PHYSIOCHEMICAL AND PHYTOCHEMICAL ANALYSIS OF FIVE VARIETIES OF ARTOCARPUS SEED OILS
HTML Full TextPHYSIOCHEMICAL AND PHYTOCHEMICAL ANALYSIS OF FIVE VARIETIES OF ARTOCARPUS SEED OILS
N. Sirisha * and T. Raghava Rao
Department of Biochemistry, College of Science and Technology, Andhra University, Visakhapatnam -530003, Andhra Pradesh, India.
ABSTRACT: Soxhlet extraction method was used for the extraction of oil from five variety seeds of the jackfruit (Artocarpus heterophyllus, Artocarpus integrifolia, Artocarpus hircitus, Artocarpus inciscus, and Artocarpus integer), planned to explore its suitability for salutary uses with a special emphasis on its physiochemical characterization, spectrophotometric spectral analysis and evaluation of phytochemical constituents. Physiochemical properties include acid value, saponification value, iodine value, peroxide value, Reichert-Meissl value (RMV) and Polenske value were examined and compared with standard oils. Spectrophotometric analysis of oils was carried out to obtain information regarding the types, numbers, and position of chromophores and auxochrome and saturated and unsaturated compounds. Phytochemical constituent’s phenols, flavonoids, alkaloids, and tannins were determined in increasing concentration from 25 mg/ml to 100 mg/ml in five varieties of jackfruit seed oils. This inquiry concludes that the seed oils can support in the maintenance of health as the trend of the future is moving towards using seed oil as medicine in the management of various chronic diseases.
| Keywords: |
Artocarpus seed oil, Auxochrome, Chromophores and Phytochemicals
INTRODUCTION: Essential oils have wide and varied applications in many industries such as cosmetics, perfumes, beverages, ice creams, confectionary and backed food products and for the scenting and flavouring of consumer’s finished products 1, 2. Currently, about 300 essential oils, out of approximately 3,000 are commercially important for the pharmaceutical, agronomic, food, sanitary, cosmetic and perfume industries. Some of the essential oils and their bioactive components such as limonene, geranyl acetate or carvone are used as an important ingredient in toothpaste and hygienic products.
These also act as food preservers and additives, as well as employed for the treatment of different ailments in the folk medicine systems 1, 3. Research is now in progress to explore the applications of essential oils for therapeutic uses and management of infectious diseases as an alternative to standard drug remedies 4, 5. Phytochemicals are not essential nutrients and are not required by the human body for sustaining life, however, have important properties to prevent or to fight some common diseases. Because of these properties, research is concentrated to reveal the beneficial health effects of phytochemicals.
The jackfruit (Artocarpus species) belonging to family Moraceae is an integral part of common Indian diet and commonly known as “Kathal.” Jackfruit appears in the Indian market in spring and is available till summer. Jackfruit pulp is eaten a fresh and used in fruit salads and possesses high nutritional value. In the present study, oil extracted from five variety seeds of the jackfruit namely Artocarpus heterophyllus, Artocarpus integrifolia, Artocarpus hircitus, Artocarpus inciscus, and Artocarpus integer, planned to explore its suitability for constructive uses with a special emphasis on its physiochemical characterization, spectrophotometric spectral analysis, & evaluation of phytochemical constituents.
MATERIALS AND METHODS:
Collection of Seeds: Five different varieties of jackfruit seeds were collected from Visakhapatnam nearby areas including Simhachalam and Kaviti. The five varieties are Artocarpus heterophyllus (A. heterophyllus), Artocarpus integrifolia (A. integrifolia), Artocarpus hirsitus (A. hirsitus), Artocarpus inciscus (A. inciscus) and Artocarpus integer (A. integer). The fruits were cut and the seeds removed from the perianth of fruits. The seeds were then sliced with a knife and dried. The dried seeds were ground to fine powder.
Extraction of oil by Soxhalation: The seed oils were extracted using Soxhlet extraction method with analytical grade hexane as refluxing solvent. After the extraction process, the oil was recovered from the mixture by distillation and stored at 4 °C and explored for physiochemical characterization, spectrophotometric spectral and for phytochemical analysis.
The percentage of oil content can be calculated as below:
% of Oil=Wt. of Oil obtained in gm / Wt. of Seed taken in gm × 100.
Oil Characterization: The crude oil samples obtained from the hexane extraction were characterized by acid value, saponification value, iodine value, peroxide value. Reichert-Meissl value (RMV) and Polenske value were based on official recommendations and Tentative Methods of the American Oil Chemist’s Society 21.
Spectrophotometric Analysis: Ultraviolet (UV) and visible absorption spectra were carried out for the Artocarpus extracted oils. Absorption was between 200-600 nm wavelengths in a quartz cell with 1 cm path length against a solvent blank in a matched cell using Shimadzu double beam UV using a visible spectrophotometer, model TCC240A with UV probe software 6.
Evaluation of Phytochemicals:
Estimation of Total Phenolics: The number of total phenolics in extracts was determined according to the 24, 22. Samples (200 μl) were introduced into test tubes. One milliliter of Folin ciocalteu reagent and 0.8 ml of sodium carbonate (7.5%) were added. The tubes were mixed and allowed to stand for 30 min. Absorption at 765 nm was measured. The total phenolic content was expressed as gallic acid equivalents (GAE) in micrograms per gram of extract as calculated from the standard gallic acid graph.
Estimation of Total Flavonoids: Total flavonoid content of the extracts was determined according to a modified colorimetric method 7. Seed extracts (1.0 ml) was mixed with 1 ml of distilled water and 75 μl of a 5% NaNO2 solution. After 5 min, 75 μl of 10% AlCl3.H2O solution was added. After 5 min, 0.5 ml of 1M Sodium hydroxide was added. The solution was mixed well and kept for 15 min. The increase in absorbance was measured at 510 nm using a UV-Visible spectrophotometer. The total flavonoid content was calculated using standard quercetin calibration curve. The results were expressed as micrograms of quercetin equivalents (QE) per gram of extract.
Estimation of Total Alkaloids: Total alkaloid content was estimated by the method of Sreevidya and Mehrotra (2003) 8. A standard solution was prepared by dissolving 5 mg of boldine and seed extract separately in 5 ml of warm distilled water each. 5 ml of boldine solution/extract was adjusted to pH 2-2.5 (with 0.01 M HCl), and 2 ml of DR (Dragendorff’s reagent) was added to form an orange precipitate that was centrifuged at 5000 rpm for 15 min.
Afterward, DR was added to the supernatant to check for complete precipitation. 2 ml amount of 1% sodium sulfide was added to the residue to form a brownish black precipitate which was centrifuged at 5000 rpm for 15 min. Complete precipitation was checked by further adding 1% sodium sulfide. The resulting residue was dissolved in 2 ml of nitric acid with warming and sonication and then made up to 10 ml with distilled water. 5 ml of 3% thiourea was added to 1 ml of the resulting solution to form a yellow bismuth complex, of which the absorbance was measured at 435 nm. All the assays were performed in triplicate. The amount of bismuth present in the boldine solution/extract was achieved from the calibration curve of bismuth nitrate. The results were expressed as boldine, considering that is a monobasic alkaloid, and therefore the complex formed with bismuth follows a 1:1 stoichiometry.
Estimation of Total Tannins: The total tannins were determined using the Folins-ciocalteau method (1927), briefly, 0.1 ml of seed extract, 6.5 ml of water and 0.5 ml of Folins-ciocalteau reagent and 1.5 ml of 20% sodium carbonate at a standard overnight solution were added and incubated at 1 h. The absorbance of the sample was measured in a spectrophotometer at 725 nm. The total tannin content was calculated using standard tannic acid calibration curve, and the results were expressed as micrograms of tannic acid equivalents per gram of extract.
Statistical Analysis: The results of in-vitro study were given as Mean ± Standard Deviation (SD) obtained from three independent experiments and analyzed with Student’s t-test for paired data and a ‘p’ value less than 0.05 was considered as a significant difference in the analysis. All the statistical analysis was resolved using SPSS software
RESULTS AND DISCUSSION:
Physiochemical Characterization: Oil extraction was carried out by Soxhlet extraction method as per the direction of AOAC (Association of Official Analytical Chemists). Hexane was used as a solvent to extract the oil from seeds, and this was passed out for 10 h. The oil obtained from all five verities that are A. heterophyllus, A. integrifolia, A. hircitus, A. inciscus, and A. integer was thick yellowish having a pungent odor with a yield of 23.3, 20.0, 16.0, 25.0 and 18.0% respectively. The yield is high in the A. inciscus may be due to its structural complexity and hardness in the seeds than other varieties.
The acid number is a measure of carboxylic acid groups in test oils. Outcomes state that A. inciscus have high acid value with 2.6 followed by A. hircitus with 2.4, A. heterophyllus with 2.2 and very low in both A. integrifolia and A. integer with 2.0 each. The high acid value of A. inciscus is a distinction of having more number of carboxylic groups (fatty acids) than the four other varieties. Saponification value is a measure of the average molecular weight (or chain length) of all the fatty acids present in the test oils.
The saponification value maximum in A. heterophyllus with 50.4, A. integrifolia with 28.0, followed by A. inciscus with 22.4 and very low in both A. hircitus and A. integer with 11.2 each. Results indicate that the low saponification values of A. hircitus and A. integer due to long chain fatty acids and with a relatively fewer number of carboxylic functional groups per unit mass of the fat. Iodine number destined to determine the degree of unsaturation test oils.
The higher the iodine number, the more C=C bonds are present in the test oils. Commencing our studies, it is clear that A. heterophyllus have maximum unsaturated fatty acid with iodine value 25.4, followed by A. inciscus with 16.4 and A. integrifolia with 12.2 and a minimum number of unsaturated fatty acids observed in both A. hircitus and A. integer with low iodine value 10.8 each. Peroxide value was an indication of the extent of oxidation suffered by oil, and it was found to be 4.0 mEqKg-1 for both A. inciscus and A. integer test oils, followed by A. integrifolia and A. hircitus with 2.4 mEqKg-1each and very low in A. heterophyllus with 2.0 mEqKg-1.
Fresh edible oils have peroxide value <10mEqKg-1, while rancid oils show a value of >20mEqKg-1. The Reichert-Meissl value of the oil was an indication of steam-volatile fatty acids. RMV of the oil was found to be extreme in A. inciscus with 0.38, followed by A. hircitus with 0.31and A. integer with 0.30, minutest RMV’s were observed in A. integrifolia and A. heterophyllus with 0.24 and 0.22 respectively. The Polonsky value of the oil was found to be extreme in A. inciscus with 0.70, followed by A. integer with 0.58 and A. hircitus with 0.52, lowest in A. integrifolia by 0.38 and A. heterophyllus with 0.36. The Polonsky value indicates the volatile alcohol-soluble fatty acids in the oil. Supporting above fallout, it is clearly known that the physiochemical characteristics of test oils were similar to that of groundnut and soybean seed oils 23. All the above results were displayed in Table 1.
TABLE 1: PHYSIOCHEMICAL CHARACTERIZATION OF FIVE VARIETIES OF ARTOCARPUS SEED OILS
| Seed
varieties |
Percentage of oil yield (%) | Acid
value (mgKOH/g) |
Saponification value
(mg KOH/g) |
Iodine value | Peroxide value
(mEqKg-1) |
Riechert-Missel value | Polensky value |
| A. heterophyllus | 23.3 | 2.2 | 50.4 | 25.4 | 2.0 | 0.22 | 0.36 |
| A. integrifolia | 20.0 | 2.0 | 28.0 | 12.2 | 2.4 | 0.24 | 0.38 |
| A. hircitus | 16.0 | 2.4 | 11.2 | 10.8 | 2.4 | 0.31 | 0.52 |
| A. inciscus | 25.0 | 2.6 | 22.4 | 16.4 | 4.0 | 0.38 | 0.70 |
| A. integer | 18.0 | 2.0 | 11.2 | 10.8 | 4.0 | 0.30 | 0.58 |
Spectrophotometric Analysis: UV - visible spectrum can be applied to identify the types, numbers, and position of chromophores as well as auxochrome and saturated and unsaturated compounds. UV-Vis absorption spectrums of five verities of Artocarpus seed oils were presented in Fig. 1. If absorption peaks between 200~400 nm were detected, there is a possibility of conjugate double bond and C=O group, demonstrating that this is most probably a saturated compound. If there is a weak peak (=10~100) between 270~350 nm and no other peaks detected over 200 nm it may contain >C=O, >C=C-O- or >C=C-N< etc. The weak peak was due to n-* transition.
FIG. 1: UV-VIS ABSORPTION SPECTRA’S OF FIVE VARIETIES OF ARTOCARPUS SEED OILS
(A. heterophyllus, A. integrifolia, A. hircitus, A. inciscus, and A. integer respectively)
If there are many peaks in the UV region, some of them are even within the visible region, and then the compounds may have long conjugation bonds. When λmax is over 250 nm and is between 1000~10000, the compound may contain aromatic structure. ε between 10000 ~ 20000 for the long wave absorption peak may be conjugated diene or carbonyl compounds. If the peaks appear at the wavelengths 425, 455 and 480 nm in addition to 525, 570 and 590 nm chromophores, they may belong to carotenoids and flavonoids.
Assuming this, five varieties of Artocarpus crude seed oils may have unsaturated fatty acids with tandem conjugated double bonds, alkaloids, carotenoids, flavonoids, tannins, and phenolic compounds, authorizing its therapeutic potential. These results agree well with the results of 9 in Ceiba pentandra seed oil.
Phytochemical Analysis of Artocarpus seeds: Phytochemicals are invaluable sources of raw material for both traditional and approved medicine. Seed oils containing bioactive agents such as alkaloids, tannins, and phenolics thus, readily present themselves as a good source of raw material in modern and outmoded medicine. Quantitative analysis of all seed oils was showed enhancement in increasing concentrations from 25 mg/ml to 100 mg/ml. The observed phenolic content of five varieties of jackfruit seeds oils at 100 mg/ml were as follows, A. hircitus 0.6 ± 0.1, A. integrifolia 0.54 ± 0.01, A. integer 0.44 ± 0.02, A. heterophyllus 0.36 ± 0.02 and A. inciscus 0.18 ± 0.02 μg gallic acid equivalents g-1. The graphical versions were shown in Fig. 2.
Phenolic compounds are a class of antioxidant agents which act as free radical terminators and also involved in retardation of oxidative degradation of lipids 10. Also, 11 has reported a strong relationship between phenolic content and antioxidant activity in selected fruits and vegetables. Thus, the presence of phenolic compounds in five varieties of Artocarpus seed oils was an added value to its nutritional and health potential.
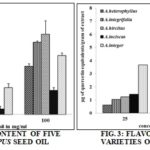
The screened flavonoid content of Artocarpus seed oils at 100 mg/ml were along these lines, A. integer 7.25 ± 0.02, A. inciscus 6.92 ± 0.03, A. hircitus 5.3 ± 0.03, A. integrifolia 4.44 ± 0.03 and low content in A. heterophyllus 3.63 ± 0.01 μg quercetin equivalents g-1. Moreover, the screened alkaloid content observed at 100 mg/ml in the seed oils were like this, high alkaloid content observed in A. hircitus 1.33 ± 0.3, A. inciscus 1.16 ± 0.02, A. heterophyllus 0.56 ± 0.2, A.integrifolia 0.53 ± 0.3 and low alkaloid content in A. integer 0.47 ± 0.02 μg boldine equivalents g-1. Graphical reports were put on view in Fig. 3 and Fig. 4 respectively.
Furthermore, the occurrence of flavonoids in the seed oils which are also phenolic compounds similarly mends the economic and health potential of the oil. This is in agreement with previous findings which suggested that flavonoids carry out antioxidant action through scavenging or chelating process and are reported to play a preventive role in cancer and heart disease 12. Alkaloids and their synthetic derivatives are being used as basic therapeutic agents for their analgesic, antispasmodic and bactericidal effects. On the other hand, alkaloids and flavonoids inhibit certain mammalian enzymatic activities such as those of phosphodiesterase, prolonging the action of cyclic-AMP.
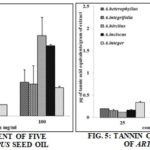
Alkaloids also affect glucagons and thyroid stimulating hormones 13. The estimated tannin content of seed oils was in this way, A. integer 2.2 ± 0.02, A. integrifolia 1.53 ± 0.02, A. heterophyllus 0.78 ± 0.05, A. hircitus 0.73 ± 0.02 and A. inciscus 0.67 ± 0.02 µg/gram of extract. The outcome was put on show in Fig. 5. Tannins revealed potential antiviral 14, antibacterial 15 and antiparasitic properties 16. When incubated with red grape juice and red wines with a high content of condensed tannins, the poliovirus, herpes simplex virus and various enteric viruses are inactivated 17.
It is believed that tannins isolated from the stem bark of Myracrodruon urundeuva may have neuro-protective functions capable of reversing 6-hydroxydopamine-induced toxicity. The plant has shown promise as a potential therapeutic agent, which may be beneficial in patients with neurological disease 20, 18 discovered that the tannins isolated from the stem bark also have anti-inflammatory and antiulcer activity in rodents, showing a strong antioxidant property with possible therapeutic applications.
Foods rich in tannins can be used in the treatment of HFE hereditary hemochromatosis, a hereditary disease characterized by excessive absorption of dietary iron, resulting in a pathological increase in total body iron stores. Tannins can also be effective in protecting the kidneys. The statistical analysis of all in-vitro (n=3) studies of Artocarpus seed oils with student ‘t’ test showed a significant difference for all tested parameters between the concentrations. Our outcomes were in a row with results of 19 in Ceiba pentandra seed oil.
CONCLUSION: Seed oils from the plants have been attributed for their nutritional, industrial and medicinal values. This study highlights physio-chemical characterization and phytochemical composition of the five varieties of seed oils from the Artocarpus. Therefore, the results of the present study support the traditional usage of the Artocarpus seed oils and these can be recommended for use as a therapeutic drug. Further investigations are anticipated to identify the active components and leads to their further clinical use.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Akiyama H, Fujii K, Yamasaki O, Oono T and Iwatsuki K: Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrob Chemother 2001; 48: 487-491.
- AOCS: Official methods and recommended practices of the AOCS. Champaign, IL, USA, American Oil Chemists' Society. Edition 5th, 1998.
- Baja YPS: Medicinal and aromatic plants. Biotechnol in Agricul Forestry, 1998.
- Bao J, Cay Y, Sun M, Wang G and Corke H: Anthocyanins, flavonols, and free radical scavenging activity of chinese bayberry (Myrica rubra) extracts and their color properties and stability. J Agric Food Chem 2005; 53: 2327-2332.
- Bozin B, Mimica-Dukic N, Simin N and Anackov G: Characterization of the volatile composition of essential oils of some Lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J Agric and Food Chem 2006; 54: 1822-1828.
- Burt S: Essential oils: their antimicrobial properties and potential application in foods-A review. Int J Food Microbiol 2004; 94: 223-253.
- Delamare APL, Moschen-Pistorello IT, Artico L, Atti-Serafini L and Echeverrigaray S: Antibacterial activity of the essential oils of Salvia officinalis and Salvia triloba L. cultivated in South Brazil. Food Chem 2007; 100: 603-608.
- Folin O and Ciocalteu V: On tyrosine and tryptophane determinations in proteins. J Biol Chem 1927; 73: 627-50.
- Guenther E: The essential oils. Krieger Publ. Co., Malabar FL III, 1985; 4000-433.
- Javanmardi J, Stushnoff C, Locke E and Vivanco JM: Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem 2003; 83: 547-550.
- Kolodziej H and Kiderlen AF: Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitized RAW 264.7 cells. Phytochem 2005; 66: 2056-2071.
- Lü L, Liu SW, Jiang SB and Wu SG: Tannin inhibits HIV-1 entry by targeting gp41. Acta Pharmacol Sin 2004; 25: 213-218.
- Odukoya AO, Jenkins MO, Ilori OO and Sofidiya MO: The use of selected Nigerian natural products in the management of environmentally induced free radical skin damage. Pak J Biol Scien 2005; 8: 1074-1077.
- Okaka JC, Enoch NJ and Okaka NC: Human nutrition: an integrated approach. ESUT publications, Enugu 1992; 57-58.
- Pourmorad F, Hosseinimehr SJ and Shahabimajd N: Antioxidant activity, phenol and flavonoids content of some selected Iranian plants. Afr J Biot 2006; 5: 1142-45.
- Ragone D: Artocarpus camansi (Breadnut).ver.2.1. In: Elevitch, C.R.(ed). Species Profiles for Pacific Island Agroforestry. Permanent Agriculture Resources (PAR). Holualoa, Haiwai’i, 2006; 1-11.
- Chekuboyina RK, Pagolu KR, Dadi BR, Nagala S and Tamanam RR: Physico-chemical characterization and antimicrobial activity of Ceiba pentandra (Kapok) seed oil. Alternat Med Studies 2012; 2: e9.43-47.
- Ravi Kiran C, Madhavi Y and Raghava Rao T: Evaluation of phytochemicals and antioxidant activities of Ceiba pentandra (Kapok) seed oil. J Bioanal Biomed 2012; 4: 068-073.
- Sacchetti G, Maietti S, Muzzoli M, Scaglianti M, Manfredini S, Radice M and Bruni R: Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in food. Food Chem 2005; 91: 621-632.
- Sreevidya N and Mehrotra S: Spectrophotometric method for estimation of alkaloids precipitable with Dragendorff’s reagent in plant materials. J AOAC Internl 2003; 86: 1124-1127.
- Souza SMC, Aquino LCM, Milach Jr AC, Bandeira MAM, Nobre MEP and Viana GS: Anti-inflammatory and antiulcer properties of tannins from Myracrodruon urundeuva Allemão (Anacardiaceae) in Rodents. Phytother Res 2006; 21: 220-225.
- Middleton EJ, Kandaswami C and Theoharides TC: The Effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 2000; 52: 673-751.
- Nobre-Junior HV, Maia FD, de Oliveira RA, Bandeira MAM and do O Pessoa C: Neuroprotective Actions of Tannins from Myracrodruon urundeuva on 6-Hydroxy dopamine-Induced Neuronal Cell Death. J Herbs Spices Med Plants 2007; 13: 41-57.
- Vogel AI: Vogel's textbook of practical organic chemistry, including qualitative organic analysis. Longman Group Ltd., London, Edition 4th, 1978.
How to cite this article:
Sirisha N and Rao TR: Physiochemical and phytochemical analysis of five varieties of Artocarpus seed oils. Int J Pharmacognosy 2014; 1(12): 785-91. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).785-91.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
7
785-791
756
2221
English
IJP
N. Sirisha٭ and T. Raghava Rao
Department of Biochemistry, College of Science and Technology, Andhra University, Visakhapatnam, Andhra Pradesh, India.
nagalasirisha@gmail.com
11 October 2014
02 November 2014
15 November 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.1(12).785-91
01 December 2014