PHARMACOGNOSTIC AND PHYSICOCHEMICAL EVALUATION OF GYNURA NEPALENSIS
HTML Full TextPHARMACOGNOSTIC AND PHYSICOCHEMICAL EVALUATION OF GYNURA NEPALENSIS
Arati Tamta * and Mahendra Rana
Department of Pharmaceutical Sciences, Bhimtal, Kumaun University, Nanital, Uttarakhand, India.
ABSTRACT: Traditional knowledge and ethnobotanical use of plants have been widely acknowledged worldwide. The cicatrizing properties of extracts obtained from this plant have been scientifically studied, attributing the main biological activity to its tannin and flavonoid content. The recent commercialization of the plant drug Gynura nepalensis. requires Pharma-cognostical and Pharmacological information to develop quality-control methods for raw materials and extracts produced with the plant drug. Macro and micro morphological parameters were established to authenticate the genuine drug that allowed detection of adulterants usually found in commercial samples of this plant material. All the parameters were studied according to WHO guidelines and Indian Pharmacopoeia. These morphological characteristics can be used for rapid identification of the drug and are particularly useful in the case of powdered materials and physicochemical parameters. TLC profiling of plant extracts gives an idea about the presence of various phytochemicals. Pharmacological activity of Gynura nepalensis ethanolic leaf extracts were analyzed for total phenolic and flavonoid contents along with the antioxidant, antimicrobial and anti-inflammatory activity. The result of the study can serve as a valuable source of information and provide suitable for the identification of this plant material in future investigation and application.
Keywords: Gynura nepalensis, Pharmacognostic standardization, Phytochemical analysis, TLC, Antioxidant, Antimicrobial, Anti-inflammatory
INTRODUCTION: Gynura nepalensis, usually acknowledged as ‘purple possion’in English, is a leaf annual belonging to the family Asteraceae 1. It is distributed in the Africa, south and east Asia and Australasia, Bangladesh 2, 3. In India, it is found growing in western Himalayas, Punjab, Kashmir, Nepal and Uttarakhand 4, 5. The tincture of fresh aerial parts is used as medicine in homeopathy. It is a `used in the treatment of vomiting, skin allergy, kidney stone, urinary tract bleeding, high cholesterol lavel, hepatoprotective, antimutagenic and antioxidant 6, 7, 8.
The development and benefit of modified analytical techniques are indispensable in drug research for identifying active compounds and characterization of chemical structure, quantifying, and laying down standards for scientific quality control. To solve complex natural matrix, it is mandatory to use a combination of advanced techniques. Chemically, the plant is reported to contain more than a few dynamic compounds such as primary alkaloids, steroids, flavonoids, saponin, and tannin 9.
Isolated and analysis of the phytochemical constituents of Gynura nepalensis using HPLC. The study was carried out for the preliminary phytochemical analysis of the ethanolic extract of Gynura formosana. HPLC analysis of the ethanolic extract of Gynura formosana to find out the bioactive compounds. In the HPLC analysis, four potent compounds were identified: quercetin 3-O-rutinoside, kaempferol 3-O-rutinoside, kaempferol-3-O-robinobioside and caffeic acid 6. Kaempferol-3-O-rutinoside, and kaempferol in the gynura procumbens methanol extract both have been determined using high-performance thin-layer chromatography 10. The same chemical analysis as (Yam et al., (2009) towards procumbens aqueous and revealed that this plant extract contains 0.76% and 2.65% of kaempferol-3-O- rutinoside and kaempferol-3-o-glucoside, respectively 11. Preliminary phytochemical analysis on gynura procumbens methanol extract and this analysis led to the isolation of flavonol and flavonol glycoside, including rutin, quercetin, kaempferol and quercetin -3-o-rhamnosyl (1-6) glucoside, quercetin-3-o- rhamnosyl (1-6) galactoside, kaempferol-3-o-rhamnosyl (1-6) glucoside and kaempferol-3-o-glucoside 12. A review of the literature reveals no pharmacognostic standards laid down for this drug, except some review works on trichome diversity and organoleptic distribution of calcium oxalate crystals in Conyza spp. 13 in view of the significance and importance of the drug, pharmacognostic and physicochemical standardization studies were carried out to lay down specific standards in homeopathic perspective.
MATERIALS AND METHODS: Aerial parts of the Gynura species were collected from a plant nursery, nearby bypass road, Bhimtal, during the month of August and October 2017, identified and confirmed by Dr. Kumar Ambrish, Botanical survey of India, Dehradun. The sample was deposited in the herbarium of the institute-wide voucher specimen number BSI/ NRC Tech. / Herb (ident.) / 2017-18 /695 (118105). The material was air-dried and then stored at 25°C in airtight container.
Preparation of Extracts: Before extraction, the plant materials were freed of adhering soil particles and washed in running tap water followed by distilled water. The plants (leaf) were shaded, dried at ambient temperature, and powdered using an electronic blender. Solvent extractions were prepared by soaking the powdered material in 600ml of the solvent ethanol in a cold maceration for 72 h. at 30°C until complete extraction.
Macroscopic and Microscopic Analysis: The macroscopy of Gynura nepalensis was described with the help of floras and books. Hand/cryostat section of 20-60µm thickness in transverse (TS) view was taken, and microscopy of the plant was studied according to the methods of C.K. Kokate and the microscopic analysis of powder was done according to the methods of Khandelwal and C.K. Kokate et al.,. The samples' leaf was boiled separately with saturated chloral hydrate for surface studies. Leaf constant, which is the stomatal number and palisade ratio, was studied according to methods of Evans.
Physico-chemical Analysis: The ethanolic Gynura nepalensis extract was determined to be phytochemical tests for secondary plant metabolites; alkaloids, saponins, tannins, phenol, and glycosides using standard procedures methods as described with little modification 14, 15. Air-dried plant material was used for the quantitative determination of ash and extractive values (WHO guidelines,)
Determination of Total Phenolic Content: A stock solution (1 mg/ml) of extract in methanol was prepared. From the stock solution suitable quantity of the extract was taken into 25 ml volumetric flask and to it 10ml of water and 1.5ml of folin ciocalteus reagent was added, the mixture was kept for 5 mint, and then 4ml of 20% Na2CO3 was added and the volume was made up to 25ml with distilled water. The mixture was kept for 30 min. and absorbance recorded at 765nm. Total phenolic content was calculated as galic acid (mg/ml) using the following equation based on the calibration curve: y = 230.1x + 0.104, r2 = 0.956, where y was the absorbance and x was the gallic acid equivalent (mg/ml) (Bray and Thorpe, 1954).
Determination of Total Flavonoid: A stock solution (1mg/ ml) of extract in methanol was prepared 0.5 ml of sample was taken from the stock solution ( methanolic extract) and added to 0.5 ml of 2 % methanolic AlCl3. Standard was prepared in the same way by using 0.2, 0.4, 0.6, 0.8, and 1ml of stock solution (quercetin solution). Yellow color indicated the presence of flavonoids. Blank was prepared by using 0.5 ml. 2% methanolic AlCl3. All the solutions were made up to 5ml. With methanol and after 1 hour, the absorbance of standard content was calculated as quercetin (mg/ml) using the following equation based on the calibration curve: y = 94.26x = 0.244, r2 = 0.977, where y was the absorbance and x was the quercetin equivalent (mg/ml).
Determination of Total Tannin: 2gm powdered plant material was extracted with 100ml distilled water by boiling in a water bath for 6-8 h; the solution was then filtered, and the volume was made up to 100ml in the volumetric flask. 1ml aliquot was taken from it and added to 5ml folin and ciocalteu’s reagent and 10ml saturated sodium carbonate, and the volume was made up to 100ml in a volumetric flask. The instrument was calibrated through the blank, the corresponding absorbance of different samples was taken, and tannin content was calculated by using the following equation based on the calibration curve: y = 75.95x + 0.017, r2 = .0999, at 760 nm, using UV-1 double beam spectrophotometer, where y was the absorbance and x the tannic acid equivalent (mg / ml) (Anonymous, 1984).
Chromatography Analysis: For the preparation of the extract, the aerial parts of dried Gynura nepalensis were powdered and sieved through.
Thin Layer Chromatography Profiling: Only plant extracts and fractions that tested positive in the amylase inhibition assay had their TLC profiled. Each of these was spotted on thin layer silica gel plates and then developed using varying solvents- for the ethanolic crude extracts, n-hexane: ethayl acetate: methyal hydro ( 6:3:1) was used. After drying, the plates were visualized using long-wave ultraviolet light and visible after staining with 5% aluminum chloride in ethanol.
Pharmacological Activity:
In-vitro Antioxidant Activity: A solution of 0.135ml DPPH in methanol was prepared, and 1.0 ml of this solution was mixed with 1ml of extract containing 0.02, 0.04, 0.06, 0.08, and 0.1 mg of the extract. The reaction mixture was vortexed thoroughly and left in the dark at room temperature for 30 min.
The absorbance of the mixture was measured spectrophotometrically at 517nm ascorbic acid, quercitin was used as references. The ability to scavenge DPPH radicals was calculated by the following equation:
Formula used:
DPPH radical scavenging activity (%) = (Abs control – Abs sample) / Abs control × 100
Where, Abs control = absorbance of DPPH radical + methanol, Abs sample = Absorbance of DPPH radical + sample / reference.
The same procedure was used for methanolic extracts of leaf Gynura nepalensis (zheng and wang, 2001).
Reducing Power Method: Reducing the extract's ability was measured according to the method of Oyaizu (Oyaizu, 1986).The process started with the preparation of phosphate buffer (0.2 M, pH 6.6) and different concentration (10,20,40,80 and 160 µg /ml) of Gynura nepalensis. 1 ml of the different concentrations of extract solution was mixed with 2.5ml of buffer and 2.5 ml of 1% potassium ferricyanide. The same reaction mixture without extract served as control, and ascorbic acid served as standard. This mixture was incubated at 50°C for 20mint. After incubation, 2.5ml of 10% trichloroacetic acid was added to each solution. It was centrifuged to 650 rpm for 10 min. after centrifugation, the upper layer of solution (2.5ml) was mixed with distilled water and 0.5ml (0.1%) of FeCl3. The absorbance was measured at 700nm. An increase in absorbance of the reaction mixture indicated an increase in reducing power. Ascorbic acid was used for comparison as a reference in place of extract.
Nitric Oxide: Sodium nitroprusside (5mm) in phosphate buffer saline was mixed with different concentration of hydromeyhanolic extract (10-640µg/ml) dissolved in DMSO and incubated at 25°C for 30min. after 30 min., 1.5ml of the incubated solution was removed and diluted with 1.5ml Griess reagent (1% sulphanilamide, 2%orthophosphoric acid and 0.1% napthylethylene diamine dihydrochloride). The absorbance of the chromophore formed during diazotization of the nitrite with sulphanilamide and subsequent coupling with napthylethylene diamine was measured at 546 nm along with a control (Sreejayan and Rao., 1997). The percentage inhibition of nitric oxide generated was measured by comparing the absorbance values of control and test sample absorbance values using the following formula.
Percentage inhibition (%) = [(Acontrol – Asample) / Acontrol)] ×100
Where, Acontrol is the absorbance of the control reaction (containing all reagents except the test compound), and Asample is the absorbance of the test compound. Ascorbic acid was used as positive control, and all tests were carried out in triplicate.
In-vitro Anti-inflammatory: The protein denaturation was performed according to the method of Ullah et al. (2014). Initially phosphate buffer saline (pH 6.4) and different concentration (10, 20, 40, 80, 160 µg/ml) of extracted were prepared. The 5ml of reaction mixture consisted of 0.2ml of egg albumin, 2.8ml of phosphate buffer saline and 2ml of different concentrations of extract.
In case of reference, acetylsalicylic acid was used in place of extract. The mixture was incubated at 37°C +2 for 15min and then heated for 5min at 70°C. it was then left for cooling, and absorbance was measured at 660nm. Ethanol was used for blank.
The protein denaturation % inhibition was calculated by:
Percentage protein denaturation inhibition = AC- AT / AC × 100
Where, AC = absorbance of control, AT= absorbance of test.
Antimicrobial:
Agar Disk Diffusion Method: The Agar disk diffusion method was used in the screening antibacterial activity described by Magaldi et al. (2004). Five bacteria testes; Bacillus Subtilis, Escherichia coli, M. luteus, P. Vulgaris, S. typhimurium . The extract obtained from the plants was used for studying their antibacterial activity. A loopful of bacterial strain was inoculated in 30ml of nutrient broth in a conical flask and incubated for 72 h to get an active stain by using the agar disk diffusion method. Nutrient agar was poured into petridishes. After solidification, 0.25 ml of test strains were inoculated in Nutrient media separately.
The experiment was performed under strict aseptic conditions in laminar airflow. After the medium solidified, a disk was pore in the plates with sterile for sheep. The extracted compound (10µl) was introduced into the disk, and plates were incubated at 37°C for 72 hours. All samples were tested in triplicates. Microbial growth was determined by measuring the diameter of the zone of inhibition (Bakht et al., 2011).
RESULTS AND DISCUSSIONS:
Macroscopic Evaluation: The result of the macroscopic study might be useful for distinguishing it from its substitutes and adulterants.
Morphologically, the stem of gynura nepalensis appeared dark, green in colour, having aromatic odour and 30-40 cm long, Corymbosely branched, smooth in texture, rough in touch, while leaves showed dark green colour, characteristic odour, linear to oblanceolate, 5-6 cm long. 2-2.5 cm breadth, rough touch, Flower purplish or maroon colour, slightly bitter taste Fig. 1 and Table 1.
FIG. 1: MORPHOLOGY OF WHOLE PLANT OF GYNURA NEPALENSIS. (A) LEAF, (B) STEM, (C) BUDS AND FLOWER, AND (D) WHOLE PLANT
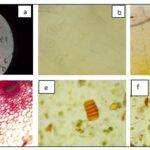
The transverse section of the stem showed the presence of thick walled, heavily cutinized epidermis and hypodermis followed by the ground tissue composed of parenchymatous cells with air spaces. The ground tissue, palisade cell, xylem, vascular bundle, collenchymas cell and trichomes Fig. 2 and 3. The powder analysis showed the presence of trichomes, lignified fibers, and crystals Fig. 4, 5, 6.
TABLE 1: MORPHOLOGY OF GYNURA NEPALENSIS, DRIED LEAF AND STEM
| Parameters | Stem | Flower | Leaf |
| Color | bright green | dark green | purple n white |
| Shape | linear to oblanceolate | smooth in texture | ovule |
| Odour | aromatic | aromatic | aromatic |
| Breadth | 2-2.5cm | 4cm | 1.5cm |
| Texture | thin | rough | soft |
| Touch | rough | rough | soft |
The leaf, stem and whole plant understudy can be utilized as a potential source of useful therapeutic. The outcome data will be beneficial for quantitative and qualitative standardization of herbal preparation containing Gynura nepalensis. Further studies are in progress on the whole plant to isolate, identify, characterize, and explore their pharmacological activity.
Phytochemical Screening: In this study, the medicinal plant Gynura nepalensis leaves were extracted in methanol to a 7.33% yield of dried grind power.
Ethanol Gynura extract was screened for the preliminary phytochemical composition such as alkaloids, phenolic, flavonoids, and saponin using colour reaction methods.
The result of phytochemical screening revealed the presence of alkaloids and phenolics, but glycosides were found to be absent, as shown in Table 2.
TABLE 2: PHYTOCHEMICAL COMPOSITION OF THE METHANOLIC GYNURA NEPALENSIS EXTRACT
| Phytocompounds | Methods | Results | References |
| Alkaloids | Dragendroff”s test Mayer’s test Hager’s test | present | 14, 15 |
| Phenolic | 5% FeCl3 solution Lead acetate solution Bromine water | present | |
| Flavonoids | sulphuric acid test | absent | |
| Saponin | foam test | absent | |
| Glycosides | keller- killiani test Baljet’s test | absent |
Physico-chemical Analysis: The physic-chemical parameters help judge the purity and quality of the drug. The percentage of active chemical constituents in crude drugs is usually mentioned on air-dried basis.
Hence, the moisture content of a drug should be controlled to make the solution of definite strength. The moisture content of the drug should be minimized in order to prevent the decomposition of crude drugs either due to chemical changes or microbial contamination. Ash values were used to detect to the presence of any siliceous contamination. These values are important quantitative standards as it is useful in determining the authenticity and purity of the drug. A lower concentration of total ashes indicates low-level f carbohydrates, phosphates, and silicate. The total ash value for a crude drug is not always reliable since there is a possibility of the presence of non-physiological substances. The water-soluble extractive indicates the presence of water-soluble matters such as alkaloids, amino acids, flavonoids, carbohydrates, and phenols. The organic ligands possess promising biological activities, which can be utilized to develop potential drugs.
TABLE 3: PHYSICO-CHEMICAL ANALYSIS
| Parameters | Result |
| LOD | 1.49% |
| Total ash
Acid Water |
1.43%
1.31% 1.608% |
| Extractive value
Ethanol water |
7.33% 14% |
Microscopic Analysis: In the present work, the microscopic study of the whole plant of Gynura nepalensis was carried out.
Microscopic evaluation allows a more detailed examination of crude drugs and enables identifying the organized structural features such as epidermis, starch grains, and parenchymatous-cells.
FIG. 2: T. S. AND POWDER MICROSCOPY OF GYNURA NEPALENSIS PLANT (A) CRYSTALS, (B) STOMATA, (C) TRICHOMES, (D) T. S. OF STEM, (E) GLOUBLES (F) FIBERS
TABLE 4: ANTIBACTERIAL EFFECT OF METHANOL, EXTRACT AND STANDARDS
| Test organism | zone of inhibition | |
| EIZ | CIZ | |
| Bacillus Subtilis | 0.833mm | 1.13mm |
| Escherichia coli, | 1.23mm | 1.16mm |
| M. luteus | 0.94mm | 0.7mm |
| P. Vulgaris | 1.1mm | 1.53mm |
| S. typhimurium | 1.33mm | 1.03mm |
FIG. 3: ANTIMICROBIAL. ZONE OF INHIBITION (A) B. SUBTILIS, (B) E. COLI, (C) M. LUTARS (D) P. VULGARIS (E) S. TYPHIMURIUM
Antioxidant:
TABLE 5: ANTIOXIDANT METHODS
| Method | % Scavenging (1000µg/ml) |
| DPPH | 54.83% |
| no activity | 56.23% |
Chromatography: In the present state of affairs, TLC profiling of crude extracts in different solvent systems indicated the presence of diverse types of phytochemicals in these plants.
Different Rf values of the compound also reflect an idea about their polarity. This information will help in the selection of an appropriate solvent system for further separation of the compound from these plant extracts.
TABLE 6: CHROMATOGRAPHY PROFILE
| Drug name | Solvent system | Colour of spot | Retention time |
| Gynura nepalensis | n-hexane, ethylacetae, methanol (30:15:5) | green | 0.571 |
FIG. 4: TLC PLANTS OF CRUDE ETHANOL EXTRACT
CONCLUSION: In the present investigation, various standardization parameters such as macroscopy, microscopy, physicochemical constants, preliminary phytochemical investigation, TLC, and pharmacological activity. This could be helpful in authantication and preparation of a suitable monograph for the proper identification of the whole plant of Gynura nepalensis. High antioxidant and antimicrobial activity is observed in the leaves of gynuranepalensis. as compaired to other extracts tested. Thus, this extract can be considered new natural antioxidant source for disease healing and health supplements.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Pogaku PR, Palani S and Penthala S: Pharmacognostic and Pharmacological evaluation of homeopathic drug. Gunura nepalensis 2017; 26-33.
- Anonymous Wealth of India Raw materials 1989; 419-21.
- Anonymous Indian herbal Pharmacopoeia 1998; 139-46.
- San AR: Phytochemical screening clastogenic and antiastogenic locally Tea cultivars 2014; 1-7.
- Afroz S and Uddin MZ: Gynura nepalensis DC (Asteraceae)- A new Angiosperm record for Bangladesh 2014; 101-104.
- Vanijajiva O: The genus gynura Asteraceae: senecioneae 2009; 25-36.
- Rahman M: Anti-inflammatory anlanalgesic activities of ethanolic extract of Gynura nepalensis (leaf) 2018; 247-253.
- Yam FM: Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin –inclued diabetic rats molecules 2001; 9008-9023.
- Akowuah GA and Sadikum A: flavonoid identification and hypoglycaemic studies of the butanol fraction from gynura procumbens 2002; 405-410.
- Meric C: calcium oxalate crystals in Conyza canadensis And Conaya bonariensis cronq 2008; 295-299.
- De S and Dey YN: Phytochemical investigation and chromatographic evaluation of the different extracts of tubers of Amorphaphallus paeonifoleus Aracese 2010; 150-157.
- Ayoola GA and Coker HAB: Phytochemical screening and antioxidant activities of some selected medicinal plants used activities of some selected medicinal plants 2008; 1019-1024.
- Russelle M and Bueno RP: Phytochemical analysis and salivary amylase inhibition activities of Carica papaya leaf and Garcinia mangostana pericarp extracts and partially purified fractions 2016.
- Kokate CK: practical Pharmacognosy 2008; 112.
- Winslow LC: Herbs as medicine 1998; 2192-9.
How to cite this article:
Tamta A and Rana M: Pharmacognostic and physicochemical evaluation of Gynura nepalensis. Int J Pharmacognosy 2022; 9(2): 41-47. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.9(2).41-47.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
41-47
1053 KB
724
English
IJP
Arati Tamta * and Mahendra Rana
Department of Pharmaceutical Sciences, Bhimtal, Kumaun University, Nanital, Uttarakhand, India.
arati.tamta12@gmail.com
20 January 2022
23 February 2022
26 February 2022
10.13040/IJPSR.0975-8232.IJP.9(2).41-47
28 February 2022