PHARMACOGNOSTIC STUDY OF ZIZIPHUS MAURITIANA LAM. (RHAMNACEAE)
HTML Full TextPHARMACOGNOSTIC STUDY OF ZIZIPHUS MAURITIANA LAM. (RHAMNACEAE)
Y. B. N. Fofie * 1, K. Coulibaly 1, K. Traore 2, 3, 4, E. Odoh 1, G. N. Zihiri 5 and D. Kone-Bamba 1
Faculty of Pharmaceutical and Biological Sciences 1, Félix Houphouët Boigny University Abidjan.
Department of Traditional Medicine 2, Faculty of Pharmaceutical Sciences 3, Faculty of Technical Sciences and Technology 4, University of Bamako, Mali.
Laboratory of Botany 5, Faculty of Biosciences, Félix Houphouët-Boigny University, Cocody, Abidjan,
ABSTRACT: Objective: To study the preliminary and pharmacognostic characteristics of Ziziphus mauritiana Lam., harvested in Côte d'Ivoire. Identify the drug by anatomy and histochemistry. Method: Macroscopic and microscopic study of the fresh and dry drug and determination of physicochemical parameters. Results: Tree, shrub or bush, 16 m long, 5 m or 4 m, Ziziphus mauritiana Lam. Has bending branches with rounded tops. Its fairly cracked bark is gray or brown, then pale red. The oval or sub-orbicular leaves are alternate and petiolate from 4 mm to 5 mm. The limb, dark green in color, is polished on the upper side and whitish and then densely tomentose on the lower side. The anatomo-histological cut of the limb showed a median rib slightly curved on the upper surface and strongly bulging on the lower side and a broader limb. Each epidermis consisted of small, visible cells more or less rectangular, is covered with a cuticle, outer lipoidal covering; impermissive and resistant, giving it a protective role. The cross-section of the stem, revealed a quadrangular shape, has two distinct zones: the bark and the central cylinder. The less developed bark comprises of 4 tissues (cuticle, epidermis, collenchyma and cortical parenchyma). The central cylinder, more developed than the bark, is composed of primary tissues (bone, wood, medullary parenchyma, sclerenchyma, and perimedullary fiber). The sclerenchyma occurs in small clusters around the conductive system. Conclusion: Pharmacognostic analysis and physicochemical characteristics can help in the efficient utilization of this medicinal plant.
| Keywords: |
Pharmacognosy, Food, Ziziphus mauritiana Lam.
INTRODUCTION: The immense knowledge of medicinal plants in the developing countries needs to be under a monographic. The World Health Organization (WHO) estimates that more than 80 percent of the population in developing countries takes to traditional medicine for their primary health care 1.
These medicinal plants also play an important role in the socio-cultural life of the peoples 2, 3 but serve as natural resources for research and development of new drugs 4.
In Africa, like in the other part of the world, several ethnobotanical surveys have been carried out and have shown that Ziziphus mauritiana Lam. in traditional medicine, is used to treat many ailments and as laxatives. Also, these plant species have anticonvulsant, anti-inflammatory, anti-cancer properties and are used as cardiac and intestinal stimulants, as central nervous system depressants and analgesics in laboratory animals. Rhamnaceae are also used for dyspepsia, against constipation and treatment of herpes. We also note the toning, astringent, clearing, purgative and emetic aspect of the species of the Rhamnaceae family. Also, they are used as digestive, diuretic, hypotensive and for the treatment of hepatic and dermatological complications. There is also a purgative action. Infusion of leaves is used in the treatment of astringent gargles. The Rhamnaceae are indicated in the treatment of furuncle, abscess, measles, general pains, dysentery.
MATERIALS AND METHODS:
Plant Material: The plant material consisted of the leaf stem of Ziziphus mauritiana Lam (Rhamnaceae) (Z m). The leaves of Ziziphus mauritiana Lam. were harvested in February 2012 in the region of Abengourou, in the eastern part of Ivory Coast. The drug was formally identified by the Laboratory of Botany, and a herbarium was constituted then deposited at the National Floristics Center (NFC) of the Félix Houphouët Boigny University Abidjan.
Methods: The harvested drug was cleaned and then dried at laboratory temperature (24-26 °C). The dry plant material was coarsely pulverized, using a RETSCH SM 2000 grinder. The powdered drug was used for pharmacognostic studies and physicochemical examination. The fresh organs were preserved for macroscopic and anatomo-histochemistry study.
Macroscopic Studies: The macroscopic study is a morphological study. This study allowed us to describe the plant and determine the shape, texture and color of the drug.
Organoleptic Character Studies: The study of the organoleptic characteristics focused on the pulverized drug and related to the taste, appearance, color and odor of the drug. The smell test was performed with 1 mg of pulverized drug taken between the thumb and index finger. The odoriferous constituents released were tested slowly and repeatedly. The intensity of the odor was first tested by the following parameters: "None, Weak, Marked, Strong". Next, was determined the type of odor: "Odorless, aromatic, fruity, characteristic". For the taste, five (5) grams of drugs were placed and kept in the mouth, without swallowing, for ten (10) to thirty (30) sec. After having spit out the sample, the mouth was rinsed, then the taste appreciated: "Peppery, fade, sour, bitter, sweet, salty, hot." This important study allows for the identification of the drug and its normalization. The appearance and color required observation.
Microscopic Studies: Anatomo - histological-chemical studies allowed the identification of tissues and localization of secondary metabolite sites, respectively. These studies were carried out on leaf and stem cuts, as well as plant powder.
Anatomo-histological Sections: It consisted mainly of observations under an optical microscope. Thin transverse sections were made using a microtome on vegetative organ fragments. Then, the sections were treated according to the technique recommended by GABE 13. The colorant used was the mirror carmine-green. The best cuts were mounted and preserved between blade and slide in glycerin for observation and taking images using a digital camera incorporated in the microscope.
Micrographic Study: A small amount of fine powder was mixed with a few drops of 5% KOH on a slide and then covered with a slide. The observation was carried out under the optical microscope with the objective 10x. The characteristic features of the drug powder were noted and photographed.
Physicochemical Study: The study of the physicochemical parameters including the determination of the water and ash content was carried out according to the following protocol:
Water Content: The determination of the water content was carried out by the gravimetric method according to the protocol carried out by Linden and Lorient 14 and Mukherjee 15: 5 test samples of 5 g of powder were introduced into 5 calibrated crucibles. The samples were dried in an oven at a temperature of 105 °C for 24 h. The crucibles were cooled in a desiccator and weighed. The masses obtained made it possible to calculate the loss of mass and to calculate the water content of the powder expressed as a percentage.
Ash Contents:
Total Ashes: The powders dried during the determination of the water content were reduced to ash in an oven at 600 °C for 6 h. After cooling in a desiccator, the ash was weighed. The masses obtained made it possible to calculate the ash masses and to calculate the total ash content, expressed as a percentage.
Ashes Insoluble in 10% Hydrochloric Acid: The total ash obtained was taken up with 20 ml of 10% hydrochloric acid. The whole was brought to a boil in a bath for 15 min. The resulting solution was filtered on Whatman paper. The residue was collected in a tempered crucible and calcined in an oven at 600 °C for 6 h. The crucible was cooled in a desiccator. The mass of the ash insoluble in hydrochloric acid was expressed as a percentage.
Sulfuric Ash at 50%: Five test samples of 5 g of powder were introduced into calibrated crucibles. It was added to the contents of each crucible 5 ml of 50% H2SO4. The whole was placed in the oven at a temperature of 600 °C for 6 h. After calcination and cooling in a desiccator, the ash was weighed, and the mass of the sulphur ash was expressed as a percentage.
RESULTS:
Macroscopic Study: This study allowed us to make an identification of the plant material, and is the first step in the characterization of the crude drug.
Ziziphus mauritiana Lam.
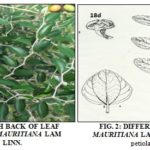
Morphological Characteristics: Tree, shrub or bush, 16 m long, 5 m or 4 m, Ziziphus mauritiana Lam. has falling branches with rounded tops. Its little cracked bark is gray to brown, then pale red. The oval or sub-orbicular leaves are alternate and petiolate from 4 mm to 5 mm. The limb, dark green in color, is varnished on the upper side and whitish and densely tomentose on the underside.
The inflorescences are sessile tomentose or woolly fascicles, from 3 to 8 flowers. The flowers are numerous, small and yellowish. The fruits are drums of 1 cm to 2 cm and containing a large nucleus coated with a whitish pulp, which is more or less floury Fig. 1 and 2.
Phytogeography: Ziziphus mauritiana Lam. is widespread in Africa in the Sudano-Sahelian region, from Senegal to Nigeria. This species has also been inventoried in tropical Asia, particularly in India, hence the name "Jujubier d'inde."
Ecology: Ziziphus mauritiana Lam. is a species of savanna zone, but is sometimes found in the Sahelo-Sudanian to Sudanian zones. A species of light, it colonizes well abandoned agricultural lands, sandy or rocky soils, and along streams or shallows.
Specimens Studied: Abengourou, February 2012, Fofié n ° 04, between Boundiali and Tengréla, 04 March 1998, Traoré D. and Dotia n ° 0185; Between Kouto and Tengréla, near Blességué, 26 July 2000, Aké-Assi and Traoré D.
Organoleptic Study: All the organoleptic characteristics revealed during this study are recorded in Table 1.
Microscopic Studies: The microscopic study of the cross section of the leaf and stem plays an important role in the diagnosis of the identification and differentiation of the drug studied.
TABLE 1: ORGANOLEPTIC CHARACTERISTICS OF THE LEAVES ZIZIPHUS MAURITIANA LAM.
| Drug / Characteristic | Ziziphus mauritiana |
| Taste | Leafy stem |
| Odor | peppery |
| Color | odorless |
| Aspect | Light green |
| Texture | Woolly |
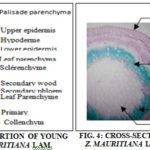
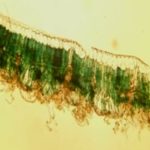
Anatomo-histological Sections: The anatomo-histological sections are shown in Fig. 3 and 4. The transverse section of the leaf blade Fig. 3 presents a median rib slightly curved on the upper surface and strongly bulging at the lower surface and the blade itself larger. Each epidermis, formed of small, visible cells more or less rectangular, are covered with a cuticle, outer lipoidal covering; Waterproof and resistant, giving it a protective role. The mesophile, comprised between the two epidermises, contains various tissues (hypodermis, palisade parenchyma, lacunous parenchyma, collenchyma, sclerenchyma, wood, and liber). The collagenase placed beneath the epidermis is formed of cells with thick, cellulosic walls; It is more abundant on the underside. This lower face is covered with filamentary, translucent, entangled and folded structures. The vascular apparatus forms a closed arc with phloem (liber) on the periphery and xylem (wood) on the inside.
FIG. 5: CROSS SECTION OF A LEAF BLADE OF ZIZIPHUS MAURITIANA LAM. (SOURCE: FOFIE YVETTE). Magnification Gx10; Mounting fluid. Glycerin water; Laboratory of Pharmacognosy (University Félix Houphouët Boigny), September 2013
The cross-section of the stem Fig. 4, of quadrangular shape, has two distinct zones: the bark and the central cylinder. The less developed bark comprises 4 tissues (cuticle, epidermis, collenchyma and cortical parenchyma). The central cylinder, more developed than the bark, is composed of primary tissues (bone, wood, medullary parenchyma, sclerenchyma, and peri-medullary fiber). The sclerenchyma occurs in small clusters around the conductive system.
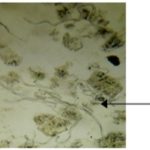
FIG. 6: MICROGRAPHIC STUDY MOUNTING IN KOH, GX10
The conductive device has a centripetal and a centrifugal wood. The medullary parenchyma, formed of large polygonal cells.
Physicochemical Study: This study is essential in that it helps identify poor handling practices and assess the quality of the proposed drug being studied.
Various parameters have been defined and recorded in Table 2.
TABLE 2: PHYSICOCHEMICAL STUDY
| Parameter | Humidity % | Total ash % | Sulphuric ashes % | HCl insoluble ashes % |
| Rate | 7.54 | 7.41 | 10.8 | 5.32 |
DISCUSSION: Our study, which was carried out in this context, focused on the pharmacognostic examination of the leaves of Ziziphus mauritiana. (Rhamnaceae). The scientific literature allowed us to review a large number of bibliographic data on drugs, including plant systematics, various domestic uses and traditional medicine. The normalization of the macroscopic and microscopic characteristics of the drug of Ziziphus mauritiana remains essential to avoid and to identify the falsification.
Thus the comparative anatomy of the cross-section of the leaf and stem showed structural similarities. The two sections present medullary parenchyma and a collenchyma. A thin cuticle is observed on the leaf on the upper and lower epidermis. Also palisadic tissues above lacunous parenchyma. At the level of the stem, secretory pockets are visible on the surface of the medullary parenchyma, as well as sclerenchymatous cells supporting primary tissue (primary lib). The distinct cortical parenchyma can be seen towards the cutting periphery. Organoleptic characteristics are important elements in the distinction of drugs, as they play a role in the detection of falsified or substituted drugs 16. The micrograph on the powder revealed several characteristic elements, namely: epidermal cells, stomate type, spiral bundles, cystoliths, hair, spiral wood bundles, and cells are diagnostic substances of crude drug of vegetable origin. These diagnostic elements are consistent with the botanical standard and WHO guidelines 17, 18.
The study of physicochemical parameters such as moisture content and ash values is useful for determining the physiological and non-physiological state of the ash, detecting the possibility of microbial growth or contaminant and finally the presence of impurities. The moisture content of the drug studied has a rate of 7.54 ± 0.00, which is less than 10%. This result respects the standards established by the international pharmacopeia because this water content would prevent oxidation, fermentation reactions and would give less possibility for microbial growth and contamination in the drug 19. Therefore, for good preservation of medicines made with the leaves of Ziziphus mauritiana, it would be desirable to use those whose water content is less than or equal to 10%.
The total ash dosage gave us a rate of 7.41 ± 0.02. This value informs us about the mineral content of the drug 20. Sulfuric ash has a rate of 10.08 ± 0.01. They result from the conversion of organic salts into sulfates 20. This value, approximately equal to the average of 10.80% found during the determination of sulfur ash in the various samples of Sclerocarya birrea (A. Rich) Hoscht 21. The ash insoluble in hydrochloric acid gave a rate of 5.32 ± 0.01. Indeed, the ash insoluble in hydrochloric acid informs us about the contamination of the drug by the siliceous elements 20.
CONCLUSION: This study allowed us to demonstrate the presence of different pharmaco-gnosic parameters in the drug of Ziziphus mauritiana Lam., by standard botanical observations and WHO guidelines. The results of the water content are considered satisfactory insofar as the content presented in the test drug allows good preservation and prevents oxidation, fermentation, and microbial growth reactions.
In the light of these results, pharmacognostic analysis andphysicochemical characteristics can help the pharmacopoeia to use this plant efficiently, within the framework of a policy of standardization, identification and research on the drug of Ziziphus Mauritiana Lam.
ACKNOWLEDGEMENT: We are grateful to the Laboratory of Applied Biochemistry of the University of Ouagadougou for their assistance in the achievement of the cuts and the Laboratory of Pharmacognosy of the Faculty of Pharmacy and Biological Sciences of the Félix Houphouët Boigny University for their technical assistance in the achievement of this work.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Nathiya S, Santhi N and Kalaiselvi S: A comparative study on the ontogenic expression of antioxidants and secondary metabolites in Withania somnifera. Int Res J Pharm 2012; 3(1): 2010-2015.
- Okwu DE: Flavouring Properties of Spices on Cassava futu. Afr. J Roots and Tuber Crops 1999; 3(2): 19-21.
- Okwu DE: Evaluation of the chemical composition of indigenous spices and flavoring Agents. Gl J of Pure and Appl Sci 2001; 7(3): 455-459.
- Kong JM, Goh NK, Chia LS and Chia TF: Recent Advances in Traditional Plant Drugs and Orchids. Acta Pharm Sci 2008; 24: 7-21.
- Orwa C, Mutua A, Kindt R, Jamnadass R and Anthony S: Agroforestree Database: a tree reference and a selection guide. Version 4.0. ICRAF Centre Mondial de l’Agroforesterie, Nairobi, Kenya.
- Adjanohoun EJ: Médecine traditionnelle et pharmacopée: contribution aux études ethnobotaniques et floristiques en république du Bénin, ACCT, Paris 1989; 274.
- Adjanahoun E, Ahyi MRA, Ake-Assi L, Elewude JA, Dramane K, Fadoju SO, Gbile ZO, Goudole E, Johnson CLA, Keita A, Morakinyo O, Ojewole JAO, Olatunji AO and Sofowora EA: Traditional medicine and Pharmacopoeia. In: Ethnobotanical and Floristic studies in Western Nigeria. Org. of Afr. Un.’s Sci Tech and Res Comm, Lagos, Nigeria. 1991; 420.
- Elujoba AA, Odeleye OM, and Ogunyemi CM: Traditional medicine development for medical and dental primary health care delivery system in Africa. Afr J Trad CAM 2005; 2(1): 46-61.
- Aké-Assi L: Abrégé de médecine et pharmacopée africaine: quelques plantes employées traditionnellement dans la couverture des soins de santé primaire. NEI-CEDA 2011; 69.
- Ekunwe INS, Melvanique ST, Xuan L, Hengshan W, Yong C, Xiaopu Z and Begonia BG: Potential cancer-fighting Ocimum gratissimum (og) leaf extracts: increased anti-proliferation activity of partially purified fractions and their spectral fingerprints. Ethni and Dis 2010; 20: 12-16.
- Aguiyi JC and Obi CI: Hypoglycaemic activity of Ocimum gratissimum in rats. Fito 2000; 71(4): 444-446.
- Abdullahi M: Phytochemical constituents and anti-microbial and grain protectant activities of clove basil (Ocimum gratissimum) Grown in Nigeria. Int J of Pl Res 2012; 2(1): 51-58.
- Gabe M: Elements de techniques histologiques (Fascicule de TP. Université Paris VI) 1968; 70.
- Linden G and Lorient D: Biochimie agro-indusrielle. Ed. Masson, Paris 1994; 360.
- Mukherjee PK: Quality control of herbal drugs, Business horizon, New Delhi, Edition 1st, 2002; 356: 187-195.
- Fouraste: Le contrôle des plantes médicinales. Actualités Pharmaceutiques; (N° 278) 1990; 55-5.
- Kumar S, Kumar V and Prakash O: Microscopic evaluation and physicochemical analysis of Dillenia indica Asian Pac J Trop Biomed 2011; 1: 337-340.
- Nasreen S and Radha R: Assessment of quality of somnifera Dunal (Solanaceae): Pharmacognostical and physicochemical profile. Int J Pharm Sci 2011; 3(2): 152-155.
- Organisation de l’unité africaine/commission scientifique technique et de la recherche (OUA/CSTR), Pharmacopée africaine, méthodes générales d’analyses. Première éd., Lagos, Nigéria 1998; 254.
- Halimatou SM: Etude du traitement traditionnel du diabète par une recette et les écorces de tronc de Manilkara multinervis Dub (Sapotaceae). Thèse de pharmacie 2006; 41.
How to cite this article:
Fofie YBN, Coulibaly K, Traore K, Odoh E, Zihiri GN and Kone-Bamba D: Pharmacognostic study of Ziziphus mauritiana Lam. (Rhamnaceae). Int J Pharmacognosy 2018; 5(6): 354-59. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(6).354-59.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
354-359
586
1378
English
IJP
Y. B. N. Fofie *, K. Coulibaly, K. Traore, E. Odoh, G. N. Zihiri and D. Kone-Bamba
Faculty of Pharmaceutical and Biological Sciences, Félix Houphouët Boigny University Abidjan.
yvette.fofie08@yahoo.fr
12 January 2018
05 February 2018
13 February 2018
10.13040/IJPSR.0975-8232.IJP.5(6).354-59
01 June 2018