PHARMACOGNOSTIC STUDIES OF STEM TUBER ON BRASSICA OLERACEA VAR. GONGYLODES
HTML Full TextPHARMACOGNOSTIC STUDIES OF STEM TUBER ON BRASSICA OLERACEA VAR. GONGYLODES
S. J. Sabannavar * and B.V. Swaroopa
Department of Botany, Mount Carmel College (Autonomous), Bangalore - 560052, Karnataka, India.
ABSTRACT: The crude extracts from stem tuber and leaf of Brassica oleracea var. gongylodes in different solvents, were subjected to physicochemical, fluorescence analysis, phytochemical and antimicrobial study. The microscopic analysis revealed that B. oleracea showed stone cells and calcium oxalate prisms. The ash value was high 7.88 % in B. Oleracea. The fluorescent analysis at 366 nm of B. oleracea under ultraviolet light revealed that powder extracted with alcohol showed pale blue colored fluorescence, with water showed yellow color, 0.1 N Sodium hydroxide showed pale yellow and dilute hydrochloric acid had greenish yellow. The extraction value with different solvents exhibited that B. oleracea maximum extractive value with chloroform followed by benzene, ethanol and petroleum ether. The phytochemical screening of the various extract of B. oleracea revealed the presence of alkaloids, flavonoids, resins, saponins, and tannins. Anti-bacterial screening in B. oleracea using stem revealed that the ethanol solvent showed maximum inhibition against gram-positive bacteria (Staphylococcus aureus) and chloroform solvent showed maximum inhibition in the case of gram-negative bacteria (Serratia marcescence).
| Keywords: |
Anti-bacterial screening, Fluorescence analysis, Phytochemical, Brassica oleracea
INTRODUCTION: World plant biodiversity is the largest source of herbal medicine and still about 60-80% world population rely on plant-based medicine which being used since ancient ages as the traditional health care system. Plant-derived substances have recently become of great interest owing to their versatile applications. Medicinal plants are the richest bio-resource of drugs of traditional systems of medicine, nutraceuticals, food supplements, folk medicines, pharmaceutical intermediates and chemical entities for synthetic drugs 1. Drugs from the plants are easily available, less expensive, safe, and efficient and rarely have side effects.
The plants which have been selected for medicinal use over thousands of years constitute the most obvious choice of examining the current search for therapeutically effective new drugs such as anticancer drugs, antimicrobial drugs 2 antihepatotoxic compounds. According to the World Health Organization (WHO), medicinal plants would be the best source to obtain a variety of drugs. However, such plants should be investigated to better understand their properties, safety, and efficiency 3.
The detection of active principles in medicinal plants plays a strategic role in the phytochemical investigation of crude plant extracts and is very important in regards to their potential pharmacological effects 4. The knowledge of the chemical constituents of plants is desirable because such information will be valuable for the synthesis of complex chemical substances. Phytochemicals are the natural bioactive compounds found in plants.
These phytochemicals work with nutrients and fibers to form an integrated part of the defense system against various diseases and stress conditions 5.
The most important of these bioactive constituents of plants are alkaloids, tannins, flavonoids, steroid, terpenoid, carbohydrate, and phenolic compounds. The majority of these bioactive compounds are alkaloids, followed by sesquiterpenes, diterpenes, triterpene saponins, triterpene aglycones, flavonoids, sterols, coumarins, quinines and monoterpenes 6. Extraction (as the term is pharmaceutically used) is the separation of medicinally active portions of plant tissues using selective solvents through standard procedures. The ash and extractive values of crude drugs help in the identification and determination of its purity and quality 7.
The products so obtained from plants are relatively complex mixtures of metabolites, in liquid or semisolid state or (after removing the solvent) in dry powder form, and are intended for oral or external use. Extraction methods used pharmaceutically involves the separation of medicinally active portions of plant tissues from the inactive/inert components by using selective solvents. During extraction, solvents diffuse into the solid plant material and solubilize compounds with similar polarity 1.
A large number of phytochemicals belonging to several chemical classes have been shown to have inhibitory effects on all types of microorganisms in vitro 8.
This study investigates the fundamental scientific bases for the use of medicinal plants by defining the crude phytochemical constituents present in these plants. The present work leads to phytochemical analysis and antimicrobial analysis in two plants Brassica oleracea var. gangolydes.
MATERIALS AND METHODS: The fresh plant materials of Brassica oleracea var. ganglyodes (stem tuber) Fig. 1 were collected from markets from Bangalore, Karnataka, India. The stem tuber of B. oleracea was cut into small fragments and shade dried till uniform and smooth. The dried plant materials were powdered by using a blender. The final uniform powder was used for the study. Physio-chemical standards such as ash, extractive values were determined as per the standard Indian Pharmacopoeia methods 9. Antimicrobial activity was also carried out using gram-positive and negative bacteria.
Powder Microscopic Analysis: For examining characters of the powder, a sufficient amount of B. oleracea was mixed in Chloral-hydrate solution on a slide and covered with a coverslip, warmed over a low flame for a short time.
Starch: To examine the presence of starch in the rhizome, two specimens were taken, one in an Iodine solution and the other in the water. With Iodine solution starch turned blue. The shape and structure of starch grains were observed in water, and their size was measured.
Fixed Oil: To examine the presence of fixed oil, a specimen was prepared in a solution of Sudan III, droplets of fixed oil were colored orange-pink.
Determination of Ash: The ash remaining on ignition of medicinal plant materials was determined by three different methods which measure the total ash, acid-insoluble ash, and water-soluble ash. The total ash method was designed to measure the total amount of material remaining after ignition. This includes both "physiological ash," which was derived from the plant tissue itself, and "non- physiological ash, which was the residue of the extraneous matter (e.g., sand).
Total Ash: About 2 gms of accurately weighed, ground plant sample was taken in a previously weighed silica dish, previously ignited and weighed. The ground dry sample was scattered in a fine even layer on the bottom of the dish and gradually increased the heat 500-600 ºC until it was white, indicating the absence of carbon. In a desiccator, it was cooled and weighed. If carbon-free ash cannot be obtained in this manner, the crucible was cooled and moistened with nitrate. Dried on a water-bath, then on a hot plate and ignite to constant weight. The residue was allowed to cool in a suitable desiccator for 30 min, later weighed without delay. The percentage of ash concerning the air-dried plant sample was calculated.
Fluorescence Analysis: The plant powder was treated with alcohol, water, 0.1 N Sodium hydroxide and dilute hydrochloric acid. They were subjected to fluorescence analysis in daylight and UV- light (254 nm and 365 nm) 10.
Extraction Value: The powdered plant sample Brassica oleracea var ganglyodes of 10 g was packed and sealed in a filter paper placed and inside a thimble into the main chamber of the Soxhlet extractor. The Soxhlet is then placed on a flask containing the extraction solvent. The solvent used for extraction is Chloroform, Petroleum ether, Ethanol, and Benzene. About 250 ml of the solvent was loaded. Previously the dried flask was weighed with pumice pieces to get empty weight. The extractor was then attached to the condenser. The water supply was then switched on for water circulation, six cycles of extraction were carried out for 8 hours at 60 ºC with required solvent. The solvent was removed and was dried in an oven at 100 ºC to a constant weight.
Calculation for the percentage of extract:
Preliminary Phytochemical Screening: The screening of the alcoholic and aqueous extracts of the plant material was carried out for qualitative determination of the groups of organic compounds present in them11 12 13.
Alkaloids:
- Dragendorff’s test: A 2 ml of alcoholic or aqueous extract of the sample was dissolved in 5 ml of distilled water, 2 M hydrochloric acid was added until an acid reaction occurred, then 1 ml of Dragendorff’s reagent was added, an orange or orange-red precipitate was formed immediately.
- Hager’s test: A few drops of Hager’s reagent added to 1 ml of alcoholic extract of the sample taken in a test tube, Formation of yellow precipitate confirmed the presence of alkaloids.
- Wagner’s test: 1 ml of alcoholic extract of the drug was acidified with 1.5% v/v of hydrochloric acid, and a few drops of Wagner’s reagent was added. A yellow or brown precipitate was formed.
- Mayer’s test: A few drops of Mayer’s reagent was added to 1 ml of acidic aqueous extract of the drug. The white or pale yellow precipitate was formed.
- Flavonoids: In a test tube containing 0.5 ml of alcoholic extract of the drug, 5-10 drops of dilute hydrochloric acid was added followed by a small piece of magnesium. In the presence of flavonoids a pink, reddish pink or brown color is produced.
- Millon’s test: A small quantity of aqueous extract of the plant sample was dissolved in 1ml of distilled water, and 5-6 drops of Millon’s reagent was added. A white precipitate was formed which turns red on heating, indicating the presence of proteins.
- Resins: The ethanol extract was dissolved in acetone and the solution was poured into distilled water. The presence of resins was indicated by turbidity.
- Saponins: In a test tube containing about 5ml of an aqueous extract of the sample and a drop of Sodium bicarbonate solution was added, the mixture was vigorously shaken and left for 3 minutes. A honeycomb-like forth was formed.
- Steroids:
(a) Liebermann-Burchard's test: To 1 ml of petroleum ether extract of the sample in chloroform 2 ml of acetic anhydride solution was added followed by 1 ml of concentrated sulphuric acid. A greenish color was developed which turned to blue.
(b) Salkowiski Reaction: To 2ml of the chloroform extract of the drug 1ml of concentrated sulphuric acid was added carefully, from the side of the tube. A red color was formed in the chloroform layer.
Tannins: To 1- 2ml of an extract of the drug a few drops of 5 % FeCl3 solution was added. A green color indicates the presence of gallotannins while brown color tannins.
Anti-bacterial Analysis: The gram-positive organisms like Staphylococcus aureus and gram-negative organism like Serratia marcescens was isolated from local isolates and maintained at the Microbiology laboratory of Mount Carmel College (Autonomous), Bangalore to use as the test organisms for the study. The chloroform, petroleum ether, ethanol and acetone extracts of B. oleracea was tested against the above-said organisms to test the antibiotic properties of the chosen extracts of the plant.
The nutrient agar media was prepared according to the composition and sterilized for 15 minutes at 121ºC temperature & 15 lbs pressure, in an autoclave. The nutrient agar is a microbial growth medium commonly used for the routine cultivation of non-fastidious bacteria. The test bacterial cultures used in work were subcultured regularly on nutrient agar slants. They were maintained by refrigeration, throughout the work period. A loopful of test organisms were dissolved in a test tube containing 5 ml of distilled water to obtain seed inoculums.
Agar Diffusion Method: Using a sterile swab the seed inoculum of the respective test organism was inoculated onto labeled plates. The plates were kept aside for 15-20 min, and then with a sterile cork borer, one central well was bored. The same method was followed for all the other test organisms. The extract (100 µl) was added into the well of a plate already inoculated with a test organism. Then the plates were incubated at 37ºC for 24 hours. This was repeated thrice, and the average of the three zones of inhibition for the test organism was measured.
RESULTS AND DISCUSSION: Plants are known to contain numerous biologically active compounds which possess curative properties. Plant, when subjected to standardization, will avoid any ambiguity in the identity of the plant. The present study was focused on microscopic analysis, preliminary phytochemical screening and in-vitro antibacterial activity of Brassica oleracea var. gongylodes. The microscopic analysis revealed that B. oleracea showed the presence of parenchyma cells with starch grains, stone cells, prisms of calcium oxalate and sieve tubes showing sieve plates which were visible in the powdered sample Fig. 2. The ash values were found to be 7.88% in B. oleracea.
The powder from the stem of B. oleracea fluoresced under ultraviolet light at 366nm. The powder extracted with alcohol showed pale blue colored fluorescence. Powder that was extracted with water showed yellow coloured fluorescence, 0.1 N Sodium hydroxide showed pale yellow coloured fluorescence and dilute hydrochloric acid had greenish yellow coloured fluorescence Table 1.
The extraction value with different solvents revealed that B. oleracea had maximum extractive value with chloroform followed by benzene, ethanol and petroleum ether Table 2. Preliminary phytochemical screening of the various extract of B. oleracea revealed the presence of alkaloids, flavonoids, resins, saponins, and tannins. Flavonoids were present in chloroform and benzene whereas saponin was found to be in ethanol. The tannins were present in petroleum ether and benzene. The plant sample was tested negative for steroids for all the solvents Table 3.
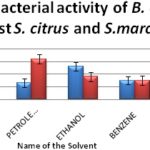
The anti-bacterial screening was carried out in B. oleracea using stem. The ethanol solvent showed maximum inhibition against gram-positive bacteria (Staphylococcus aureus), and chloroform solvent showed maximum inhibition in the case of gram-negative bacteria (Serratia marcescence) is presented in Table 4. The minimum zone of inhibition was observed in chloroform and ethanol solvents against gram positive and negative bacteria respectively Fig. 3.
TABLE 1: FLUORESCENCE ANALYSIS OF POWDERED STEM TUBER BRASSICA OLERACEAE VAR. GONGYLODES
| Powdered drug | UV 366nm |
| Powder extracted with alcohol | Pale blue color |
| Powder extracted with water | Yellow color |
| Powder extracted with 0.1N sodium hydroxide | Pale yellow color |
| Powder extracted with dilute hydrochloric acid | Greenish yellow color |
TABLE 2: EXTRACTION VALUES OF BRASSICA OLERACEA VAR. GONGYLODES
| Name of solvent | Initial weight of beaker | Final weight with extract | Extraction value |
| Chloroform | 179.721 | 209.5 | 297.79 |
| Petroleum ether | 49.149 | 64.994 | 158.45 |
| Ethanol | 82.92 | 101.56 | 186.4 |
| Benzene | 198.545 | 179.494 | 190.51 |
TABLE 3: PHYTOCHEMICAL SCREENING OF BRASSICA OLERACEA VAR. GONGYLODES
| Test | Chloroform | Petroleum ether | Ethanol | Benzene |
| Alkaloids | + | + | + | + |
| Flavonoids | + | - | - | + |
| Resins | - | - | - | - |
| Saponins | - | - | + | - |
| Steroids | - | - | - | - |
| Tannins | - | + | - | + |
TABLE 4: ANTIMICROBIAL ACTIVITY OF BRASSICA OLERACEA VAR. GONGYLODES
| Staphylococcus aurecus (Gram-positive) | Serratia marcescence (Gram-negative) | |
| Name of the solvent | Zone of inhibition (mm) | Zone of inhibition (mm) |
| Chloroform | 12 ± 0.251 | 20 ± 0.251 |
| Petroleum ether | 13 ± 0.152 | 17 ± 0.550 |
| Ethanol | 18 ± 0.208 | 15 ± 0.513 |
| Benzene | 15 ± 0.3 | 18 ± 0.435 |
FIG. 1: VARIETY OF BRASSICA OLERACEA VAR. GONGYLODES
FIG. 2: POWDER MICROSCOPIC ANALYSIS OF BRASSICA OLERACEAE VAR. GONGYLODES
FIG. 3: ANTIBACTERIAL ACTIVITY OF B. OLERACEAE AGAINST STAPHYLOCOCCUS CITRUS (GRAM POSITIVE) AND SERRATIA MARCESCENCE (GRAM NEGATIVE
The identification of plants involves the study of morphological features of the vegetative and floral parts. Generally, the herbal drugs are used in powder forms and adulteration in the powder drug is very easy but it can be detected by observing the powder under the ultraviolet light because the fluorescence characteristics of any powdered drug are very distinctive and helpful in distinguishing features for the determination of a drug. This parameter may be utilized not only for the identification of the drug but also to establish its purity and standard 14. The powder analysis carried out on B. oleracea revealed the presence of parenchyma cells with starch grains, stone cells, prisms of calcium oxalate and sieve tubes showing sieve plates.
The ash value of medicinal plants reflects the carbonate, phosphates, oxides, silicates, and silica. The ash values are particularly important in the evaluation of purity of drugs 15. The total ash is particularly essential in the evaluation of purity of drugs i.e. the presence or absence of foreign organic matter such as metallic salts and or silica. The ash value can also detect the nature of the material added to the drug for adulteration. The ash value of B. olercea was found out to be high.
Fluorescence analysis is the phenomenon exhibited by various chemical constituents present in the plant material under UV light. This can be used to characterize crude drugs. Thus crude drug is often assessed qualitatively and forms an important parameter of pharmacognostical evaluation16. Many phytocompounds fluorescences when suitably illuminated. The fluorescence color is specific for each compound. A non-fluorescent compound may fluoresce if mixed with fluorescent impurities.
The powder from the stem of B. oleracea fluorescence under ultraviolet light at 366nm which showed different color indicating the purity of the sample which could be made upon different color observed.
The extractive value gives a rough idea of the chemical constituents extracted from a specific amount of air-dried material. Extractive values of the plant parts with various solvents can be used to determine the exhausted and adulterated drugs. Extractive values are primarily useful for the determination of exhausted or adulterated drug17. The variance in the extractive value may be possible due to the presence of a specific compound, according to the solubility, soil condition, atmospheric condition and water content of the sample. It was found that B. oleracea had maximum extraction with chloroform and least extraction in petroleum ether.
The detection of active principles in medicinal plants plays a strategic role in the phytochemical investigation of crude plant extracts and is very important in regards to their potential pharmacological effects 4. Plant synthesizes a wide variety of chemical compounds, which can be sorted by their chemical class, biosynthetic origin, and functional groups into primary & secondary metabolites. Knowledge of the chemical constituents of plants is desirable, not only for the discovery of therapeutic agents but also because such information be of value in disclosing new resources of such chemical substances. Presence or absence of certain compounds in an extract is determined by the color reactions of the compounds with specific chemicals which act as dyes. This procedure is a simple preliminary pre-requisite before going for the detailed phytochemical investigation.
Preliminary phytochemical of the various extracts of B. oleracea revealed to contain alkaloids, flavonoids, resins, saponins, and tannins. The plant sample was tested negative for steroids for all the solvents. Vegetables belonging to Brassicaceae family are rich in polyphenols, flavonoids, and glucosinolates and they have antibacterial, antioxidant and anticancerous properties 18.
Flavonoids and tannins are associated with many biological effects such as antibacterial, antiviral, anti-inflammatory, antiplatelet, antioxidant, and free radical scavenging activity. High activity of flavonoids and antioxidant was found in intensely colored vegetables, such as red cabbage, red onion, etc. The plant extracts were also revealed to contain saponins which are known to produce an inhibitory effect on inflammation 19. Saponins have the property of precipitating and coagulating red blood cells. Some of the characteristics of saponins include the formation of foams in aqueous solutions, hemolytic activity, cholesterol binding properties, and bitterness.
The samples tested were negative for resins in all the solvents. Steroids present in the plant extract are of special interest in cancer studies. Steroids have considerable values in aiding the control of cancer in man. Steroids in modern clinical studies have supported their role as anti-inflammatory and analgesic agents 20.
Tannins, phenolics, saponins, alkaloids, and flavonoids have been linked or suggested to be involved with antibacterial and anti-viral activity while tannins and flavonoids are thought to be responsible for antidiarrheal activity 21. The remedial values of tannins include application on burns to heal the injury and on cuts to stop bleeding. Tannins ability to form a strong ‘leather’ resistance on the exposed tissues helps in protecting the wounds from being affected further. Tannin has several industrial uses as preservatives22. So, the identification of tannins in medicinal plants screened could be very advantageous in healing various diseases, and these plants could be potentially used for a varied range of applications.
Moreover, acting by several different mechanisms, particular flavonoid can exert significant anticancer activity including anti-carcinogenic properties and even a pre-differentiated activity, amongst other modes of action. Certain flavonoids possess potent inhibitory activity against a wide array of enzymes 23. Evidence suggests that only activated cells are susceptible to the modulating effects of flavonoids that is, cells which are responding to a stimulus. So the presence of this type of phytochemical compounds in the screened medicinal plants has a wide range of applications and could be certainly used for a variety of applications.
Anti-bacterial screening in B. oleracea with ethanol solvent showed maximum inhibition against S. aureus, and chloroform solvent showed maximum inhibition in S. marcescence. The activity is higher in these two solvents compared to other solvents may be due to the additive effect of antibacterial property of the solvent themselves along with the plant extract. In general gram-positive bacteria are more resistant than gram-negative bacteria. The resistance is due to the difference in the cell wall composition.
The gram-positive bacterium has its cell wall made up of several peptidoglycan layers joined together forming a thick and rigid structure. By contrast, gram-negative bacteria have only a thin peptidoglycan layer. The cell wall of a gram-positive bacterium has teichoic acids, which mainly consist of alcohols and phosphate. In the gram-negative bacteria, the outer membrane acts as a great barrier to much environmental substance. Presence of thick murine layer in the cell wall prevents the entry of inhibitors. The outer membrane is permeable to nutrients due to the presence of porins, proteins that form channels toward the cytoplasm 24.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Ncube NS, Afolayan AJ and Okoh AI: Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. African Journal of Biotechnology 2008; 7(12): 1797-1806.
- Phillipson JD and Wright CW: Plants with Antiprotozoal Activity: Tease and Evans, Pharmacognosy, WB Saunders Company 1996; 14: 612.
- Arunkumar S and Muthuselvam: Analysis of phytochemical constituents and antimicrobial activities of Aloe vera against clinical pathogens. World J Agril Sc 2009; 5(5): 572-576.
- Pascual ME, Carretero ME, Slowing KV and Villar A: Simplified screening by TLC of plant drugs. Pharmaceutical Biology 2002; 40(2): 139-143.
- Koche D, Shirsat R and Imran S: Phytochemical screening of eight traditionally used ethnomedicinal plants from Akola district (MS) India. International Journal of Pharma and Bio Sciences 2010: 1(4).
- Pascaline J, Charles, M, Lukhoba, C and George O: Phytochemical constituents of some medicinal plants used by the Nandis of South Nandi district Kenya. Journal of Animal & Plant Sciences 2011; 9(3): 1201- 1210.
- Kokate CK, Purohit AP and Gokhale SB: Pharmacognosy. Nirali prakashan, Mumbai India 2010: 64.
- Cowan MM: Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 564-582.
- Anon: "The Wealth of India - Raw Materials" Vol II C., CSR. New Delhi 1950: 66.
- Chase CR and Pratt RJ: Fluorescence of powdered vegetable drugs with particular reference to the development of a system of identification. J Amer Pharm Assoc 1949; 38: 324-331.
- Sofowara AE: Medicinal plants and traditional medicine in Africa. Vol. 2. Spectrum Books Ltd, Ibadan, 1993: 288.
- Trease GE and Evans WC: Pharmacognosy. Braille Tiridel and Macmillan Publishers, 2nd 1989.
- Harborne JB: Methods of plant analysis. In: Phytochemical Methods. Chapman and Hall, London 1973.
- Nasreen J, Afaque SH, Khan NA, Ghufran A and Abid Ali A: Physico-chemical studies of the gum Acacia. Nature Product Radiance 2008; 7(4): 335-337.
- Shruthi SD, Ramachandra YL, Rai PS and Jha PK: Pharmacognostic evaluation of the leaves Kirganelia reticulate Baill (Euphorbiaceae). The Asian and Asian & Australasian J Plant Sci Biotech 2010; 4(1): 62- 65.
- Dominic VJ and Manju M: Pharmacognostic and preliminary phytochemical screening of the rhizome of Anaphyllum wightii schott, an endemic and threatened genus of the Western ghats. Journal of Herbal Medicine and Toxicology 2012; 6(2): 55-60.
- Shrivastwa S and Leelavathi S: Int. J Pharm. Sci. Review and Research 2010; 3: 114-118.
- Kumar JA, Gaurav R and Shilpi G: Phenolic composition, antioxidant capacity and antibacterial activity of selected Irish Brassica vegetables. Nat Prod Commun 2011; 6: 1-6.
- Just MJ, Recio MC, Giner RM, Cueller MU, Manez S, Billia AR and Rios JL: Antiinflammatory activity of unusual lupine saponins from Bupleurum fruticescens 1998; 64: 404-407.
- Singh AP: Short Review: Distribution of Steroid like Compounds in Plant Flora. Pharmacognosy Magazine 2006; 2(6): 87-89.
- Enzo AP: Traditional plants and herbal remedies used in the treatment of diarrheal disease: Mode of action, quality, efficacy, and safety considerations. In: Ahmad I, Aqil F, Owais M, editors. Modern Phytomedicine Turning Medicinal Plants into Drugs, WILEY- VCH Verlag GmbH & Co. KGaA, Weinheim 2007; 248-260.
- Arshad MA: Ethnopharmacological application of medicinal plants to cure skin diseases and in folk cosmetics among the tribal communities of North-West Frontier Province. Pakistan. J Ethnoph 2010; 128: 322-35.
- Lena G: Phenolic compounds, antioxidant activity and in-vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bio Resour. Technol 2010; 10: 4676-4689.
- Wheelis M: Principles of Modern Microbiology. Published by Jones & Bartlett Publishers
How to cite this article:
Sabannavar SJ and Swaroopa BV: Pharmacognostic studies of stem tuber on Brassica oleracea Var. Gongylodes. Int J Pharmacognosy 2016; 3(5): 221-28. doi: 10.13040/IJPSR.0975-8232.3(5).221-28.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.