | 66.87 | 70 | 73.5 | 75.5 |
| 6 | 64.50 | 64.5 | 68 | 66,5 |
| Mean | 64.53 | 72.66 | 67.91 | 75.66 |
| SD | 1.69 | 5.68 | 5.56 | 6.01 |
| SEM | 0.69 | 2.3 | 2.27 | 2.45 |
| T-value | - | 3.36 | 1.42 | 4.364 |
| P-value | - | 0.0072*** | 0.1849* | 0.0014*** |
TABLE 6: SHOWING % CLOSURE OF ORIGINAL WOUND AREA ON 16th DAY
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 78.94 | 89.5 | 84.5 | 91.5 |
| 2 | 79.47 | 94.5 | 83.5 | 90 |
| 3 | 80.10 | 88.5 | 79.5 | 90.5 |
| 4 | 80.07 | 88.5 | 84.5 | 93.5 |
| 5 | 80.00 | 92 | 86.5 | 99.5 |
| 6 | 79.50 | 85 | 83.5 | 99.5 |
| Mean | 79.68 | 89.58 | 86.66 | 94.91 |
| SD | 0.4593 | 3.26 | 8.08 | 4.36 |
| SEM | 0.1875 | 1.33 | 3.30 | 1.78 |
| T-value | - | 7.41 | 0.1976 | 8.04 |
| P-value | - | 0.0001*** | 0.8473* | 0.0001*** |
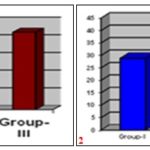
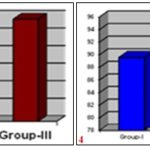
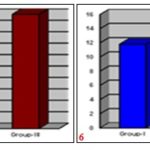
GRAPH 1-2: SHOWING MEAN PERCENTAGE CLOSURE OF ORIGINAL EXCISION WOUND AREA ON 4th AND 8th POST WOUNDING DAY RESPECTIVELY
GRAPH 3-4: SHOWING MEAN PERCENTAGE CLOSURE OF ORIGINAL EXCISION WOUND AREA ON 12th AND 16th POST-WOUNDING DAY RESPECTIVELY
GRAPH 5-6: SHOWING MEAN PERCENTAGE CLOSURE OF ORIGINAL EXCISION WOUND AREA ON 21th POST WOUNDING DAY AND EPITHELIZATION IN NUMBER OF DAYS
TABLE 7: SHOWING % CLOSURE OF ORIGINAL WOUND AREA ON 18th DAY
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 90.78 | - | 94.5 | - |
| 2 | 91.20 | - | 94.5 | - |
| 3 | 90.70 | - | 90 | - |
| 4 | 91.42 | - | 95 | - |
| 5 | 92.50 | - | 93.5 | - |
| 6 | 92.00 | - | 94.5 | - |
| Mean | 91.43 | - | 93.58 | - |
| SD | 0.70 | - | 1.86 | - |
| SEM | 0.2847 | - | 0.7601 | - |
| T-value | - | - | 2.74 | - |
| P-value | - | - | 0.0205* | - |
TABLE 8: SHOWING PERIOD OF EPITHELISATION (IN NO OF DAYS)
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 21 | 16 | 18 | 16 |
| 2 | 22 | 15 | 17 | 17 |
| 3 | 21 | 16 | 19 | 15 |
| 4 | 20 | 16 | 18 | 14 |
| 5 | 20 | 15 | 17 | 14 |
| 6 | 22 | 16 | 18 | 15 |
| Mean | 21 | 15.66 | 17.83 | 15.16 |
| SD | 0.8944 | 0.5164 | 0.7528 | 1.16 |
| SEM | 0.365 | 0.210 | 0.3073 | 0.4773 |
| T-value | - | 12.64 | 6.63 | 9.70 |
| P-value | - | 0.0001*** | 0.001** | 0.0001*** |
TABLE 9: SIZE OF SCAR AREA (sq. mm %)
| S. no. | Group I | Group II | Group III | Group IV |
| 1 | 16.91 | 10.49 | 15.22 | 9.61 |
| 2 | 15.26 | 12.63 | 14.56 | 9.69 |
| 3 | 17.36 | 11.23 | 12.21 | 8.94 |
| 4 | 17.21 | 12.51 | 14.35 | 9.32 |
| 5 | 14.92 | 11.97 | 13.78 | 8.83 |
| 6 | 15.25 | 11.61 | 12.52 | 8.76 |
| Mean | 16.15 | 11.74 | 13.77 | 9.19 |
| SD | 1.12 | 0.8096 | 1.188 | 0.450 |
| SEM | 0.4575 | 0.3305 | 0.4851 | 0.1654 |
| T-value | - | 7.81 | 3.56 | 14.30 |
| P-value | - | 0.0001*** | 0.0051* | 0.0001*** |
Leaf Constant Values: Stomatal number: 12 - 18 mm2; stomatal index 16.5 m; vein islet no. 20 mm2; vein termination no.: 12 mm2.
CONCLUSION: The leaf extract had exhibited more significant wound healing promotion activity. The healing activity of leaves may be due to anti-septic property of essential oils or protein precipitating property of tannins. Further, study needs an investigation to pinpoint the mechanism of activity.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Shashi A, Jain SK and Pandey M: In-vitro evaluation of the antilithiatic activity of seeds of Dolichos biflorus and roots of Asparagus racemosus. International Journal of Plant Sciences 2008; 1: 67-71.
- Johnson M, Sophia A, Babu A and Raja DP: Iso-peroxidase profile as taxonomic criteria in the morphologically related species of Jasminum (Oleaceae). BCAIJ 2010; 4(1): 163-166.
- Wei FH, Chen FL and Tan XM: Gas Chromatographic-Mass Spectrometric Analysis of Essential Oil of Jasminum officinale var Grandiflorum Flower. Tropical Journal of Pharmaceutical Research January 2015; 14(1): 149-152.
- Esaki S and Vidyalakshmi A: Protease activity of floral extracts of Jasminum grandiflorum, a wound healing herb. J of Medicinal Plants Studies 2013; 1(4): 11-15.
- Sandeep, Padmaa M and Paarakh: grandiflorum Linn. (Chameli): Ethnobotany, phytochemistry &pharmacology - A review. Pharmaco Online 2009; 2: 586-595.
- Hirapara H, Ghori V, Anovadiya A, Baxi S and Tripathi C: Effects of ethanolic extract of grandiflorum Linn. Flowers on wound healing in diabetic Wistar albino rats. Avicenna J of Phytomedicine AJP 2017; 7(5): 401-408.
- Swati S, Swati S and Vadi R: Jasminum sambac (motia): A review. International Journal of Pharmaceutical research and bio-science 2013; 2(5): 108-130.
- Johnson M, Sophia A, Babu A and Raja DP: Iso-peroxidase profile as taxonomic criteria in the morphologically related species of Jasminum sp. (Oleaceae). BCAIJ 2010; 4(1): 17-20.
- Umamaheswari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadradevi V and Ravi TK: Antiulcer and in-vitro antioxidant activities of Jasminum grandiflorum J of Ethnopharmacol 2007; 110: 464-470.
How to cite this article:
Hunasagi BS, Somashekhar M, Kalyane NV and Gaviraj EN: Phytochemical investigation and wound healing activity of Jasminum grandiflorum. Int J Pharmacognosy 2018; 5(6): 364-68. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(6).364-68.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
8
364-368
763
1718
English
IJP
B. S. Hunasagi *, M. Somashekhar, N. V. Kalyane and E. N Gaviraj
Department of Phytochemistry, BLDEA’S SSM College of Pharmacy and Research Centre, Vijaypur, Karnataka, India.
bsavabjp73@gmail.com
09 February 2018
25 March 2018
30 March 2018
10.13040/IJPSR.0975-8232.IJP.5(6).364-68
01 June 2018