PHARMACOGNOSTICAL STUDY OF PICRORHIZA KURROA ROOT
HTML Full TextPHARMACOGNOSTICAL STUDY OF PICRORHIZA KURROA ROOT
V. Sandhiya
Department of Pharmaceutics, C. L. Baid Metha College of Pharmacy, OMR, Rajiv Gandhi Salai, Jyothi Nagar, Thoraipakkam, Chennai - 600097, Tamil Nadu, India.
ABSTRACT: Introduction: Picrorhiza kurroa is a well-known plant used in Ayurvedic medicines belonging to the family, Scrophul-ariaceae. This herb has been traditionally used in treating liver disorders, upper respiratory tract disorders, reduce fever, scorpion stings, and treat dyspepsia and chronic diarrhea. Experiment: The macroscopy, microscopy, powder microscopy, physiochemical screen-ing was done on Picrorhiza kurroa roots. TLC, HPTLC. Results: The macroscopy, microscopy, and powder microscopy study reveals the identification of P. kurroa root. The phytochemical studies showed the presence of secondary active constituents such as phenols, glycosides, etc., TLC and HPTLC analysis showed that the chloroform fraction of P. kurroa has a higher number of peaks and spots compared to total extract.
| Keywords: |
P. kurroa, Hepatoprotective activity, Cell-line study
INTRODUCTION: Picrorhiza kurroa (P. kurroa; Family Scrophulariaceae), a well-known herb in the Indian traditional Ayurveda system of medicine 1. The leaves are 2-4 inches long, oval in shape with a sharp apex, flat, and serrate. The rhizome of picrorhiza is manually harvested in October through December. Like many species of medicinal plants, picrorhiza is threatened to near extinction due to over-harvesting. The P. kurroa has been used to treat disorders of the liver and is an important ingredient of many herbal preparations used for the treatment of liver ailments. Picroliv is a standardized iridoid glucoside mixture isolated from the roots and rhizomes of P. kurroa. It contains at least 60% of 1:1.5 mixture of picroside I and kutkoside and has been used as a hepato-protective agent in diseases such as jaundice 2.
In addition, the nitric oxide scavenging activity, cardioprotective effect, anticancer effect, anti-diabetic activity, and anti-viral effect of P. kurroa extract have been reported. Oxidative stress is caused due to the imbalance between reactive oxygen species (ROS) generation and antioxidant defence of the body.
Increasing levels of ROS like hydroxyl radical (OH•), superoxide anion (O2 •−) and hydrogen peroxide (H2O2) reduce the antioxidant levels such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX) and glutathione reductase (GR) and glutathione (GSH) and further damage the cellular components like DNA, proteins, and lipids 3, 4. Oxidative stress is well reported in ischemia, hypoxia, Parkinson's, Huntington's, and Alzheimer's diseases. Supple-mentation of a diet rich with antioxidant principles such as polyphenols and flavonoids can protect the cell from the damage of ROS. Herbal supplements rich in flavonoids, polyphenols, and terpenoids are used as a source of natural antioxidants to reduce or control symptoms associated with chronic or stress-related illnesses in the liver, picroliv decreased the levels of lipid peroxides and hydroperoxides and facilitated the recovery of superoxide dismutase and glycogen 5.
Hepatotoxicity mainly caused by Galactosamine intoxication, which disrupts the membrane permeability of the plasma membrane, causing leakage of the enzymes to form the cell, which leads to the elevation of serum enzymes. Hence, a significant rise in the transaminase levels could be taken as an index of liver damage.
Galactosamine has great liver specificity compared to other toxic groups, such as paracetamol, acetaminophen, and carbon tetrachloride, because hepatocytes have high levels of galactokinase and galactose-1-uridylyltransferase and galactosamine does not affect other organs. Galactosamine induces hepatotoxicity with spotty hepatocytes, necrosis, and marked portal and parenchymal infiltration.
Galactosaimine also induces the depletion of uridine diphosphate (UDP) by increasing the production of UDP-sugar derivates, which causes inhibition of RNA and protein synthesis, leading to cell membrane deterioration 6, 7. The focus of the current study was to evaluate the effects of chloroform fraction of P. kurroa root for its anti-oxidant and hepatoprotective activity using cell-line study
Methods of Collection and Extraction:
Collection: The root of Picrorhiza kurroa was purchased from the commercial shops in Paris, Chennai, Tamil Nadu. The identification was made on botanical and morphological basis. The macro-scopical study of drugs was conducted with the naked eye. The size, shape, color, and organoleptic characters were observed, and the plant was confirmed on the basis of the literature description.
Extraction: The dried root was coarsely powdered. The powdered material was extracted by cold maceration method. The methanolic extract of roots was prepared by soaking the powdered material in the 90% methanol for 24 h. After that, the filtrate was collected and preserved. The marc is again is treated with methanol for 24 h. Again the filtrate was collected. Both the 1st filtrate and 2nd filtrate are mixed together.
The mixed filtrate is concentrated by evaporation, which produced brown color sticky residue, which was stored in the airtight container. The concentrated extract was weighed and further used for the experiments whenever required.
Fractionation: Methanol extract is fractionated with chloroform in the ratio of 1:4. That is, one part of extract is treated with 4 parts of chloroform in the separating funnel. The chloroform layer is collected. The chloroform fraction is concentrated by the evaporation method. The concentrated extract is used for the experiments.
Pharmacognostical Studies:
Macroscopic Evaluation or Organoleptic Study: Collected and authentified roots of P. kurroa were dried, and various organoleptic characters viz., color, odor, taste, texture, fracture, and shape were studied 8, 9.
Microscopic Evaluation: Freehand sections of root of P. kurroa were taken and stained using safranin. The section was observed under a compound microscope, and photographs were taken
Powder Microscopy: A pinch of P. kurroa root powder was taken in a watch glass and stain with 1-2 drops of staining agents such as iodine and wait for few minutes, then place a pinch on a glass slide and view under the microscope.
Phytochemical Screening: The phytochemical screening, such as the presence of alkaloids, tannin, glycosides, terpenoids, flavonoids, steroids etc. are carried out as per the procedure given in the standard book 10.
Physiochemical Analysis: The physiochemical parameters such as Ash value, loss on drying, extractive value, swelling index, were carried out as per the procedure given in standard books.
TLC Study: The technique is used to separate the compounds and mainly used for the identification of the compounds 11.
Principle: Adsorption
Procedure:
For Total Methanolic Extract:
Mobile Phase:
- Chloroform: hexane: acetic acid (50: 50:1).
- Chloroform: ethyl acetate: acetic acid (50: 50:1).
Detecting Agent: Iodine vapors, vanillin –sulphuric acid
For Iridoid Glycosides:
Mobile Phase:
Chloroform: Methanol (8:2), (9:1).
Detecting Agent: Iodine vapors.
HPTLC Study: High-Performance Thin Layer Chromatography is used to establish reference “fingerprints” of herbs, which is one of the most powerful tools to link the botanical identity to the chemical constituent profile of the plant. HPTLC technique applied for the compilation of profiles pertaining to a varied range of bioconstituents such as alkaloids, glycosides, terpenoids, flavonoids, saponins, resins, coumarins, plant hormones, anti-biotics and number of other compounds of natural origin 12.
High-Performance Thin Layer Chromatography, also known under the synonym Planar Chromato-graphy, is a powerful analytical technique with separation power, performance, and reproducibility superior to classical TLC. Today most HPTLC instruments are computer-controlled and can, therefore, offer dramatically improved reproduci-bility of the analytical result.
HPTLC Finger Print Chromatogram of Chloroform fraction:
Aim: To develop the HPTLC fingerprint of Chloroform fraction of Picrorhiza kurroa Chro-matographic condition for HPTLC fingerprint.
Sample: Chloroform fraction of Picrorhiza kurroa.
Stationary Phase: HPTLC plates silica gel 60 f 254 nm and 365 nm
Mobile Phase: Chloroform: Methanol (8:2) Sample concentration: 10 mg/ml
Applied Volume: 2.0 μl, 4.0 μl and 6.0 μl
Development Chamber: Glass tank twin trough chamber
Development Mode: ascending
Scanning Wavelength: 254 nm and 365 nm
Documentation: 254 nm and 365 nm using CAMAG TLC scanner 3
RESULTS AND DISCUSSION:
Macroscopy:
Color: The rhizomes are deep grayish-brown in color, externally white, blackish internally with whitish wood.
Odor: Slight and unpleasant.
Taste: Bitter.
Size: 3 to 5 cm in length and 0.5 to 1 cm in diameter.
Shape: Cylindrical pieces with longitudinal wrinkles and annulations at the tip.
Features: Conical, buds, and stems along with the roots also constitute the drug. The roots are longitudinally wrinkled with transverse cracks. Fracture is tough. The result was shown in Fig. 1.
FIG. 1: PICRORHIZA KURROA ROOT
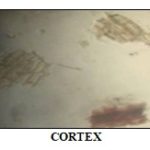
Microscopy: The transverse section of root showed 20-25 layers of cork consisting of tangentially elongated, submersed cells and cork cambium. The cortex is multilayered and vascular bundles are present in the cortex. The vascular bundles are surrounded by single layer endodermis of thick-walled cells. The secondary phloem is composed of phloem parenchyma and a few scattered fibers and 2-4 layered cambium. The secondary xylem consists of vessels, tracheids, xylem fibers, and xylem parenchyma. The tracheids are long, thick-walled, lignified, and more or less cylindrical. The xylem parenchyma is thin-walled, polygonal in shape, and center occupied by small pith consisting of thin-walled cells. It is a simple round to oval shape containing starch grains.
FIG. 2: MICROSCOPY OF PICRORHIZA KURROA ROOT
The periderm of the root constitutes 8-10 layers of thin-walled cork. The phelloderm cells are full of brownish contents.
The cortical cells are rounded with occasional inter-cellular spaces the secondary phloem is many-layered and xylem is made up of vessels, fibers and tracheids. Pith consists of thick parenchymatous cells. The result was shown in Fig. 2.
Powder Microscopy:
FIG. 3: POWDER MICROSCOPY OF P. KURROA
TABLE 1: PRELIMINARY PHYTOCHEMICAL SCREENING OF THE ROOT EXTRACT
| Chemical tests | Chloroform fraction | Plant extract |
| Alkaloids
Mayer’s test Dragendorff’s test Hager’s test Wagner’s test |
-
- - - |
-
- - - |
| Carbohydrates
Molish’s test Fehling’s test Benedict’s test |
-
- - |
-
- - |
| Glycosides
Anthrone test Borntrager’s test Legal’s test Baljet’s test Keller-killiani test |
+
+ + + + |
+
+ + + + |
| Proteins
Biuret’s test Million’s test |
-
- |
-
- |
| Amino acids
Ninhydrin test |
- | - |
| Saponins
Foam test |
- | - |
| Flavanoids
Shinoda test |
+ | + |
| Phenolic compounds
Ferric chloride test Lead acetate solution test |
+
+ |
-
- |
| Tannins
Ferric Chloride test Lead Acetate test Gelatin solution test |
+
+ + |
-
- - |
Microscopy of the powder Fig. 3 showed Cortex cells were found circular to oval. Cork cells in transversely cut mode and in surface view were found to contain fragments of pitted vessels and tracheids and groups of cells with orange-colored content, medullary rays, xylem, cork cells are observed in the powder microscopy.
Preliminary Phytochemical Screening: The chloroform fraction of P. kurroa showed the presence of glycosides, flavonoids, phenols and tannins and the total plant extract of P. kurroa showed presence of glycosides and flavanoids. Which indicates based on the polarity of the solvents the active constituents are solubilized; therefore higher the polarity of the solvent increase the solubility nature of active constituents. The result was showed in Table 1.
Physiochemical Analysis:
TABLE 2: PHYSIOCHEMICAL ANALYSIS STUDY
| S. no. | Parameters | Results |
| 1 | Ash Values:
Total ash |
6%w/w |
| Acid insoluble ash | 2%w/w | |
| Water soluble ash | 1%w/w | |
| 2 | Extractive Values:
Water soluble extractive |
17.4% |
| Alcohol soluble extractive | 13.7% | |
| 3 | Loss on drying | 0.11 |
| 4 | Swelling index | 0.13 |
Report: The physicochemical analysis of Picrorhiza kurroa was carried out, and total ash was found to be 6% w/w, acid insoluble ash was found to be 2% w/w, water-soluble ash was found to be 1% w/w, alcohol soluble extractive was found to be 17.4%, water-soluble extractive was found to be 13.7%, loss on drying was found to be 0.11 and swelling index was found to be 0.13. The result was showed in Table 2.
TLC Study:
FIG. 4: TLC OF P. KURROA
For Total Extract: Rf value of spot A was found to be 0.241, Rf value of spot B was found to be 0.354, Rf value of spot C was found to be 0.548.
For Chloroform Fraction: Rf value of spot A was found to be 0.627, Rf value of spot B was found to be 0.788.
The Rf value of chloroform fraction of Picrorhiza kurroa were found to be higher than total extract of Rf values, At 254 nm, the samples showed 2-3 spots towards the baseline indicating the highly polar nature of the compounds. After dipping the plate in a vanillin-sulphuric acid solution, the plate showed different colored compounds indicating the presence of compounds with strong chromophoric groups.
This shows the solubility of the active constituents was based on the polarity of the solvent. The result was showed in Fig. 4.
HPTLC Fingerprint:
FIG. 5: FINGERPRINT OF CHLOROFORM FRACTION OF PICORRHIZA KURROA
TABLE 3: HPTLC OF CHLOROFORM FRACTION OF PICORRHIZA KURROA
| Peak | Start
Rf |
Start height | Max
Rf |
Max height | Max
% |
End
Rf |
End height | Area | Area % | Assigned substance |
| 1 | -0.01 | 7.7 | 0.01 | 48.3 | 3.37 | 0.06 | 4.6 | 1104.8 | 2.88 | Unknown * |
| 2 | 0.36 | 0.8 | 0.38 | 18.3 | 1.28 | 0.39 | 8.5 | 290.3 | 0.76 | Unknown * |
| 3 | 0.39 | 8.7 | 0.41 | 12.3 | 0.86 | 0.42 | 0.0 | 198.5 | 0.52 | Unknown * |
| 4 | 0.44 | 1.5 | 0.48 | 190.7 | 13.33 | 0.52 | 3.0 | 3911.0 | 10.18 | Unknown * |
| 5 | 0.52 | 3.2 | 0.54 | 61.0 | 4.26 | 0.55 | 13.7 | 876.0 | 2.28 | Unknown * |
| 6 | 0.55 | 14.0 | 0.57 | 66.7 | 4.66 | 0.59 | 7.7 | 1109.2 | 2.89 | Unknown * |
| 7 | 0.59 | 8.5 | 0.61 | 20.2 | 1.41 | 0.62 | 12.0 | 307.3 | 0.80 | Unknown * |
| 8 | 0.62 | 10.9 | 0.68 | 226.0 | 15.79 | 0.70 | 17.9 | 7206.3 | 18.75 | Unknown * |
| 9 | 0.70 | 20.8 | 0.73 | 311.8 | 21.78 | 0.75 | 66.2 | 7272.7 | 18.93 | Unknown * |
| 10 | 0.76 | 68.1 | 0.77 | 114.7 | 8.02 | 0.79 | 91.8 | 2583.8 | 6.72 | Unknown * |
| 11 | 0.79 | 31.9 | 0.85 | 251.5 | 17.57 | 0.88 | 95.8 | 11187.3 | 29.11 | Unknown * |
| 12 | 0.88 | 97.0 | 0.89 | 109.6 | 7.66 | 0.91 | 39.8 | 2377.9 | 6.19 | Unknown * |
FIG. 6: HPTLC OF PICRORHIZA KURROA CHLOROFORM FRACTION
The HPTLC fingerprinting of chloroform fraction of P. kurroa showed the maximum number of UV active compounds, and that was detected in 254 nm and 365 nm.
Totally 12 peaks were observed with Rf value ranges from 0.06 to 0.91 at respective nm. Peak 10 shows the maximum height of 21.78%, with area of 18.93%. Peak 3 shows a minimum height of 12.3% with area of 0.52%, the result was showed in table 3 and Fig. 5 and 6.
CONCLUSION: Kutaki (P. kurroa) has been used in the indigenous system of medicine since a long time. Kutaki is considered to be a valuable bitter tonic and a favorite remedy in bilious dyspepsia accompanied with fever.
It is antipyretic, anthelmintic, and slightly laxative and is useful in asthma, blood troubles, burning sensation, piles, inflammations, ringworm. but some other species such as root of Picrorhhiza scrophularia are sold in drug market under the name kutaki or kuru so, there is a need to standardize the authentic source of genuine drug by using different parameters. This paper is an attempt of the author to generate identity and purity standards for P. kurroa to prevent its adulteration in the herbal drug market.
ACKNOWLEDGEMENT: Grateful to thank the institute and friends to support the study.
CONFLICTS OF INTEREST: No conflict of interest from the institution.
REFERENCES:
- Akhil Bhardwaj and Pankaj Khatri: Potent Herbal hepato-protective drugs- A review. J Adv Sci Res 2011; 2(2): 15-20.
- Ansari RA, Aswal BS, Chander R, Dhawan BN, Garg NK and Kapoor NK: Hepatoprotective activity of Kutkin- the iridoid glycoside mixture of Picrorhiza kurroa. Indian J Med Res 2007; 87: 401-7.
- Saraswat B, Visen PK, Patnaik GK and Dhawan BN: Ex-vivo and in-vivo investigations of picroliv from Picrorhiza kurroa in an alcohol intoxication model in rats. J Ethnopharmacol 2012; 66: 263-9.
- Díaz-Castro J, García Y, López-Aliaga I and Alférez MJ: Influence of several sources and amounts of iron on DNA, lipid and protein oxidative damage during anaemia recovery. Biological Trace Element Research 2013; 15(3): 403-10.
- Saluk-Juszczak J, Olas B, Nowak P, Wachowicz B, Bald E and Głowacki R: Extract from Conyza canadensis as a modulator of plasma protein oxidation induced by peroxynitrite in-vitro. Central European Journal of Biology 2010; 1(5): 800-07.
- Halliwell B: Oxidative stress and neurodegeneration: where are we now? Journal of Neurochemistry 2013; 97: 1634-58
- Mueller MM and Fusenig NE: Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2014; 4: 839-49.
- Pharmacognosy –CK Kokate and AP Purohit edition 42nd90-8.92.
- Textbook of Pharmacognosy –T E Wallis (edition5th). No.426.
- Practical Pharmacognosy –Khandalwal K.R (edition12th). 149-155 and 157-160.
- Rui Wang, Ai-Zhen Xiong: Radix Paeoniae rubra and Radix raeioniae alba attenuate CCl4-induced acute liver injury: An ultra-performance liquid chromatography-mass spectroscopy based metabolic approach for the pharmaco-dynamic study of traditional Chinese medicines. Int J Mol Sci 2012; 13: 14634-47.
- Srivastava V and Dubey S: High performance thin layer chromatography- a modern analytical separation technique for natural products. World Journal of Pharmacy and Pharmaceutical Sciences 2016; 2: 525-31.
How to cite this article:
Sandhiya V: Pharmacognostical study of Picrorhiza kurroa root. Int J Pharmacognosy 2020; 7(6): 148-54. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(6).148-54.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
148-154
619
1199
English
IJP
V. Sandhiya
Department of Pharmaceutics, C. L. Baid Metha College of Pharmacy, Thoraipakkam, Chennai, Tamil Nadu, India.
sandhiyavaithi@gmail.com
05 April 2020
23 June 2020
28 June 2020
10.13040/IJPSR.0975-8232.IJP.7(6).148-54
30 June 2020