PHARMACOLOGICAL ASPECTS OF CURCUMIN: REVIEW ARTICLE
HTML Full TextPHARMACOLOGICAL ASPECTS OF CURCUMIN: A REVIEW
Ali Alsamydai * and Nisrein Jaber
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Jordan, Amman, Jordan.
ABSTRACT: Turmeric (Curcuma longa) is a widely used popular Indian medicinal plant which belongs to the family of Zingiberaceae. Curcumin, an important constituent of turmeric, is known for various biological activities, primarily due to its antioxidant mechanism. Epidemiological observations are suggestive that turmeric consumption may reduce the risk of some form of cancers and render other protective biological effects in humans like antidiabetic, anti-inflammatory, anti-angiogenic, anti-oxidant, wound healing and anti-cancer effects. This review summarizes the most interesting biological effects of curcumin.
| Keywords: |
Curcumin, Curcuma longa, Zingiberaceae, Pharmacological uses, Anticancer, Antidiabetic, Antioxidant
INTRODUCTION: Turmeric is an Indian rhizomatous herbal plant (Curcuma longa) of the ginger family (Zingiberaceae) of well-known medical benefits 1, 2. Fig. 1 shows the Curcuma longa. The medicinal benefits of turmeric could be attributed to the presence of active principles called curcuminoids. One of the most interesting components of curcuminoid is curcumin, which is a small molecular weight polyphenolic compound and lipophilic in nature, hence insoluble in water and also in ether but soluble in ethanol, dimethylsulfoxide, and other organic solvents 3. Curcumin is stable at the acidic pH of the stomach 4. The other constituents present are volatile oils including tumerone, atlantone, and zingiberone and sugars, proteins and resins 2. The active constituent of turmeric- curcumin is isolated from Curcuma longa, and it provides color to turmeric.
FIG. 1: CURCUMA LONGA
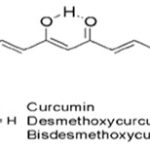
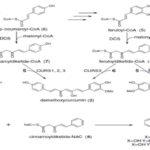
Such bioactive component has been thoroughly investigated 5. Curcumin (1, 7-bis (4-hydroxy-3-methoxyphenyl)-1, 6-heptadiene-3, 5-dione) is also called diferuloylmethane 6. It is a tautomeric compound existing in enolic form in organic solvents and as a keto form in water Fig. 2. It was found that curcuminoids in the herb C. longa is synthesized by a collaboration of two types III Polyketide synthases, diketide-CoA synthase (DCS) and curcumin synthase 1 (CURS1, the first identified CURS) (Fig. 3A). DCS catalyzes the formation of feruloyldiketide CoA (4) from feruloyl-CoA (5) and malonyl-CoA. CURS1 catalyzes the formation of curcumin from feruloyl-CoA (5) and the feruloyldiketide-CoA produced by the action of DCS (4). Thus, DCS and CURS1 catalyze the formation of curcumin. Both enzymes accept p-coumaroyl-CoA (6), but at low efficiency, and are also capable of synthesizing bisdemethoxy curcumin (3) from p-coumaroyl-CoA (6) and malonyl-CoA via p-coumaroyldiketide-CoA (7) formation. Although, a pair of DCS and CURS produces a mixture of Curcumin oids; i.e., Curcumin (1), demethoxyCurcumin (2) and bisdemethoxycurcumin, from feruloyl-CoA (5), p-coumaroyl-CoA (6) and malonyl-CoA in-vitro, it yields the mixture of products with a composition different from that of an ethyl acetate extract of the rhizome of turmeric; the rhizome of turmeric contains a relatively larger amount of bisdemethoxycurcumin (3) than the in-vitro reaction products by a pair of DCS and CURS. Therefore, it was assumed that the composition of curcuminoids in the mixture might be regulated by the concentrations of p-coumaroyl-CoA and feruloyl-CoA in-vivo.
FIG. 2: CHEMICAL STRUCTURES OF CURCUMINOIDS
CURSs catalyzes the formation of curcuminoids (1-3) from cinnamoyl-CoA (10), p-coumaroyl-CoA (6) and feruloyl-CoA (5) when incubated with cinnamoyldiketide-N-acetylcysteine (NAC) (8), an analog of diketide-CoA Fig. 3 123.
FIG. 3: THE BIOSYNTHESIS PATHWAY OF CURCUMIN OIDS
Turmeric is the boiled, dried, cleaned and polished rhizomes of Curcuma longa. After harvesting the whole rhizomes are collected. These rhizomes are transported as whole rhizomes. They are usually like fingers 2 to 8 cm long and 1 to 2 cm wide having bulbs and splits. The dried rhizomes are further processed and reprocessed to obtain the turmeric powder 2. The use of turmeric dates back nearly 4000 years to the Vedic culture in India, where it was used as a culinary spice and had some religious significance 7. It has different names in different cultures and countries. In North India, turmeric is commonly called “haldi,” and in the south, it is called “manjal,” It is known as terre merite in French and simply as “yellow root” in many languages. In Arabic, it is called Kurkum, Uqdah safra. In Sanskrit, turmeric has at least 53 different names 7.
Curcumin has been used in tradition as a medicinal herb due to its various advantages such as antioxidant 8, anti-inflammatory 9 antimutagenic 10, antimicrobial 11 and several therapeutic properties 12. Curcumin shows poor absorption, rapid metabolism, and rapid elimination. Several agents have been introduced to improve the bioavailability of curcumin. The most interesting one is piperine, it enhances curcumin bioavailability by blockage of the metabolic pathway of curcumin 13. Piperine results in an increase of 2000% in the bioavailability of curcumin 14. Curcumin is available in several forms including capsules, tablets and ointments 15. Curcuminoids have been approved by the US Food and Drug Administration (FDA) as “Generally Recognized as Safe” (GRAS) 16. It is the purpose of this review to provide a brief overview of the potential health benefits of curcumin.
Medicinal Uses of Curcumin:
Anti-diabetic Activity: Curcumin was reported to possess anti-diabetic activity. The effect of anti-diabetic activity could be attributed to the antioxidant property of curcumin 17. In their study, researchers demonstrated positive curcumin effect through the improvement of diabetes-induced endothelial dysfunction by decreasing superoxide production and vascular protein kinase C inhibition. Interestingly, recent studies demonstrated the ability of curcumin to have the capacity to directly quench reactive oxygen species (ROS) that can contribute to oxidative damage 18.
This property is known to contribute to the overall protective effects of curcumin. Curcumin can attenuate cell death caused by oxidative stress, indirectly through induction and activation of antioxidant/ cytoprotective enzymes, such as heme oxygenase-1 (HO-1). The protective mechanisms of HO-1 in diabetes could present some emerging therapeutic options for HO-1 expression in treating diabetic diseases 18.
Curcumin was evaluated for the prevention of type 2 diabetes in pre-diabetic human population 19. The subjects received curcumin capsules for 9 month period versus placebo capsule group. The curcumin-treated group showed a better overall function of β-cells, with higher HOMA-β and lower C-peptide. The curcumin-treated group showed a lower level of HOMA-IR and higher adiponectin when compared with the placebo group. The results indicated that curcumin intervention might have a positive effect to a prediabetic population 19.
Wound Healing Activity: Wound healing includes the repair of tissues in a complex process that involves inflammation, granulation, and remodeling of the tissue 20. Enhancement of wound healing was reported by curcumin in animals. The mechanisms of action of the wound healing effect of curcumin include: immunohistochemical localization of transforming growth factor-β1 showed an increase in curcumin-treated wounds as compared with untreated wounds 22 and modulating collagen and decreasing reactive oxygen species 21. Also, curcumin showed earlier re-epithelialization, improved neovascularization, increased migration of various cells including dermal myofibroblasts, fibroblasts, and macrophages into the wound bed, and higher collagen content 22, 23.
Anti-arthritis Activity: Rheumatoid arthritis (RA) is a chronic inflammatory disease that is characterized by hyperplasia of the synovial fibroblasts. Curcumin is known to possess potent anti-inflammatory and anti-arthritic properties 24. Curcumin treatment was carried out on patients with active rheumatoid arthritis and compared with diclofenac sodium reference group. Interestingly, the curcumin group showed the highest percentage of improvement in overall rheumatoid arthritis scores and these scores were significantly better than the patients in the diclofenac sodium group. More importantly, the curcumin group was found to be safe and did not relate to any adverse events compared to diclofenac sodium group 25.
It is believed that curcumin antioxidant anti-proliferative, anti-inflammatory and immune-suppressive activities shared in the improvement of symptoms to patients suffering from rheumatoid arthritis 26. One of the important consequences of RA could be decreased apoptosis. Exposure of the synovial fibroblasts to curcumin resulted in growth inhibition and the induction of apoptosis, as measured by MTT assay, fluorescent microscopy, and Annexin-V-based assay. These results show that curcumin might help against hyperplasia of the synovial fibroblasts in RA 27.
Anti-Alzheimer Activity: Alzheimer disease (AD) is by far the most common cause of dementia globally. This neurodegenerative disorder of the brain is chronic and progressive, characterized clinically by the deterioration in the key symptoms of behavioral and cognitive abilities. Researchers reported the advantages of curcuminoids as anti-Alzheimer agents 28.
Curcumin action was demonstrated through the inhibition of the accumulation of amyloid β-peptide (Aβ) and the formation of β-amyloid fibrils (fAβ) from Aβ, as well as the destabilization of preformed fAβ in the central nervous system. Consequently, curcumin would be an attractive therapeutic target for the treatment of Alzheimer's disease 29.
Anti-Parkinson Activity: Oxidative stress has been implicated in the degeneration of dopaminergic neurons in the substantia nigra (SN) of Parkinson's disease (PD) patients. An important biochemical feature of presymptomatic PD is a significant depletion of the thiol antioxidant glutathione (GSH) in these neurons resulting in oxidative stress, mitochondrial dysfunction, and ultimately cell death. Curcumin restores depletion of GSH levels, protects against protein oxidation, and preserves mitochondrial complex I activity which normally is impaired due to GSH loss. Thus, it helps in the treatment of PD 30. Overexpression and abnormal accumulation of aggregated α-synuclein (αS) have been linked to Parkinson's disease (PD) and other synucleinopathies. αS can misfold and adopt a variety of morphologies, but recent studies implicate oligomeric forms as the most cytotoxic species. Curcumin can alleviate αS-induced toxicity, reduce intracellular reactive oxygen species ROS levels and protect cells against apoptosis. Thus, curcumin could be used as anti-Parkinson 31.
Anti-inflammatory Activity: Curcumin possesses significant anti-inflammatory activity in acute as well as in chronic models of inflammation. It is as potent as phenylbutazone in the carrageenan edema test but only half as potent in chronic tests 32. Curcumin has been demonstrated to be safe in six human trials and has demonstrated anti-inflammatory activity. It may exert its anti-inflammatory activity by inhibition of several different molecules that play a role in inflammation 33. Curcumin has been shown to regulate numerous transcription factors, cytokines, protein kinases, adhesion molecules, redox status and enzymes that have been linked to inflammation 24.
Anti-Venom Activity: Curcumin was listed as a herbal plant metabolite that can be effective against snake venom PLA2 34. Researchers studied the structural relationship between medicinally important herbal compounds such as acalyphin, chlorogenic acid, stigmasterol, curcumin and tectoridin and PLA2 from Russell's viper. The molecular modeling studies revealed favorable interactions with the amino acid residues at the active site of venom PLA2 that could result in the inhibition 35.
Anti-angiogenesis Activity: Curcumin was tested for its ability to inhibit the proliferation of primary endothelial cells in the presence and absence of basic fibroblast growth factor (bFGF), as well as its ability to inhibit proliferation of an immortalized endothelial cell line. Curcumin was tested for its ability to inhibit phorbol ester-stimulated vascular endothelial growth factor (VEGF) mRNA production 36. Curcumin effectively inhibited endothelial cell proliferation in a dose-dependent manner. Curcumin demonstrated significant inhibition of bFGF-mediated corneal neo-vascularization in the mouse. Curcumin did not affect phorbol ester-stimulated VEGF production. These results indicate that curcumin has direct anti-angiogenic activity in-vitro and in-vivo 37.
Anti-oxidant Activity: Curcumin demonstrated the antioxidant activity by evaluation curcumin using various in-vitro antioxidant assays such as 1,1-diphenyl-2-picrylhydrazyl free radical (DPPH) scavenging, 2, 2′-and-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging activity, N, N-dimethyl- p- phenylenediamine dihydro-chloride (DMPD) radical scavenging activity, total antioxidant activity determination by ferric thiocyanate, total reducing ability determination by the Fe3+– Fe2+ transformation method, superoxide anion radical scavenging by the riboflavin/ methionine/ illuminate system, hydrogen peroxide scavenging and ferrous ions (Fe2+) chelating activities 38.
Protective against Cardio Toxicity and Liver Toxicity: Researchers investigate the protective effects of curcumin on experimentally induced hepatotoxicity, and cardiotoxicity using various animal models with biochemical parameters like serum marker enzymes and antioxidants in target tissues. The increased relative weight of liver and heart in CCl4 induced liver injury and isoproterenol-induced cardiac necroses were also reduced by curcumin treatment. Elevated serum marker enzymes, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) increased lipid peroxidation, decreased glutathione (GSH), glutathione peroxidase (GPx) and superoxide dismutase (SOD) in edematous, granulomatous, liver and heart tissues during liver injury and cardiac necrosis, respectively. The study demonstrated the in-vitro and in-vivo protective effect of curcumin on experimentally induced hepatotoxicity and cardio- toxicity in rats 39.
Anti-bacterial Activity: The antibacterial study of curcumin shows the ability to inhibit the growth of a variety of periodontopathic bacteria and Porphyromonas gingivitis Arg- and Lys-specific proteinase (RGP and KGP, respectively) activities 63. Also, curcumin suppressed P. gingivitis homotypic and Streptococcus gordonii biofilm formations in a dose-dependent manner 64. Bacterial growth was suppressed almost completely at very low concentrations of curcumin. A concentration of 20 µg/mL of curcumin inhibited these P. gingivitis biofilm formations by more than 80%. On the other hand, 100 µg/mL of curcumin did not suppress the growth of Aggregatibacter actinomycetemcomitans 63. Furthermore, at relatively high concentrations, curcumin targets bacterial membranes (Escherichia coli).
Additionally, many features of a bacterial apoptosis-like response were observed after treatment with curcumin at the MIC, including membrane depolarization, Ca2+ influx, PS exposure, and DNA fragmentation. A bacterial apoptosis-like response, induced by curcumin, by causing reactive oxygen species generation and DNA damage 65. The study on E. coli and B. subtilis demonstrated that curcumin by the inhibitory effect against FtsZ polymerization could suppress the FtsZ assembly leading to disruption of prokaryotic cell division 66.
On another hand, Curcumin - Polymyxin B used clinically for topical therapy to treat or prevent traumatic wound infections of the skin. It would not only increase the spectrum of activity to include Gram-positive bacteria but also combat those isolated resistant. The use of the combination may also reduce the emergence of resistant isolates during treatments, due to the multiple antimicrobial targets of dual drug therapy and ease the selective pressure produced by broad-spectrum antibiotics 67.
Additionally, curcumin loaded in zein (zein-CUR) fibers showed good antibacterial activity towards S. aureus, and E. coli and the inhibition efficiency increased with the increase of curcumin contents. Due to the different cell membrane constituent and structure, the antibacterial activity towards S. aureus was better than that towards E. coli. The study displayed that the zein-CUR fibers might have potential as a promising material for antimicrobial applications to inhibit bacterial growth and propagation in food packaging 68. Also, the antibacterial activity of curcumin-chitosan film against Staphylococcus aureus and Rhizoctonia solani was studied by the zone inhibition method 69. A better antibacterial activity was certified compared to PCH film, which is an important consideration in food packaging. The natural blend films of curcumin and chitosan could be as a promising antimicrobial packaging for food and agriculture products 70.
Novel fibrous materials from cellulose acetate (CA) and polyvinylpyrrolidone (PVP) contain curcumin. The incorporation of PVP resulted in increased hydrophilicity of the fibers and faster curcumin release. Likewise, curcumin was found in the amorphous state in the curcumin-containing fibers, and these mats exhibited antibacterial activity against Staphylococcus aureus (S. aureus). The Curc/CA+Curc/PVP mat prepared by dual-spinneret electrospinning killed all the bacteria at the 4 h. Curcumin fibrous materials are potential antibacterial for wound dressing applications 71.
Also, surface charge, as well as the small size of curcumin nanoparticles, plays a key role in enhancing cell-antimicrobial interaction and anti-microbial efficacy. The fabricated curcumin nanoparticles showed the best antimicrobial activity against Listeria monocytogenes. A size reduction to nano-scale is a recently developed strategy used to improve drug/food delivery and matching the public demand for effective and safe antimicrobial formulations for control of foodborne pathogen 72. In-vivo study of antibacterial effect of curcumin on H. pylori compared to OAM (Omeprazole, Amoxicillin, and Metronidazole) treatment revealed poor activity for the eradication of H. pylori (5.9% vs. 78.9% for OAM treatment). The reduction in inflammatory cytokine production was not reported from pylori-infected patients treated with curcumin 73. The in-vivo study of 1-week nonantibiotic therapy comprised of curcumin, pantoprazole, N-acetylcysteine, and lactoferrin against H. pylori infection was not effective for the eradication of H. pylori. However, the decrease in immunological criteria of gastric inflammation and dyspeptic symptoms was reported after 2 months of treatment schedule 74.
Nevertheless, the curcumin administration to the rats with H. pylori-induced gastric inflammation revealed a significant reduction in macromolecular leakage and NF activation 75. In an in-vivo study of H. pylori-infected C57BL/6 mice administered with curcumin exhibited immense therapeutic potential and pronounced eradication effect against H. pylori infection associated with restoration of gastric damage 76.
Anti-Fungal Activity: Substances and extracts isolated from different natural resources especially plants have always been a rich arsenal for controlling the fungal infections and spoilage. Due to extensive traditional use of curcumin in food products, various researches have been done to study curcumin with the aspect of controlling fungal related spoilage and fungal pathogens 77.
The study of addition the curcumin powder in plant tissue culture showed that curcumin at the 0.8 and 1.0 g/L had appreciable inhibitory activity against fungal contaminations 78. The possible mechanism underlying the mentioned antifungal effect was found to be the downregulation of desaturase (ERG3) leading to a significant reduction in ergosterol of fungal cell. Reduction in production of ergosterol results in accumulations of biosynthetic precursors of ergosterol which leads to cell death via generation of ROS 118. Reduction in proteinase secretion and alteration of membrane-associated properties of ATPase activity are other possible critical factors for antifungal activity of curcumin 79. Finding new anti-candida substances seems to be crucial due to the development of resistant strain against existing antifungal drug 56. The study of curcumin, against 14 strains of Candida, showed that curcumin is a potent fungicide compound against Candida species with MIC values ranging from 250 to 2000 μg/mL 79.
In another study, anti-candida activity of curcumin was demonstrated against 38 different strains of Candida including some fluconazole resistant strains and clinical isolates of C. albicans, C. glabrata, C. tropicalis, C. guilliermondii, and C. krusei. The MIC90 values for sensitive and resistant strains were 250-650 and 250-500 μg/mL, respectively. Intracellular acidification via inhibition of H+-extrusion was identified as a possible mechanism for cell death of Candida species 80. The development of hyphae was proved to be inhibited by curcumin through targeting the global suppressor thymidine uptake 1 (TUP1). Curcumin exhibited potent antifungal effect via mechanisms associated with disruption of the plasma membrane in Candida albicans 81.
Curcumin also showed an inhibitory effect on Cryptococcus neoformans and C. dubliniensis with MIC value of 32 mg/L 79. One of the major complications during therapies against chronic asthma is oropharyngeal candidiasis. Curcumin as a potential candidate for the treatment of candidiasis with anti-inflammatory activity was studied in a murine model of asthma. The oral administrator of curcumin is more effective than dexamethasone in reducing fungal burden in BALB/c mice. It also significantly decreased pathological changes in asthma 82. Adhesion of Candida species isolated from AIDS patients to buccal epithelial cells is also markedly inhibited by curcumin, and it was found to be more effective compared to fluconazole 83.
The investigation of curcumin mediation for photodynamic therapy can reduce the biofilm biomass of C. albicans, C. glabrata, and C. tropicalis. The results demonstrated that the association of four LED influences for light excitation with 40 μM concentration of curcumin at 18 J/cm2 inhibited up to 85% metabolic activity of the tested Candida species. The use of curcumin with light proved to be an effective method for noteworthy improvement in the antifungal activity against a planktonic form of the yeasts 84. Photodynamic effect considerably decreased C. albicans viability in either planktonic or biofilm cultures probably through increasing the uptake of curcumin by cells.
However, to a lesser extent, photodynamic therapy was found to be phototoxic to the macrophages 85. The strong antifungal activity of C. longa rhizome and its low side effect were the main reasons to investigate its probable synergistic effect with existing fungicides. The synergistic activity of curcumin with five azole and two polyene drugs including voriconazole, itraconazole, ketoconazole, miconazole, fluconazole, amphotericin B and nystatin showed a 10-35-fold reduction in the MIC values of the fungicides against 21 clinical isolates of C. albicans. The synergistic activity of curcumin with amphotericin B and fluconazole could be associated with the accumulation of ROS which will be suppressed by adding an antioxidant 46.
Anti-viral Activity: Lack of effective therapeutics for the most of viral diseases, the emergence of antiviral drug resistance and high cost of some antiviral therapies necessitate finding new effective antiviral compounds 57, 58. Additionally, the existing antiviral therapies are not always well-tolerated or quite effective and satisfactory 46. Hence, the increasing requirement for antiviral substances will be more highlighted. Plants as a rich source of phytochemicals with different biological activities including antiviral activities are in the interest of scientists 59.
It has been demonstrated that curcumin as a plant derivative has a wide range of antiviral activity against different viruses: papillomavirus virus (HPV), influenza virus, Hepatitis B virus (HBV), Hepatitis C virus (HCV), adenovirus, coxsackie-virus, Human norovirus (HuNoV), Respiratory syncytial virus (RSV) and Herpes simplex 1 (HSV-1) 86, 87, 88, 89, 90. Curcumin functionalized graphene oxide shown synergistic antiviral effect against respiratory syncytial virus infection 87. Respiratory syncytial virus (RSV), which is considered as the major viral pathogen of the lower respiratory tract of infants, has been implicated in severe lung disease 86.
Developing a β-cyclodextrin (CD) functionalized graphene oxide (GO) composite, which displayed excellent antiviral activity and curcumin loading efficiently, showed that the composite could prevent RSV from infecting the host cells by the directly inactivating virus and inhibiting the viral attachment, which possessed the prophylactic and therapeutic effects towards virus 86. The antiviral effect of curcumin was a dose-dependent manner 91. Curcumin inhibits the activity of inosine-mono phosphate dehydrogenase (IMPDH) enzyme in an either noncompetitive or competitive manner. By inhibition of IMPDH, this led to reducing the level of intracellular guanine nucleotides which required for adequate RNA and DNA synthesis 86, 88, 92. Curcumin mechanism involves in viral entry or other life cycle stages rather than the replication of viral RNA 91. Therefore, by inhibition of IMPDH Curcumin have potential anti-proliferative, antiviral and antiparasitic effects 92.
Anti-cancer Activity: Cancer is the second largest single cause of death claiming over six million lives every year worldwide 93. Scientific studies of plants used in various types of ethnic medicine have led to the discovery of many valuable drugs, including taxol, camptothecin, vincristine and vinblastine 94, 44. Many studies pointed out anticancer activities of curcumin alone or in combination with conventional chemotherapy drugs in the treatment of cancer and its cancer-related complications 94, 95, 96, 97.
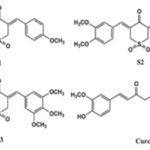
In-vitro and in-vivo studies have indicated that curcumin prevents carcinogenesis by affecting two primary processes: Angiogenesis and tumor growth 96, 97 98. Curcumin has exhibited efficient anticancer and antifungal activities alone or in combination with conventional chemotherapy drugs and antifungal agents 99. Curcumin analogs S1- S3 is containing sulfone strongly inhibited the growth of human prostate, colon, lung and pancreatic cancer cells 100, 101.
FIG. 4: STRUCTURE OF CURCUMIN ANALOGS CONTAINING SULFONE
Curcumin significantly inhibited the growth of human breast cancer cell by inducing apoptosis in a dose and time-dependent manner, accompanied by a decrease in MCF-7 cell viability 98. The antitumor action of curcumin is mediated via its anti-proliferative effect in multiple cancers, inhibitory action on transcription factors and downstream gene products, modulatory effect on growth factor receptors and cell adhesion molecules involved in angiogenesis, tumor growth and metastasis, while recent works showed the possibility curcumin could exert its antitumor potential by telomerase inhibition 102, 103, 104. Curcumin oil has bi-functional effects by blocking anti-apoptotic signaling but also blocking anti-oncogenic signaling and interferon-γ production 105, 106. Moreover, Curcumin showed higher uptake in tumor cells compared to normal cells, suggesting potential diagnostic applications in this field 107.
In a study, the Gallium-Curcumin complexes showed an uptake in A549 lung cancer cells, at least equivalent to the respective free curcumin, confirming potential applications as cancer-detecting radiotracers. Natural products play a major role in chemotherapy drugs and primarily target proliferating tumor cells 94. Their use could be of great interest and is considered to be an inexpensive, safe and accessible approach to cancer control and management. However, in spite of the useful biological activities of curcumin but it limited due to its poor bioavailability, water solubility and some possible adverse effects 96, 109.
The development of formulations of curcumin in the form of nanoparticles, liposomes, micelles, or phospholipid complexes to enhance its bio-availability and efficacy is still in its early stages 110. Various nano-sized curcumin delivery systems, such as nanoparticles, nanospheres, solid lipid nanoparticles, micelles, and liposomes have been shown to overcome these shortcomings and significantly improve the anticancer and antifungal activities of curcumin. Many studies on curcumin and its nanoformulations are still in the preclinical stage at present 110, 111.
PLGA curcumin nanoparticles efficiently inhibit the growth of prostate cancer cells both in-vitro and in-vivo. This was achieved through lysosomal activity, apoptosis, and inhibition of Androgen receptor and nuclear b-catenin activity. PLGA-CUR NPs significantly modulate the expression of miR-21 and miR-205 genes. Shown significant prostate tumor-specific targeting in a xenograft mouse model 112. Curcumin exhibits the ability to modulate multiple targets via the regulation of diverse transcription factors, inflammatory cytokines, growth factors, different protein kinases, and various other enzymes. Furthermore, safety and tolerability as evidenced by multiple clinical trials carried out thus far together with cost-effectiveness are some other added yet inevitable advantages offered by this agent 113.
Delay of Cataract Development: Cataract is responsible for more than one-third of blindness worldwide. Twenty-five percent of people over the age of 65 and 50% of people larger than an age of 80 have a serious defeat of vision due to cataracts 114, 115. Cataract extraction surgery is the majority treatment for cataract. Whereas cataract surgery is considered to be not dangerous and mature, irreversible blindness is a possible risk. There is no recognized drug which can treat or overturn cataract. If cataract onset is late by 10 years, it is expected to decrease the risk for cataract surgery by 50%. Thus, much emphasis is being laid on identifying compounds with high effectiveness and low toxicity that can either avoid the onset or delay cataract progression.
It is supposed that oxidative damage to the eye lens responsible for the development of different kinds of cataracts 116. The antioxidant characteristics of curcumin are the main anti-cataract mechanism 117. In cultured human lens epithelial cells (hLECs) in-vitro, curcumin inhibits peroxiredoxin 6 (a pleiotropic oxidative stress-response protein). By reversing the activity of increasing the activities of superoxide dismutase (SOD), decreasing ROS, and antioxidant enzymes, the bioactive derivatives of curcumin were reported to inhibit the selenite inducing cataract 118, 119, 120.
Additionally, curcumin was found to have a protective effect against cataract development and progression of diabetic cataract in numerous in-vitro and in-vivo cataract models 120, 121. Vitamin C is a potent non-enzymic antioxidant, and the level of vitamin C is high in the human lens, suggesting that vitamin C may have a preventive role in cataract progression. The decreased levels of vitamin C linked with selenite-induced rat cataracts. So by the administration of curcumin was found to increase vitamin C levels so protect rat eyes 122. Pre-treatment of curcumin may prevent oxidative damage and delay the development of cataracts 118.
Hepatoprotective Activity: The liver is one of the most important organs of the body, that plays an important role in maintaining various physiological processes and is involved in numerous vital functions, such as metabolism, secretion, and storage 123. Also participating in the biochemical processes of growing, providing nutrients, supplying energy, and reproducing. In addition, it aids in the metabolism of carbohydrates and fats, in the secretion of bile, and in the storage of Vitamins It plays a central role in detoxifying endogenous (waste metabolites) and/or exogenous (toxic compounds) substances of organisms, as well as for synthesizing useful agents, has been analyzed since the 1970s by many researchers. Curcumin has been discussed by various researchers for their hepatoprotective. New evidence has proven the hepatoprotective activity of curcumin, but its underlying mechanisms remain to be elucidated.
Phytosome Curcumin had a strong protective effect against paracetamol-induced with acute hepatic damage in mice. The hepatoprotective effect of phytosome curcumin may be explained by increasing levels of antioxidant enzymes and decreasing the lipid peroxidation and liver enzyme on paracetamol-induced damage in mice. Furthermore, an investigation of the protective effect of curcumin on hepatic damage via measuring the antioxidant capacity and regulation of different enzymes. Curcumin treatment of bile duct ligated rats led by elevation of antioxidant (thiols, SOD, and catalase) and hepatic enzymes (ALP, AST, and ALT). And curcumin attenuated liver damage through down-regulating of Ras-related C3 botulinum toxin substrate 1, Rac1-GTP, and NADPH oxidase 1 as well as reducing oxidative stress in serum and liver tissue of BDL rats.
Curcumin may serve as effective hepatoprotective agents for mercuric chloride-induced hepato-toxicity. The protective effect is due to their free radical scavenging activities and recovery of antioxidant enzymes and function markers of the liver. In additionally, the protective effects of curcumin against Diethyl nitrosamine induced hepatocarcinogenesis in albino rats is due to modulated the hepatic pathological alteration, liver function enzymes serum levels, induced the hepatic anti-oxidant system and suppressed the proinflammatory cytokines.
Anti-fibrotic Activity: Idiopathic pulmonary fibrosis (IPF) is a progressive disease of unknown etiology that can result in respiratory failure. The resulting fibrotic changes in lung architecture lead to decreased gas exchange and pulmonary compliance. Notably, curcumin effectively reduces profibrotic effects in fibroblasts in-vitro via the inhibition of key steps in the signaling pathway of transforming growth factor beta (TGF-b) a multifunctional cytokine belonging to the transforming growth factor.
It was reported that the activation of peroxisome proliferator-activated receptor gamma (PPAR-g) by curcumin blocked platelet-derived growth factor (PDGF) signaling pathway in hepatic satellites cells. However, the relationship of PPAR- g and PDGF signaling pathway is unclear in TGF-b induced differentiation of lung fibroblasts to myofibroblasts. Curcumin inhibits TGF-β2 driven differentiation of mouse lung fibroblasts to myofibroblasts. Curcumin and PPAR-γ could potentially be used for effective treatment of IPF.
Anti-atherosclerosis and Anti-hypertension Activity: Atherosclerosis and hypertension can potentially progress into dangerous cardiovascular diseases such as myocardial infarction and stroke. Statins are widely used to lower cholesterol levels while antihypertensive agents such as captopril are widely prescribed to treat high blood pressure. Curcumin, a phenolic compound isolated from Curcuma domestica, has been proven effective for a broad spectrum of diseases, including hypertension and hypercholesterolemia. Therefore, curcumin is quite promising as an alternative therapeutic compound. By studying the effects of Curcumin on hyperlipidemia and hepatic steatosis in high-fructose-fed Wistar rats, the results showed the ability of curcumin in treatment high-fructose induced fatty liver, lipid derangements and obesity through modulation of lipid metabolism in the liver as evidenced by decreased expression of lipogenic enzymes and transcription factors. Therefore, it is suggested that the use of curcumin may be beneficial as an adjuvant in the prevention and management of diet-induced obesity and its associated complications.
Another study reported that encapsulated of curcumin in a nanoemulsion showed significant cholesterol-lowering activity compared to a standard drug, pravastatin and this encapsulated increased not only the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition but also Angiotensin-converting enzyme inhibitors like effect by producing vasodilation by inhibiting the formation of angiotensin II. These effects are suggested to be the result of improved solubility in the nanoemulsion system.
CONCLUSION: The wisdom and scientific knowledge of curcumin, a highly pleiotropic agent, which were used for its therapeutic effects in many countries as traditional medicine. For that, the pharmacological properties and applications of curcumin are a rapidly growing, progressing, and expanding enterprise, as evidenced by the studies reviewed above and the many more being reported every day.
Of the most obvious therapeutic weight of curcumin, researchers typically pointed at diseases like diabetes, wound healing, arthritis, Alzheimer, Parkinson, inflammatory, venom, angiogenesis, cataract, cancer, atherosclerosis, and hypertension, etc, which is in use since ages owing to its multiple pharmacological activities. Curcumin is enriched with many useful phytoconstituents, which are responsible for its efficacy proven by experimentally and clinically. It has been established beneficial in treating anti-inflammatory, anti-allergic, anti-oxidant, anti-hyperglycaemic, anti-cancer, anti-microbial, anti-atherosclerosis and anti-hypertension properties. Because of curcumin facility to affect a large range of molecular targets and a good safety profile, was established to be a potential candidate for the avoidance or/and treatment of several diseases.
ACKNOWLEDGEMENT: We respect and thank Prof. Dr. Mohammad Hudaib for providing us an opportunity to do the project work in (Pharmacological Aspects of Curcumin: Review article) and giving us all support and guidance which made us complete the project duly. We are extremely thankful to Dr. Mayada Shihadeh for providing such a nice support and guidance.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Panpatil VV, Tattari S, Kota N, Nimgulkar C and Polasa K: In-vitro evaluation on antioxidant and antimicrobial activity of spice extracts of ginger, turmeric and garlic. J of Pharmacognosy and Phytochemistry 2013; 2(3): 143-148.
- Pawar H, Karde M, Mundle N, Jadhav P and Mehra K: Phytochemical evaluation and curcumin content determination of turmeric rhizomes collected from Bhandara District of Maharashtra (India). Med Chem 2014; 4(8): 588-591.
- Aggarwal BB, Kumar A and Bharti AC: Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Research 2003; 23(1/A): 363-398.
- Kharat M, Du Z, Zhang G and McClements DJ: Physical and chemical stability of curcumin in aqueous solutions and emulsions: Impact of pH, temperature and molecular environment. Journal of Agricultural and Food Chemistry 2017; 65(8): 1525-1532.
- Hewlings SJ and Kalman DS: Curcumin: a review of its’ effects on human health. Foods 2017; 6(10): 92.
- Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M and Sahebkar A: Antioxidant and anti-inflammatory effects of Curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clinical Nutrition 2015; 34(6): 1101-1108.
- Kant V, Gopal A, Pathak NN, Kumar P, Tandan SK and Kumar D: Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin - induced diabetic rats. International Immunopharmacology 2014; 20(2): 322-330.
- Rheim FA, Ragab AA, Hamdy HED and Hammam FM: Evaluation of DNA damage in-vivo by comet assay and chromosomal aberrations for pyrethroid insecticide and the antimutagenic: Role of cThe Egyptian Journal of Hospital Medicine 2015; 59: 172-181.
- Gómez-Estaca J, Balaguer MP, López-Carballo G, Gavara R and Hernández-Muñoz P: Improving antioxidant and antimicrobial properties of curcumin using encapsulation in gelatin through electrohydrodynamic atomization. Food Hydrocolloids 2017; 70: 313-320.
- Noorafshan A and Ashkani-Esfahani S: A review of the therapeutic effects of cCurrent Pharmaceutical Design 2013; 19(11): 2032-2046.
- Prasad S, Tyagi AK and Aggarwal BB: Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer research and treatment: Official Journal of Korean Cancer Association 2014; 46(1): 2.
- Gupta SC, Patchva S and Aggarwal BB: Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS Journal 2013; 15(1): 195-218.
- Hu S, Maiti P, Ma Q, Zuo X, Jones MR, Cole GM and Frautschy SA: Clinical development of curcumin in neurodegenerative disease. Expert Review of Neuro Therapeutics 2015; 15(6): 629-637.
- Rungseesantivanon S, Thenchaisri N, Ruangvejvorachai P and Patumraj S: Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complementary & Alternative Medicine 2010; 10: 57-57.
- Chuengsamarn S, Rattanamongkolgul S, Luechapudiporn R, Phisalaphong C and Jirawatnotai S: Curcumin extract for prevention of type 2 diabetes. Diabetes Care 2012; 35(11): 2121-2127.
- Akbik D, Ghadiri M, Chrzanowski W and Rohanizadeh R: Curcumin as a wound healing agent. Life Sciences 2014; 116(1): 1-7.
- Panchatcharam M, Miriyala S, Gayathri VS and Suguna L: Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen Molecular and Cellular Biochemistry 2006; 290(1): 87-96.
- Chereddy KK, Coco R, Memvanga PB, Ucakar B, des Rieux A, Vandermeulen G and Préat V: Combined effect of PLGA and curcumin on wound healing activity. Journal of Controlled Release 2013; 171(2): 208-215.
- Aggarwal BB and Harikumar KB: Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. The International Journal of Biochemistry and Cell Biology 2009; 41(1): 40-59.
- Chandran B and Goel A: A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytotherapy Research 2012; 26(11): 1719-1725.
- Jackson JK, Higo T, Hunter WL and Burt HM: The antioxidants Curcumin and quercetin inhibit inflammatory processes associated with arthritis. Inflammation Research 2006; 55(4): 168-175.
- Kloesch B, Becker T, Dietersdorfer E, Kiener H and Steiner G: Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like syno-International Immunopharmacology 2013; 15(2): 400-405.
- Villaflores OB, Chen YJ, Chen CP, Yeh JM and Wu TY: Curcumin oids and resveratrol as anti-alzheimer agents. Taiwanese Journal of Obstetrics and Gynecology 2012; 51(4): 515-525.
- Kim J, Lee HJ and Lee KW: Naturally occurring phytochemicals for the prevention of Alzheimer's disease. Journal of Neurochemistry 2010; 112(6): 1415-1430.
- Fu W, Zhuang W, Zhou S and Wang X: Plant-derived neuroprotective agents in Parkinson’s disease. American Journal of Translational Research 2015; 7(7): 1189a.
- Ghosh N, Ghosh R and Mandal SC: Antioxidant protection: a promising therapeutic intervention in neurodegenerative disease. Free Radical Research 2011; 45(8): 888-905.
- He Y, Yue Y, Zheng X, Zhang K, Chen S and Du Z: Curcumin, inflammation and chronic diseases: how are they linked?. Molecules 2015; 20(5): 9183-9213.
- Ghosh S: Gomes A. Russell’s viper (Daboia Russelli Russelli) venom toxicity neutralizing the efficacy of Curcumin -Gold Nanoparticle (C-GNP) in an experimental animal model. J Toxins 2016; 3(2): 6.
- Sebastin Santhosh M, Hemshekhar M, Sunitha KM, Thushara R, Jnaneshwari S, Kemparaju K and Girish SK: Snake venom-induced local toxicities: secondary plant metabolites as auxiliary therapy. Mini Reviews in Medicinal Chemistry, 2013; 13(1): 106-123.
- Perry MC, Demeule M, Regina A, Moumdjian R and Beliveau R: Curcumin inhibits tumor growth and angiogenesis in glioblastoma xenografts. Molecular Nutrition and Food Research 2010; 54(8): 1192-1201.
- Li KK, Liu CL, Tam JCW, Kwok HF, Lau CP, Leung PC, Ko CH and Ye CX: In-vitro and in-vivo mechanistic study of a novel proanthocyanidin, GC-(4→ 8)-GCG from cocoa tea (Camellia ptilophylla) in antiangiogenesis. The Journal of Nutritional Biochemistry 2014; 25(3): 319-328.
- Naik SR, Thakare VN and Patil SR: Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: evidence of its antioxidant property. Experimental and Toxicologic Pathology 2011; 63(5): 419-431.
- Hilles AR and Mahmood S: A review on phytochemistry and pharmacological effects of Trigonella foenum-graecum. Advanced Herbal Medicine 2016; 2(3): 61-67.
- Wise R, Hart T, Cars O, Streulens M, Helmuth R, Huovinen P and Sprenger M: Antimicrobial resistance: is a major threat to public health. BMJ: British Medical Journal 1998; 317(7159): 609.
- Samy PR and Gopalakrishnakone P: Therapeutic potential of plants as anti-microbials for drug discovery. Evidence-based Complementary & Alternative Medicine 2010; 7(3); 283-294.
- Dias DA, Urban S and Roessner U: A historical overview of natural products in drug discovery. Metabolites 2012; 2(2): 303-336.
- Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss EH and Rollinger JM: Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances 2015; 33(8): 1582-1614.
- David B, Wolfender JL and Dias DA: The pharmaceutical industry and natural products: historical status and new trends. Phytochemistry Reviews 2015; 14(2): 299-315.
- Scannell JW, Blanckley A, Boldon H and Warrington B: Diagnosing the decline in pharmaceutical R and D efficiency. Nature Review Drug Discovery 2012; 11(3): 191-200.
- Butler MS: The role of natural product chemistry in drug discovery. Journal of Natural Products 2004; 67(12): 2141-2153.
- Cragg GM and Newman DJ: Natural products: a continuing source of novel drug leads. Biochimica et Biophysica Acta (BBA)-General Subjects 2013; 1830(6): 3670-3695.
- Lahlou M: The success of natural products in drug discovery. Pharmacol Pharm 2013; 4(3A): 17-31.
- Abreu AC, McBain AJ and Simoes M: Plants as sources of new antimicrobials and resistance-modifying agents. Natural Product Reports 2012; 29(9): 1007-1021.
- World Health, Organization: WHO’s first global report on antibiotic resistance reveals serious, worldwide threat to public health. Antimicrobial resistance-global surveillance report. Virtual Press Conference 2014.
- Lister PD, Wolter DJ and Hanson ND: Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clinical Microbiology Reviews 2009; 22(4): 582-610.
- Liu Y, Xu Z, Yang Z, Sun J and Ma L: Characterization of community-associated Staphylococcus aureus from skin and soft-tissue infections: a multicenter study in China. Emerging Microbes and Infections 2016; 5(12): e127.
- Rao Q, Shang W, Hu X and Rao X: Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. Journal of Medical Microbiology 2015; 64(12): 1462-1473.
- Thakre AD, Mulange SV, Kodgire SS, Zore GB and Karuppayil SM: Effects of cinnamaldehyde, ocimene, camphene, curcumin and farnesene on Candida albicans. Advances in Microbiology 2016; 6(09): 627.
- Petersen LR, Jamieson DJ, Powers AM and Honein MA: Zika virus. New England Journal of Medicine 2016; 374(16): 1552-1563.
- De Clercq E and Li G: Approved antiviral drugs over the past 50 years. Clinical Microbiology Reviews 2016; 29(3): 695-747.
- Razonable RR: Antiviral drugs for viruses other than human immunodeficiency virus. In Mayo Clinic Proceedings, Elsevier 2011; 86(10): 1009-1026.
- Al Akeel R, Al-Sheikh Y, Mateen A, Syed R, Janardhan K and Gupta VC: Evaluation of the antibacterial activity of crude protein extracts from seeds of six different medical plants against standard bacterial strains. Saudi Journal of Biological Sciences 2014; 21(2): 147-151.
- Ekor M: The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology 2014; 4: 177.
- Rahnavard R and Razavi N: A review on the medical effects of Capparis spinosaAdvanced Herbal Medicine 2016; 2(1): 44-53.
- Izui S, Sekine S, Maeda K, Kuboniwa M, Takada A, Amano A and Nagata H: Antibacterial activity of curcumin against periodontopathic bacteria. Journal of Periodontology 2016; 87(1): 83-90.
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S and Aggarwal BB: Curcumin , the golden nutraceutical: multitargeting for multiple chronic diseases. British Journal of Pharmacology 2017; 174(11): 1325-1348.
- Yun DG and Lee DG: Antibacterial activity of curcumin via an apoptosis-like response in Escherichia coli. Applied Microbiology and Biotechnology 2016; 100(12): 5505-5514.
- Kaur S, Modi NH, Panda D and Roy N: Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ–a structural insight to unveil antibacterial activity of c European Journal of Medicinal Chemistry 2010; 45(9): 4209-4214.
- Betts JW, Sharili AS, La Ragione RM and Wareham DW: In -vitro antibacterial activity of curcumin - polymyxin B combinations against multidrug - resistant bacteria associated with traumatic wound infections. Journal of Natural Products 2016; 79(6): 1702-1706.
- Wang H, Hao L, Wang P, Chen M, Jiang S and Jiang S: Release kinetics and antibacterial activity of curcumin loaded zein fibers. Food Hydrocolloids 2017; 63: 437-446.
- Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D and Zhai G: Oral bioavailability of curcumin: problems and advancements. Journal of Drug Targeting 2016; 24(8): 694-702.
- Liu Y, Cai Y, Jiang X, Wu J and Le X: Molecular interactions, characterization and antimicrobial activity of curcumin-chitosan blend films. Food Hydrocolloids 2016; 52: 564-572.
- Tsekova PB, Spasova MG, Manolova NE, Markova ND and Rashkov IB: Electrospun curcumin -loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Materials Science and Engineering: C, 2017; 73: 206-214.
- No DS, Algburi A, Huynh P, Moret A, Ringard M, Comito N, Drider D, Takhistov P and Chikindas ML: Anti-microbial efficacy of curcumin nanoparticles against Listeria monocytogenes is mediated by surface charge. Journal of Food Safety 2017; 37(4):
- Koosirirat C, Linpisarn S, Changsom D, Chawansuntati K and Wipasa J: Investigation of the anti-inflammatory effect of C. longa in H. pylori-infected patients. International Immunopharmacology 2010; 10(7): 815-818.
- Di Mario F, Cavallaro LG, Nouvenne A, Stefani N, Cavestro GM, Iori V, Maino M, Comparato G, Fanigliulo L, Morana E and Pilotto A: A curcumin ‐based 1‐week triple therapy for eradication of Helicobacter pylori infection: Something to Learn From Failure?. Helicobacter 2007; 12(3): 238-243.
- Sintara K, Thong-Ngam D, Patumraj S, Klaikeaw N and Chatsuwan T: Curcumin suppresses gastric NF-κB activation and macromolecular leakage in Helicobacter pylori-infected rats. World Journal of Gastroenterology: WJG 2010; 16(32): 4039.
- De R, Kundu P, Swarnakar S, Ramamurthy T, Chowdhury A, Nair GB and Mukhopadhyay AK: Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrobial Agents and Chemotherapy 2009; 53(4): 1592-1597.
- Shuping DSS and Eloff JN: The use of plants to protect plants and food against fungal pathogens: a review. African Journal of Traditional, Complementary and Alternative Medicines (AJTCAM) 2017; 14(4): 120-127.
- Upendra RS, Khandelwal P and Reddy AM: Turmeric powder (Curcuma longa) as an antifungal agent in plant tissue culture studies. International Journal of Engineering Science 2011; 3(11): 7899-7904.
- Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M and Khan LA: Curcumin as a promising anticandidal of clinical interest. Canadian Journal of Microbiology 2011; 57(3): 204-210.
- Khan N, Shreaz S, Bhatia R, Ahmad SI, Muralidhar S, Manzoor N and Khan LA: Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012; 83(3): 434-440.
- Lee W and Lee DG: An antifungal mechanism of curcumin lies in membrane‐targeted action within Candida albicans. IUBMB Life 2014; 66(11): 780-785.
- Karaman M, Ayyıldız ZA, Fırıncı F, Kiray M, Bağrıyanık A, Yilmaz O, Uzuner N and Karaman Ö: Effects of curcumin on lung histopathology and fungal burden in a mouse model of chronic asthma and oropharyngeal candidiasis. Archives of Medical Research 2011; 42(2): 79-87.
- Dovigo LN, Pavarina AC, Carmello JC, Machado AL, Brunetti IL and Bagnato VS: Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers in Surgery and Medicine 2011; 43(9): 927-934a.
- Dovigo LN, Pavarina AC, Ribeiro APD, Brunetti IL, Costa CADS, Jacomassi DP, Bagnato VS and Kurachi C: Investigation of the photodynamic effects of curcumin against Candida albicans. Photochemistry and Photo-biology 2011; 87(4): 895-903b.
- Blanco JC, Pletneva LM, Otoa RO, Patel MC, Vogel SN and Boukhvalova MS: Preclinical assessment of safety of maternal vaccination against the respiratory syncytial virus (RSV) in cotton rats. Vaccine 2017; 35(32): 3951-3958.
- Yang, XX, Li CM, Li YF, Wang J and Huang CZ: Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale 2017; 9(41): 16086-16092.
- Buckley D, Fraser A, Huang G and Jiang X: Recovery Optimization and Survival of the human norovirus surrogates feline calicivirus and murine norovirus on carpet. Applied and Environmental Microbiology 2017; 83(22): e01336-17.
- Camini FC, da Silva Caetano CC, Almeida LT and de Brito Magalhães CL: Implications of oxidative stress on viral pathogenesis. Archives of Virology 2017; 1-11.
- Gasparini R, Amicizia D, Lai PL, Bragazzi NL and Panatto D: Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part I: influenza life-cycle and currently available drugs. Journal of Preventive Medicine and Hygiene 2014; 55(3): 69.
- Yang M, Lee G, Si J, Lee SJ, You HJ and Ko G: Curcumin Shows Antiviral Properties against Norovirus. Molecules 2016; 21(10): 1401.
- Dairaku I, Han Y, Yanaka N and Kato N: Inhibitory effect of curcumin on IMP dehydrogenase, the target for anticancer and antiviral chemotherapy agents. Bioscience, Biotechnology and Biochemistry, 2010; 74(1): 185-187.
- World Health Organization. Cancer 2017. http://www.who.int/mediacentre/factsheets/fs297/en/.
- Siveen KS, Uddin S and Mohammad RM: Targeting acute myeloid leukemia stem cell signaling by natural products. Molecular Cancer 2017; 16(1): 13.
- Gupta AP, Khan S, Manzoor MM, Yadav AK, Sharma G, Anand R and Gupta S: Anticancer curcumin: Natural analogues and structure-activity relationship. In Studies in Natural Products Chemistry, Elsevier 2017; 54: 355-401.
- Dulbecco P and Savarino V: Therapeutic potential of curcumin in digestive diseases. World Journal of Gastroenterology: WJG 2013; 19(48): 9256.
- Maheshwari RK, Singh AK, Gaddipati J and Srimal RC: Multiple biological activities of curcumin: a short review. Life Sciences 2006; 78(18): 2081-2087.
- Koohpar ZK, Entezari M, Movafagh A and Hashemi M: Anticancer activity of Curcumin on human breast adenocarcinoma: role of Mcl-1 gene. Iranian Journal of Cancer Prevention 2015; 8(3).
- Anon: Yeast Is A Cause of Cancer And Turmeric Can Kill Both, Research Confirms. Yeast Is A Cause of Cancer And Turmeric Can Kill Both, Research 2015. http://www. com/blog/yeast-cause.
- Zhang Q, Li D, Liu Y, Wang H, Zhang C, Huang H, He Y, Chen X, Du Z and Zheng X: Potential anticancer activity of curcumin analogs containing sulfone on human cancer cells. Archives of Biological Sciences 2016; 68(1): 125-133.
- Siegel R, Ma J, Zou Z and Jemal A: Cancer statistics, 2014. CA: A Cancer J For Clinicians 2014; 64(1): 9-29.
- Fu Z, Chen X, Guan S, Yan Y, Lin H and Hua ZC: Curcumin inhibits angiogenesis and improves defective hematopoiesis induced by tumor-derived VEGF in tumor model through modulating VEGF-VEGFR2 signaling Oncotarget 2015; 6(23): 19469b.
- Bayomi SM, El-Kashef HA, El-Ashmawy MB, Nasr MN, El-Sherbeny MA, Abdel-Aziz NI, Magda AA, Suddek GM, El-Messery SM and Ghaly MA: Synthesis and biological evaluation of new curcumin analogues as antioxidant and antitumor agents: Molecular modeling study. European Journal of Medicinal Chemistry 2015; 101: 584-594.
- Wilken R, Veena MS, Wang MB and Srivatsan ES: Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular Cancer 2011; 10(1), p.12.
- Fiala M: Curcumin and omega-3 fatty acids enhance NK cell-induced apoptosis of pancreatic cancer cells but Curcumin inhibits interferon-γ production: benefits of omega-3 with Curcumin against cancer. Molecules 2015; 20(2): 3020-3026.
- Attari F, Zahmatkesh M, Aligholi H, Mehr SE, Sharifzadeh M, Gorji A, Mokhtari T, Khaksarian M and Hassanzadeh G: Curcumin as a double-edged sword for stem cells: dose, time and cell type-specific responses to Curcumin. DARU Journal of Pharmaceutical Sciences 2015; 23(1): 33.
- Rubagotti S, Croci S, Ferrari E, Orteca G, Iori M, Capponi, PC, Versari A and Asti M: Uptake of Ga-Curcumin derivatives in different cancer cell lines: Toward the development of new potential 68 Ga-labelled Curcumin oids-based radiotracers for tumor imaging. Journal of Inorganic Biochemistry 2017; 173: 113-119.
- Asti M, Ferrari E, Croci S, Atti G, Rubagotti S, Iori M, Capponi PC, Zerbini A, Saladini M, Versari A. Synthesis and characterization of 68Ga-labeled curcumin and curcuminoid complexes as potential radiotracers for imaging of cancer and Alzheimer’s disease. Inorganic chemistry. 2014; 53(10): 4922-33.
- Stanić Z: Curcumin, a compound from natural sources, a true scientific challenge-a review. Plant Foods for Human Nutrition 2017; 72(1): 1-12.
- Allegra A, Innao V, Russo S, Gerace D, Alonci A and Musolino C: Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Investigation 2017; 35(1): 1-22.
- Chen J, He ZM, Wang FL, Zhang ZS, Liu XZ, Zhai DD and Chen WD: Curcumin and its promise as an anticancer drug: an analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. European Journal of Pharmacology 2016; 772: 33-42.
- Yallapu MM, Khan S, Maher DM, Ebeling MC, Sundram V, Chauhan N, Ganju A, Balakrishna S, Gupta BK, Zafar N and Jaggi M: Anti-cancer activity of Curcumin loaded nanoparticles in prostate cancer. Biomaterials 2014. 35(30): 8635-8648.
- Anon, Blindness 2: Major Causes Worldwide. WHO. http://www.who.int/mediacentre/factsheets/fs143/en/
- Anon, Vision impairment and blindness. World Health Organization. http://www.who.int/mediacentre/factsheets/ fs282/en/
- Kapoor S: Curcumin and its emerging intraocular benefits. Journal of Zhejiang University 2013; 14(1): 85.
- Raman T, Ramar M, Arumugam M, Nabavi SM and Varsha MKNS: Cytoprotective mechanism of action of Curcumin against cataract. Pharmacological Reports 2016; 68(3): 561-569.
- Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K and Reddy GB: Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Investigative Ophthalmology and Visual Science 2005; 46(6): 2092-2099.
- Travica N, Ried K, Sali A, Scholey A, Hudson I and Pipingas A: Vitamin C status and cognitive function: A systematic review. Nutrients 2017; 9(9): 960.
- Ilyas U, Katare DP, Aeri V and Naseef PP: A review on hepatoprotective and immunomodulatory herbal plants. Pharmacognosy Reviews 2016; 10(19): 66.
- Adewusi EA and Afolayan AJ: A review of natural products with hepatoprotective activity. Journal of Medicinal Plants Research 2010; 4(13): 1318-1334.
- Ahsan MR, Islam KM, Bulbul IJ, Musaddik MA and Haque E: Hepatoprotective activity of methanol extract of some medicinal plants against carbon tetrachloride-induced hepatotoxicity in rats. Eur J Sci Res 2009; 37(2): 302-310.
- Kabirifar R, Safari F, Karimollah A, Moradi A and Eskandari-Nasab E: Hepatoprotective effects of Curcumin in rats after bile duct ligation via downregulation of Rac1 and NOX1. Nutrition 2017; 36: 72-78.
- Joshi D, Mittal DK, Shukla S, Srivastav SK and Dixit VA: Curcuma longa extract and curcumin protect CYP 2E1 enzymatic activity against mercuric chloride-induced hepatotoxicity and oxidative stress: A protective approach. Experimental and Toxicologic Pathology 2017; 69(6):373-382.
- Kadasa NM, Abdallah H, Afifi M and Gowayed S: Hepatoprotective effects of Curcumin against diethyl nitrosamine-induced hepatotoxicity in albino rats. Asian Pac J Cancer Prev 2015; 16: 103-108.
- Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, Kondapi AK and Reddy RC: Curcumin inhibits fibrosis-related effects in IPF fibroblasts and mice following bleomycin-induced lung injury. American Journal of Physiology-Lung Cellular and Molecular Physiology 2010; 298(5): L616-L625.
- Hayes BJ, Riehle KJ, Shimizu-Albergine M, Bauer RL, Hudkins KL, Johansson F, Yeh MM, Mahoney JWM, Yeung RS and Campbell JS: Activation of platelet-derived growth factor receptor alpha contributes to liver fibrosis. PloS One 2014; 9(3): e92925.
- Fenton R, Brook-Barclay L, Delaney CL, Spark JI and Miller MD: Do Medications Commonly Prescribed to Patients with Peripheral Arterial Disease Have an Effect on Nutritional Status? A Review of the Literature. Annals of Vascular Surgery 2016; 32:145-175.
- Rachmawati H, Soraya IS, Kurniati, NF and Rahma A: In- vitro study on anti-hypertensive and antihyper-cholesterolemic effects of a curcumin nanoemulsion. Scientia Pharmaceutica 2016; 84(1): 131-140.
- Maithilikarpagaselvi N, Sridhar MG, Swaminathan RP, Sripradha R and Badhe B: Curcumin inhibits hyper-lipidemia and hepatic fat accumulation in high-fructose-fed male Wistar rats. Pharmaceutical Biology 2016; 54(12): 2857-2863.
- Katsuyama Y, Kita T and Horinouchi S: Identification and characterization of multiple Curcumin synthases from the herb C. longa. FEBS Letters 2009; 583(17): 2799-2803.
- Sharma M, Manoharlal R, Puri N and Prasad R: Antifungal Curcumin induces reactive oxygen species and triggers early apoptosis but prevents hyphae development by targeting the global repressor TUP1 in Candida albicans. Bioscience Reports 2010; 30(6): 391-404.
- Loomis-King H, Flaherty KR and Moore BB: Pathogenesis, current treatments and future directions for idiopathic pulmonary fibrosis. Current Opinion in Pharmacology 2013; 13(3): 377-385.
How to cite this article:
Alsamydai A and Jaber N: Pharmacological aspects of curcumin: A review. Int J Pharmacognosy 2018; 5(6): 313-26. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(6).313-26.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
313-326
668
1991
English
IJP
A. Alsamydai * and N. Jaber
Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Jordan, Amman, Jordan.
Phalimahmoud2012@yahoo.com
09 February 2018
21 March 2018
30 March 2018
10.13040/IJPSR.0975-8232.IJP.5(6).313-26
01 June 2018