NUTRACEUTICAL: A REVIEW
HTML Full TextNUTRACEUTICAL: A REVIEW
Sejal Patel
Department of Pharmacognosy, Nootan Pharmacy College, Sankalchand Patel University, Visnagar - 384315, Gujarat, India.
ABSTRACT: Nutraceuticals have received considerable interest because of their presumed safety. The Present article focuses on the need for consuming appropriate diets, health issues surrounding the failure to adhere to the known healthy eating models, development of new nutraceuticals / functional foods/food supplements with novel health benefits, elucidation mechanisms of action of these products, to define and understand the analytical, formulation and regulatory aspects of nutraceutical. This article may act as a tool to abreast of the recent developments in nutraceutical research.
| Keywords: |
Nutraceutical, Food supplement, Functional food
INTRODUCTION: The word of nutraceutical was derived from words of "nutrition" and "pharma-ceutical and which was coined in 1989 by Dr. Stephen L. Defelice, He was founder and chairman of the Foundation of Innovation Medicine. Nutraceuticals are products derived from food sources that provide extra health benefits, in addition to the basic nutritional value found in foods. Depending on the jurisdiction, products may claim to prevent chronic diseases, like cancer, cardiovascular disease and diabetes improve health, delay the aging process, increase life expectancy, or support the structure or function of the body 1.
The Reasons for the Shift towards Nutraceuticals are: 2-6
Increasing numbers of consumers, concerned about healthcare costs.
- Dissatisfied with pharmaceutical agents in promoting health, are turning to nutraceuticals to improve their health and prevent chronic disease.
- Health care providers recognizes the fact that our heavily processed food supply, coming from crops grown with chemical fertilizers, pesticides, herbicides, and often genetically modified seeds, lack sufficient nutrients necessary for optimum health.
- People are believing more in prevention than a cure.
- People who have chronic diseases and have found no solution in allopathic medicines.
- Economically challenged patients.
With few exceptions, the U. S. Food and Drug Administration (FDA) has not approved nutraceuticals for health benefits or disease prevention; nonetheless, the manufacturers of nutraceuticals have been touting them as health-promoting agents.
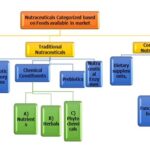
Categories Based on Natural Source: 7, 8
- Carbohydrates & Fiber
- Fat & Essential fatty acids
- Protein
- Minerals like Macrominerals & Trace minerals
- Vitamins
- Water
- Other nutrients like Antioxidants, Phyto-chemicals & Intestinal bacterial flora Recombinant nutraceuticals.
They are simply natural with no changes to the food. The food contains several natural components that deliver benefits beyond basic nutrition, such as lycopene in tomatoes, omega-3 fatty acids in salmon, or saponins in soy.
Dietary Supplements: A Dietary supplement is a product that contains nutrients derived from food products that are concentrated in liquid or capsule form. Dietary supplement products include vitamins, minerals, herbs, or other botanicals, amino acids, and substances such as enzymes and metabolites. Dietary supplements can also be extractor concentrates and may be found in many forms such as tablets, capsules, soft gels, gelcap-sule, liquids, or powders. Dietary vitamin B supplements are typically sold in pill form. With a few well-defined exceptions, dietary supplements may only be marketed to support the function of the body and may not claim to treat a disease or condition,
They are grouped on the Basis of Chemical Constituents:
- Nutrients
- Herbals
- Phytochemicals
Phytochemicals basically are plant nutrients with particular biological activities in supporting human health; they work by the following way.
- Substrate for biochemical reactions.
- Cofactors of enzymatic reactions.
- Inhibitors of enzymatic reactions.
- Absorbents that bind to and eliminate undesirable constituent in the intestine.
- Enhance the absorption and stability of essential
- Selective growth factor for beneficial
- Fermentation substrate for beneficial
- Selective inhibitors of deleterious intestinal
- Scavengers of reactive or toxic
- Ligands that agonize or antagonize cell surface or intracellular receptors 91.
2. Probiotic Microorganisms: Metchnikoff coined the term “probiotic. Probiotics’ mean ‘for life’ and are defined as live microorganisms, which when consumed in adequate amounts, it confer a health effect on the host Probiotic are very important nutraceutical for removing the toxic flora from the intestine and maintaining a friendly environment, for example, useful consumption of Bacillus bulgaricus which obtained from yogurt they act to crowd out pathogens, like yeasts, other bacteria and viruses that may otherwise cause disease and develop a mutually advantageous symbiosis with the human gastrointestinal tract. They have an antimicrobial effect through modifying the microflora action, and it preventing adhesion of pathogens to the intestinal epithelium, which necessary for producing a toxic effect and reversing some of the consequences of infection on the intestinal epithelium, such as secretory changes and neutrophil migration. Probiotics can cure lactose intolerance by the production of the specific enzyme (ß-galactosidase); in the selection benchmarks for probiotics, one should consider safety, functional and technological aspects as follows Show a potential health benefit.
Probiotics should have a human origin.
- Commonly gram-positive organisms.
- It can survive after passage through acid and bile.
- Can adherence to the human intestinal cells and grow in the gut.
- It can show antagonist action against pathogenic or carcinogenic bacteria.
- It has clinically proven documented beneficial health effects 9.
List of Bacteria and Their Beneficial Effects: 10-18
| Name of bacteria | Action |
|
L. rhamnosus (High tolerance to bile salts, surviving in less than favorable environments) |
· Reduction of viral-associated pulmonary damage
· Prevention and reduction of severity of atopic dermatitis in children · Reduction of risk for developing allergic disease · Anti-diabetic potential · Prevention of necrotizing enterocolitis in newborns · Prevention or treatment of bacterial vaginosis · Aid in a weight loss of obese women · Treatment of acute gastroenteritis in children · Reduction of risk for rhinovirus infections in preterm infants · Protection of human colonic muscle from lipopolysaccharide-induced damage · Produces lactic acid in the large intestine. · Protection of human colonic muscle from lipopolysaccharide-induced damage · Produces lactic acid in the large intestine. |
| L. acidophilus (Present in the linging of the intestine,
Acidophilus can also take up residence in the vagina, cervix or urethra.) |
· Used in treatment of travellers’ diarrhoea, acute diarrhea
· Used in treatment of bacterial vaginosis · Reduction risk of febrile urinary tract infections in children · Reduction of irritable bowel syndrome symptoms · Inhance Immunity by inhibiting pathogens and producing lactocidin and acidophilin. · Also show anti-microbial effects against Staphylococcus aureus, Salmonella, E. coli, Candida albicans. |
|
L. plantarum (Synthesis of lactolin and L-lysine: anti- viral amino acid) |
· Prevention of endotoxin production, antifungal activity
· Reduction of irritable bowel syndrome symptoms and reduce abdominal pain, bloating flatulence, and constipation · Eliminates nitrate, promoting nitric oxide levels · Reduces risk of bleeding · Positive effect on immune responses |
|
L. casei |
· Treatment of functional constipation in adults, reduction of irritable bowel syndrome symptoms, antibiotic-associated diarrhea
· Restoration of vaginal flora of patient with bacterial vaginosis and use in intravaginal staphylococcosis which reduce cervix tumors · Protection against Salmonella infection, rotavirus infections, clostridium difficile infection, synovitis and show immunomodulatory action and decrease lactose intolerance · Impovement in cholesterol levels, decrease triglycerides, decrease blood pressure and also decreases systemic inflammatory response syndrome · Show positive effects in Allergy Benefits like Pollen Allergies, Newborn Allergies. · Produce vitamins B1 and B2 |
|
L. delbrueckii (bulgaricus) |
· Enhancement of systemic immunity
· Antimicrobial action against E. coli, Helicobacter pylori · Exhibited antimutagenic activities against 4NQO, a typical mutagen, and faecal mutagen · Protective action by producing lactic acid |
| L. brevis | · Protective role in bile salt tolerance reduction in plague acidogenicity
· Synthesis of Lactic acid, Vitamins D /K. |
| L. johnsonii | · Antimicrobial action against Helicobacter pylori, S. sonnei
· Treatment of perennial allergic rhinitis |
|
L. fermentum |
· Prevention or treatment of bacterial vaginosis
· Potential for reduction of insulin resistance and hypercholesterolemia · Relieve symptoms associated with occasional gastrointestinal (GI)discomfort, occasional bowel irregularity, diarrhea, and other common digestive and non-digestive discomforts |
| L. reuteri
(found in human |
· Reduction of low-density lipoprotein, triglyceride, cholesterol
· Treatment of acute gastroenteritis and diarrhea |
| breast milk) | · Immunosupportive and anti-gas effects are associated with breastfeeding. |
|
B. infantis |
· Reduction of irritable bowel syndrome symptoms
· Reduction of necrotizing enterocolitis in preterm infants · Simulates the production of cytokines that affect the immune system, · Antimicrobial action against clostrida, salmonella and shigella. B. longum colonizes the large intestine. · This can decrease the frequency of gastrointestinal problems, such as diarrhea and nausea during antibiotic use. |
|
B. animalis ( lactis) |
· Reduction of the incidence of febrile urinary tract infections in children
· Reduction of necrotizing enterocolitis in preterm infants · Reduction of total microbial counts in dental plaque also protect from enterohemolytic pathogen like Escherichia coli · Reduction of total cholesterol · Reduction of risk of upper respiratory illness · Usefull in Crohn's disease · Improvements in immunity · Protection from Salmonella infection · reduce the severity of weanling diarrhea associated with rotavirus and E. coli · Used in animal feed (stimulate animal growth, reduce coliform counts by the production of antimicrobial metabolites |
| B. bifidum (second most prominent species that identified in breast-fed infants) | · Used in the treatment of acute diarrhea
· Reduction of necrotizing enterocolitis · Reduction of total cholesterol · Boosted immune functions. · Shown anti-ulcer activities, anticancer activity |
|
B. longum (It is commonly found in the GI tracts and vagina) |
· Prevention and treatment of necrotizing enterocolitis in newborns
· Reduction of irritable bowel syndrome symptoms · Perinatal intervention against the onset of allergic sensitization · Anti-inflammatory properties that protect the cells lining your mucous membranes from toxins and help immune cells to mature and function properly. · Present in breast milk, and colonize the infant's gut · Able to ferment carbohydrates and digest protein · Useful in Seasonal allergies, Bone health, Pathogen infections, and also prevent Colon cancer |
| · Intestinal injury and inflammation. By inhibits the activation of extracellular signal-regulated ½ and mitogen-activated protein (MAP) kinases, thus modulating host signaling pathways for protection against diarrhoeal diseases
· Treatment of travelers’ diarrhea, irritable bowel syndrome, ulcerative colitis, recurrent pseudomembrane colitis infection, acute gastroenteritis |
|
|
L. lactis |
· Treatment of antibiotic-associated diarrhea
· Adhesion to vaginal epithelial cells · production of bacteriocins I as lacticins, nisin A, lactococcins · modulation of brain activity · Wide spectrum of bactericidal and fungicidal action to the pathogens like activity against C. difficile · Use for cytokine delivery · Formation of acetaldehyde, diacetyl, acetoin, and 2-3 butylene-glycols during fermentation which lead to typical flavour in cheese. · Can able to degrade methionine to methonethiol, dimethyledisulphide (DMDS), citrate, and dimethyltrisulphide (DMTS) · Utilize in the formulation of animal food products |
|
E. faecium |
· Treatment of antibiotic-associated diarrhoea
· Decreased duration of acute diarrhea from gastroenteritis · Prevent infection by Salmonella enteric ssp. · Stimulate animal growth, reduce coliform counts by the production of antimicrobial metabolites and therefore utilize in the formulation of animal food products · Production of bacteriocin-like inhibitory substances that show antimicrobial activity against Gram (+) bacteria. |
|
S. thermophilus |
· Reduction of irritable bowel syndrome symptoms
· Used in fermented milk products deliver enough bacterial lactase to the intestine and stomach where lactose is degraded to prevent symptoms in lactase nonpersistent individuals · reduction of necrotizing enterocolitis in preterm infants · Reduce the risk of bleeding |
|
P. acidilactici |
· Exert antagonism action against pathogens by the production of lactic acid and bacteriocins, pediocins elimination of H. pylori infections and help combat viruses, fungi, and microbes
· Used in the treatment of constipation, diarrhea, relieving stress, enhancing immune response · Generate accelerated food decomposition and nutrient absorption, as well as more regular bowel movements and increased energy levels. · Prevent colonization of pathogens like Shigella, Salmonella, Clostridium difficile, and Escherichia coli in the small intestine · Regulate glucose readings and potentially aid in weight management and diabetes prevention over time. · Normalize mental stability by stimulating the presence of gamma-aminobutyric acid (or GABA, for short), a neurotransmitter responsible with coordination, stress management, pain, and anxiety receptors. |
| L. mesenteroides | · Intestinal injury and inflammation. By inhibits the activation of extracellular signal-regulated ½ and mitogen-activated protein (MAP) kinases, thus modulating host signaling pathways for protection against diarrhoeal diseases
· Treatment of travelers’ diarrhea, irritable bowel syndrome, ulcerative colitis, recurrent pseudomembrane colitis infection, acute gastroenteritis |
| · Produce acids, Leucoin, and bacteriocins, which reduce pathogens in ferments and in your body. | |
| B. coagulans (Lactobacillus sporogenes or "spore- forming lactic acid bacterium. ") | · Treatment of antibiotic-associated diarrhea, bacterial vaginosis
· Immunological support, increased immune response to a viral challenge, prevents respiratory infections. · Decrease Irritable bowel syndrome, Clostridium difficile colitis, Clostridium difficile colitis, abdominal pain, and bloating symptoms. · Also used to prevent cancer or the formation of cancer-causing agents. |
| E. coli | · Treatment of functional constipation in adults
· treatment of inflammatory bowel disease, gastrointestinal disorders · pro-inflammatory potential · reduction of salmonella enterica Typhimurium intestinal colonization by iron competition · Promote immune, digestive (produce various digestive enzymes), reproductive health |
3. Nutraceutical Enzymes: Enzymes are an essential part of life, without which our bodies would cease to function. Those people who are suffering from medical conditions such as hypoglycemia, blood sugar disorders, digestive problems, and obesity, eliminate the symptoms by enzyme supplements to their diet. These enzymes are derived from microbial, plant, and animal sources.
4. Prebiotics: Prebiotics” are a more recent addition to our vocabulary and are substances which when consumed are not digested by us. Instead, they act as a nutrient source for the good probiotic bacteria.
This encourages the probiotic bacteria to grow in a favourable environment, which in turn reduces the chances that harmful microorganisms may start to grow in our digestive tract. Inulin is prebiotic that has been widely used in processed foods. Essentially, it is a type of fiber obtained from the roots of plants such as chicory, Jerusalem artichoke and even dandelions 19.
Non-traditional Nutraceuticals: Artificial foods prepared with the help of biotechnology. They are arranged into.
- Fortified nutraceuticals.
- Recombinant nutraceuticals.
Fortified Nutraceuticals: They are enriched with vitamins, minerals, usually at a range up to 100 percent of the Dietary Reference Intake for that nutrient. It constitutes fortified food from agricultural breeding or added nutrients and/or ingredients added folic acid. Some examples are milk fortified with cholecalciferol used in vitamin D deficiency 20.
Recombinant Nutraceuticals: Preparation of various food materials by fermentation process such as cheese and bread to extract the enzyme which are useful for providing necessary nutrients at an optimum level. The production of probiotics and the extraction of bioactive components by enzyme/fermentation technologies as well as genetic engineering technology, are achieved through biotechnology.
Commercial Nutraceuticals: New molecule is difficult to discover and more expensive and risky than ever before. Many pharmaceutical companies are now trying to manufacture nutraceutical because there is undoubtedly a very huge and growing market. Nutraceuticals cover most of the therapeutic areas, like anti-arthritic, cold and cough, sleeping disorders, digestive disease and prevention of certain cancers, osteoporosis, disease related to cardiovascular like blood pressure, cholesterol control, and pain killers, depression, and diabetes. Recognition of health benefits from the consumption of omega-3 fatty acids rich seafood is one of the most promising developments in human nutrition and disease prevention research in the past three decades.
- Dietary supplements,
- Functional food,
- Medicinal food
Medicinal Food: Medicinal food a food which is formulated to be consumed or administered internally under the supervision of a physician and which is intended for the specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation also without any components that promote disease condition or contain a specific nutrient that the body cannot normally produce due to specific disease condition. It is prescribed by physicians for various health conditions that lead to impaired ingestion, digestion, absorption, or metabolism of traditional foods like phenyl-ketonuria, coeliac disease, and lactose intolerance 21.
Functional Foods: The term was first used in Japan in the 1980s .Functional foods are "any food or food ingredient that may provide a health benefit and disease prevention by adding new ingredients or more of existing ingredients. Consumed as part of a regular diet, Functional foods have been either enriched or fortified, and this process is called nitrification.
In Japan, All Functional Foods have Three Established Requirements:
- Food should be Present in their naturally occurring form, rather than a capsule, tablet, or powder.
- Functional food consumed in the diet as daily
- (Should regulate a biological process in hopes of preventing or controlling disease 22.
Dietary Fibers are of two Types:
- Water-insoluble fibers.
- Water-soluble fibers.
Daily recommended intake is 30-40 gms. Sources: Whole grain cereals, wheat products, Oats, dried beans, legumes.
Table 1: THE BEST HIGH-FIBER FOODS
| Fibrous Food | Content of Fiber (gms) |
| Split Peas | 16. 3 grams per cup |
| Lentil | 15. 6 grams per cup |
| Black Beans | 15 grams per cup |
| Lima Beans | 13. 2 grams per cup |
| Brussels Sprouts | 10. 3 grams per medium vegetable |
Antioxidants are of 3 Categories:
- True antiosxidants.
- Reducing agents.
- Antioxidant synergists.
Deficiency causes diseases like Cancers, rheumatoid arthritis, Alzheimer's disease, cardio-vascular diseases.
TABLE 2: EXAMPLES OF ANTIOXIDANT & THEIR SOURCES
| Antioxidant | Source |
| Vitamins | |
| Vitamin C | Citrus fruits, vegetables |
| Vitamin E | Grains, nuts, oils |
| Carotenoids | |
| Lycopene | Tomatoes |
| Beta carotene | Carrots, sweet potato |
| Xanthophylls | |
| Beta cryptoxanthin | Mango, papaya, oranges |
| Flavanoids | |
| Rutin | Tobacco, eucalyptus species |
| Luteolin | Lemon, red pepper, olive |
| Quercitin | Onion, apple skin, black grapes |
| Kaempferol | Grape fruit, tea |
| Liquiritin | Liquorice |
Lipids: Fats are highly energy sources for body.
- Saturated fatty acids.
- Monosaturated (MFA).
- Polyunsaturated (PUFA).
- Eicosapentaenoic acid -EPA (20:5 n-3).
- Docosahexaenoic acid - DHA (22:6 n-3).
- Saturated fats- animal based products.
- MFA&PUF plant origin.
Trans fatty acids are products of partial hydrogenation of PU fats and are typically solids at room temperature. MFA & PUFA do not promote the formation of fatty deposits that cannot clog the arteries.
Saturated Fatty Acids: Palmitic, lauric, myristic acids are major cholesterol-elevating fatty acids in our diets. Eskimos diet is rich in cholesterol and fat therefore they are free from heart diseases. Fish rich linolenic acid, found in fish + soyabean oils. Linoleic acid - corn, soy bean oils.
Linolenic Acid- Omega 3 fatty Acids: Linolenic acid (18:3 n-3) 18C, 3 double bonds, the 1st being at C-3 from the methyl end. CH3CH2CH = CHCH2CH = CHCH2CH = CH (CH2)7 COOH
Precursor of:
- Eicosapentaenoic acid -epa (20:5n-3)
- Docosahexaenoic acid - dha (22:6n-3)[23]
Challenges in Formulation of Nutraceutical Dosage Form:
Analytical Challenges
- The nutraceuticals are a cluster of a chemical entity, and it is comparatively difficult to identify and quantify all the ingredients in the products.
- Defining and identifying the impurities and ensuring that these impurities are not harmful to the consumer.
- Having a Structural Analysis of each entity in a formulation is difficult.
Formulation Challenges:
Tablet Dosage Form:
- Botanicals are complex with multiple chemical components, Can contain up to 50 active. Ingredients; 70- 90% of the formula can be actives.
- There are no of active ingredients and excipients
- Natural Nutraceutical Ingredients challenges which related to particle size, flow, compressibility, moisture sensitivity, in-gredient interaction, content uniformity and quality control (QC) parameter. Botanicals and extracts can vary which based on region the crop was grown, season grown in and other factors.
- Quantity of each ingredient to enable sufficient delivery of the beneficial ingredients, dose size of the active constituent is large hence very less space for excipients in the final formulation. -Nutraceutical formulations normally have more actives ingredient present in higher weights than pharmaceutical formulas. A typical nutraceutical formulation has 70–90% actives ingredient with the balance as excipients, whereas traditional pharmaceutical formulations have 70-90%
- Excipients and 10-30% active ingredient. The fewer excipients and variety of actives in the same formulation make it difficult to achieve certain desired outcomes, like disintegration time, hardness, and friability parameter.
- Careful design of the tablet shape and form needs to be considered when choosing suitable tooling-Adding to the challenge; many nutraceutical tablets tend to be produced using neutral colors such as browns and greys with mottled, textured or granular appearances, which can make any embossing difficult to read.
- The addition of natural ingredients in nutraceuticals, which have a tendency to be unrefined, abrasive, corrosive, and hard, which results in the utilize components damage during the process.
Liquid Dosage Form: Most of nutraceuticals are phytoconstituents, fatty acids, flavonoids, volatile oils etc., Problems faced by these ingredients are.
- Solubility of these ingredients. Example: carotenoids.
- Stability of these ingredients. Example: Coenzyme Q10, Omega 3 fatty acids. The oral delivery of probiotics is a slowdown by the low instability of the bacteria in the GIT and consequent of loss of viability under the effect of high acidity and bile salt concentrations.
- Bioavailability and permeability of these ingredients. Example: Curcumin. Even the bioavailability of the lipophilic antioxidant coenzyme Q10 was challenged by its.
- Low aqueous solubility and slow dissolution rate in GI fluids which furnished by its highly lipophilic character (log P=21).
- And permeability is limited by its large molecular weight (863),
- P-glycoprotein efflux and active transport by a number of transporters (including peptide transporters (PEPT1), cation/camitine tran-sporters (OCT1, OCTN1, OCTN2 and OCT3) and organic anion transporters (AE2 and MCTl) 24
Interactions:
- Active constituent and excipient interaction.
- Active constituent and Active constituent interaction.
- Processing challenges: Large variation in heat, light, and moisture sensitivity of ingredients within one formula. Example in Probiotic encapsulation technology Conditions that maintain cell viability like.
- Biomaterial selection-natural and synthetic polymers are used; factors to be addressed are:
- Physicochemical properties like chemical composition, morphology, mechanical strength, stability in GI fluids.
- Toxicity assay.
- Manufacturing and sterilization processes.
- Solvent type and.
- Toxicity and.
- Choice of proper technology.
Psychological Challenges: Nutraceuticals manu-facturers must first separate the products and treat nutraceuticals differently from functional foods.
- Tailoring products to domestic tastes and preferences. It includes vegetarian, Hindu dietary practices, traditional remedies, flavor and formulation preferences which reflecting social and cultural diversity.
- Choice of Study Population is difficult. (Based on age, disease condition ) 25
Regulatory Challenges:
- Need to furnish adequate information with scientific evidence to prove that the product is safe, reproducible, and therapeutically efficient and whether it offers such effects for a definite period of time, say two or three years.
- The need is to create a mechanism to prove that the product quality is reproducible, and this mechanism needs to be in place with solid, scientific support experimentally that can be proved using a reliable technique.
- Certification requirements often apply to excipients as well as active ingredients.
- GMO-Free
- Halal
- Kosher
- WADA Compliance (World Anti Doping Agency) country and product-specific
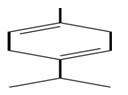
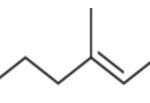
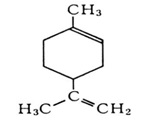
Gamma Terpinene
Registration Category / Classification:
- According to ingredients, the formula may fit into different categories by country.
- Registration complexity varies by category and country; dossier requirements vary greatly.
- Testing requirements for finished products, as well as ingredients and excipients, are not uniform.
Product Gamma Terpinene:
IUPAC Name: 1-Methyl-4-(1-methylethyl)-1, 4-cyclohexadiene.
Plant Sources:
- Cuminum cyminum.
- Melaleuca alternifolia.
- Cannabis sativa.
- Origanum syriacum.
Uses:
- Antibacterial, Antifungal, Analgesic, Anti-inflammatory, Antioxidant & spasmolyticis.
- Gyamma terpinene is a perfume and flavoring chemical used in the cosmetics and food industries.
- Its use in both the pharmaceutical and the electronics semi-conductor manufacturing industries has also proven to be valuable.
| Properties | |
| Chemical formula | C10H16 |
| Molar mass | 136. 24 g·mol−1 |
| Density | Γ: 0. 853 g/cm3 |
| Boiling point | Γ: 183 °C |
Formulation Still Now:
- Cold gel.
- Essential oils.
- Unasni kulzam.
TABLE 3: FORMULATION OF GYAMMA TERPINENE 26-32
| S.
no. |
Title of the Paper | Type of
Formulation |
Journal Name | Materials & Methods | Therapeutic Effect
Proposed |
Conclusion | Authors and Year
of Publications |
| 1 | Study of the composition of Thymus vulgaris essential oil, developing of topic formulations and evaluation of antimicrobial efficacy | Cream gel | Journal of Medicinal Plants Research | Extraction of the essential oil of T. Vulgaris, Determining compounds in the oil of T. Vulgaris, Antimicrobial activity, Disk diffusion test | Antimicrobial | The essential oil obtained presented as majority components geraniol, thymol, gama-terpinene, para-cymene, citral, 3-octanone, and 3- octenol. Thus, the essential oil should be used in formulations at a concentration of at least 4. 5% to produce effective antimicrobial activity against the three strains. The cream gel formulation containing essential oil of T. vulgaris is a promising alternative for cosmetic and phytotherapeutic use. It is not possible to state that the formulations are absolutely stable. Therefore, after some adjustments to improve stability, the formulation could be used as an ally in the fight against topical infections. However, like all antimicrobial agents, it must be used with care to avoid increasing the number of
strains resistant to therapeutic agents. |
Gisele Mara Silva Gonçalves
(2015) |
| 2 | The Use of Two New Formulations of Ocimum Canum Sims And Cymbopogon Schoenanthus L. In The Control of Amitermes Evuncifer Silvestri (Termitidae:
Termitinae), In Togo |
Mixture of Essential oils | International Journal of Natural Sciences Research | Extraction, Analysis by GC-MS, Statistical analysis | Biopesticide | The results of this study showed that the formulations from the essential oils of C. schoenantus and O. canum mixed with starch possessed some toxic properties on workers of A. evuncifer at low concentrations (0. 5 mg/cm² and 1 mg/cm²). At 2 mg/cm² a total mortality of 100% was recorded. A survey of the persistence of the formulations needs to be carried out in order to determine how longthe product remains effective after field application. Following this survey, new formulations of essential oils with starch could
Potentially be used as biopesticide against pestiferous insects. |
Nyamador Wolali Seth (2014) |
|
3 |
Formulation and Evaluation of Mucoadhesive Anti Infective Solution Containing Solubilised Tea Tree Oil for Vaginal Infections. |
Cold gel |
International journal of advances in pharmacy, Biology and chemistry |
Evaluation studies |
Anti-infective mucoadhesive |
The present research work indicated that a stable aqueous mucoadhesive system containing antiinfective essential oils like tea tree oil and dragosantol oil in a solubilised form and a mucoadhesive like Poloxamer 407 can be formulated using a synergistic approach of solubilisation by Cold gel method and cosolvency. The resultant anti- infective solution can be used as an efficient bacteriostatic as well as aid to balance the fluctuated vaginal pH in conditions like vaginosis,
vaginitis, candidiasis etc without adversely affecting the inherent microenvironment of the vagina. |
H. Desai ⃰, A. Sav and P. Amin (2013) |
|
4 |
Determination of Chemical Composition of Essential Oil Portion of Reputed Marketed Unani Formulation Zinda Tilismath |
Essential oils |
International Journal of Pharmacy and Pharmaceutical Sciences |
GC-MS Analysis |
Antibacterial, Antifungal, Analgesic, Anti‐inflammatory, Antioxidant & Spasmolyticis | The outcome of this study is essential oil portion of the Zinda Tilismath contain terpenes and their oxygenated derivatives, which are believed to be highly effective antibacterial, antifungal, analgesic, anti‐inflammatory, immunomodulatory, antioxidant & spasmolyticis.
The eight major compounds of the formulation can be regularly be checked for their detection in routine quality control of this herbal formulation by GC‐MS technique |
K. Ashok Kumar 2011 |
|
5 |
Preparation and Characterization of Liposomes Containing Essential Oil
of Eucalyptus camaldulensis Leaf |
Liposomes |
Jundishapur Journal of Natural Pharmaceutical Products |
The leaf of E. Camaldulensis, GC- MS Analysis of Essential Oil |
Antimicrobial |
Liposomal gel formulation of the essential oil may lead to improved antifungal activity. |
Eskandar Moghimipour (2012) |
|
6 |
Determination of antibacterial, antifungal activity and chemical composition of essential oil portion of unani formulation
kulzam |
Unani Kulzam (Aromatic Oil) |
International Journal of Green Pharmacy |
The formulation was subjected to antibacterial, antifungal studies and was carried out by agar cup plate method. |
Antibacterial and Antifungal |
The kulzam exhibited strong in vitro inhibition of growth against all the test microorganisms at both 100 and 150 µl levels of undiluted formulation (test sample). It also draws attention that, gram-negative micro-organism are more susceptible to inhibitory action than gram- positive organisms. |
K. Ashok Kumar, Ram Kumar Choudhary (2011) |
|
7 |
The Development of Anti-Acne Products From Eucalyptus Globulus And Psidium Guajava Oil |
Oil in water Cream |
Journal Health Resources |
agar Diffusion and micro- dilution methods. |
Anti-acne |
Both eucalyptus and guava oil creams showed good texture and have proper pH to be used topically. After stored under freeze thaw condition, phase separation was not observed.
Their efficacy was decreased after stored under accelerated conditions (-4° C, 45° C, freeze thawing) |
Sirivan Athikomkulcha, and et al (2008) |
Product Name:
Lycopene IUPAC Name: 2, 6, 10, 14, 19, 23, 27, 31 – Octamethyldotriaconta - 2, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 30 – tridecaene.
Lycopene
Biological Source: Lycopene from the neo-latin lycopersicum, the tomato species.
Other Sources: Carrots, watermelons, gac and papayas, although not in strawberries, or cherries.
Uses:
- Treatment of the leukopkia (oral cancer),
- Anticancer and antidiabtic activity,
- Antioxidant action
Formulation Still Now:
- Mucoadhesive film
- Noisome
- Emulgel
- Osmotical control capsule
- Powder (confectionary)
- Microemulsion
| Properties | |
| Chemical formula | C40H56 |
| Molar mass | 536. 89 g·mol−1 |
| Appearance | Deep red solid |
| Density | 0. 889 g/cm3 |
| Melting point | 172–173 °C (342–343 °F; 445–446 K) |
| Boiling point | 660. 9 °C (1, 221. 6 °F; 934. 0 K) at 760 mmhg[1] |
| Solubility in water | Insoluble |
TABLE 4: FORMULATION OF LYCOPENE: 33-38
| S.
no. |
Title of the Paper | Type of Formulation | Journal Name | Material and Method used | Therapeutic Effect Proposed | Conclusion | Author and Year of Publication |
| 1 | Novel encapsulation of Lycopene in noisome & assessment of its anticancer activity | Niosome | Journal o bio- equivalanc and bio avaliblity | Lycopene, span60, cholesterol, n-hexane ethanol, acetone, cisplatin
Method –adsorption hydration technique |
Anticancer and antidiabtic activity | The lycopene niosome formulation prepared by adsorptionhydration method was found to be efficient and has preserved the lycopene’s activity. This method promises to be a novel technique for enhancing entrapment efficiency by niosome formulation. The formulated nano-niosomes have potential to play a vital role in efficient herbal delivery of a broad spectrum of anticancer agents. The technique is simple and reproducible for further application, and could be
Useful for different therapeutic applications. |
Sharma PK*, and et al, 2016 |
| 2 | Formulation and evalution of Lycopene Emulgel | Emulgel | Indo American journal of pharmaceuticle sciences | Lycopene, carbopol, 934p, Na cmc, HPMC, LV 15, SPAN 20, SPAN
80, triethanolamine, methyl paraben, Method -addition of emulsion agent to gelling agent |
Antioxidant effect |
This work was conducted to develop an emulgel of lycopene using three different gelling agents i. e. Carbopol 934P, HPMC LV-15 and NaCMC.
Oleic acid was used as a penetration enhancer. The gellified emulsions were characterized for their physical appearance, rheology, spreadability, drug content and stability. In-vitro release studies were conducted to check the drug release through egg membrane. Formulation F1 was found to have fallen within the stipulated criteria of all the evaluation parameters. Hence, it was concluded that formulation F1, containing carbopol 934P (1% w/w), was the optimized formulation. It exhibited the maximum drug release and antioxidant activity, in addition to the least skin irritation potential. |
A. Kumari, and et al, 2015, |
|
3 |
Development and Optimization of Osmotically Controlled Asymmetric Membrane Capsules for Delivery of Solid Dispersion of
Lycopene |
Osmotical control capsule |
Scientific world journal |
Nacl, acetone, ethanol, glycerol, ethyl cellulose, Method –Dip coating method of assmetric mem. Capsule |
Antioxidant effect |
Asymmetric membrane capsule for the solid dispersion of lycopene with 𝛽-cyclodextrin was prapared using dip coating method and optimized using central composite design, design method proves to determine influence of formulation factors on drug release pattern. |
Nitin Jain, Rashni Sareen, Neeray Mohin, K. L Dhar, 2014 |
|
Fresh tomato, sugar, butter milk, Mehod –prepration of the fudge |
Three variations of tomato fudge | ||||||
| namely TC-1, TC-2 and TC-3 were | |||||||
| tested for sensory quality, consumer | |||||||
| acceptability, antioxidant activity | |||||||
| Development & | and microbial load determination. | ||||||
|
4 |
evaluation if the
antioxidant activity of tomato based |
Powder(confectionary) |
Internationa food journal | Antioxidant activity | Among the three samples TC-1 was
found best acceptable based on sensory scores. TC1 & TC2 Contains |
Soma. s, 2013. | |
| confectionary | 1 & 3gm of tomato powder | ||||||
| respectively. | |||||||
| Lower sensory scores states that | |||||||
| level more than 3gm is not | |||||||
|
5 |
Topical delivery of Lycopene using micro-emulsion |
Microemulsion |
Willay Science journal |
PEG, Brij 97, Capric acid, amm. acetate Method – purification and extraction |
Antioxidant activity |
Lycopene was incorporated (0. 05%, w/w) in two microemulsions containing BRIJ-propylene glycol (2:1, w/w, surfactant blend) but different oil phases: mono/diglycerides of capric and caprylic acids (MG) or triglycerides of the same fatty acids (TG). the antioxidant activity of skin treated with MG-containing microemulsion was determined by CUPRAC assay, and found to be 10-fold higher than untreated skin. These results demonstrate that the MG-containing microemulsion is an efficient and safe system to increase lycopene
delivery to the skin and the antioxidant activity in the tissue. |
Luciana B. Lope Hillary Vande, Vijay Venugopal, Stanay 2010. |
|
6 |
Formulation of water soluble mucoadhesive film of lycopene |
Mucoadhesive |
Intrrnational journational journal of pharmaceuticle science and research |
Lycopene PEG400, carbopol 934, giycerine, isoprpyl achhol, propylene glycol Method 1)using vehicle 2)using surfactant |
Treatment of the leukopkia (oral cancer) |
The main advantage of this formulation is that it contains a less drug dose, provides effect as it is located directly on the site of the patch, The film has high mucoadhesion force, and thus not easily remove from site by tongue. The time required to dissolve is also high compare to other formulations and thus, the concentration of
lycopene can be achieved in higher amount. |
Shah Divyen gaud R. S, Mishra A. Nparkin Rima 2010. |
Aloein
- Product name:
Aloein Biological Sources: Scientific names given to include.
- Aloe perryi,
- Barbadensis,
- Ferox, and
- Hybrids of A. ferox with A. africana and A. spicata.
Uses: It is used as a stimulant-laxative, Treating constipation by inducing bowel movements.
Formulations till now:
- Chewing gum
- Gel
- Gel powder
- Suppositories
- Cosmetic herbal hydrogel
| Properties | |
| Formula | C21H22O9 |
| Molar mass | 418. 39 |
| Melting point | 148 °C (298 °F) (70–80 °C for monohydrate) |
TABLE 5: FORMULATIONS OF ALOEIN 39-44
| S. no. | Title of Paper | Type of formulation | Journal name | Material and method | Therapeutic effect | Conclusion | Author and journal with year of
published |
||
| 1. | Design, formulation and evaluation of Aloe Vera chewing gum. | Chewing gum | Journal of Advanced biomedical research | Aloe vera powder, sugar, liquid glucose, glycerin, sweeteners der Latin square method | Antioxidant, anti- inflammatory, healing, antiseptic, anticancer and antidiabetic effects mouth abscesses as well as
reducing mouth dryness caused by chemotherapy. |
the best formulation considering the organoleptic properties was F16 formulation. Based on the views of participants, from six flavors which tested at first mint and cinnamon were selected as better flavors and
in next stage between these two mint was chosen as the best flavoring agent. |
Abolfazl Aslani, Alireza Ghannadi 2015 | ||
|
2. |
Formulation Design of Micronized Silver Sulfadiazine Containing Aloe vera Gel for Wound Healing |
Gel |
Benthamscience |
Silver sulfadiazine, Aloe vera, Spreadability, Viscosity, Wound healing. Aloe Vera Gel Extraction |
SSD is one of the most widely used topical antibacterial agents for the treatment of burns. |
MSSD containing AV-gel showed enhanced antibacterial activity in opposition to pathogens commonly invading burn wounds, and also exhibited excellent potential for more rapid burn wound healing which may decrease the trauma of the patients. SSD is one of the most widely used topical antibacterial agents for the treatment of burns. It has confirmed deleterious effects on
burn wound healing (wound healing retardant). |
Farhan J. Ahmad1* And et al 2016 |
||
|
3. |
Formulation And Evaluation Of Hydrogel With Ascorbic Acid Using Aloe Vera Gel Powder As A Drug Carrier |
Gel powder |
Innovare journal of sciences |
ascorbic acid, poly vinyl pyrrolidone, gelatin, starchaloe vera gel powder, distilled water, Preparation of hydrogel with drug by chemical cross-linking method |
Approach to deliver antioxidants in a controlled manner Bio-available, bio- compatible with non- toxicity. |
Ascorbic acid hydrogel preparation represents a feasible and productive approach to deliver antioxidants in a controlled manner. Polymers with desired hydrophilicity and hydrophobicity can be chosen to impart the desirable dissolution and drug release patterns in the present study. In addition, the materials used in the hydrogel’s preparation are bio-available, bio- compatible with non-toxicity. From the results it can be clearly concluded that the diffusion of ascorbic acid from the hydrogel
has gradually increased with respect to time suggesting that the drug is released at a pre- |
Suseem S R, Ojhakhyati, Shenoyvranda, 2013. |
||
|
4 |
Formulation evaluation and in-vitro drug release characteristics of aloe vera herbal suppositories |
Suppositories |
Scholars Research Library |
Extract of Aloe Vera was done by soxhlet using methanol as solvent. Heat molding method was used for the preparation of suppositories |
Laxative |
All five formulations showed more than 50% drug release within 25min. This is due to the addition of Tween 80 in the formulation.
Based on the in- vitro release rate studies, it can be concluded that polyethylene glycol 4000 can be used as a base which were easily soluble in aqueous medium, disperses rapidly and has higher rate of release for immediate release of aloe Vera herbal suppositories. |
Tarkase K. N. And Danve A. V. *2015. |
||
|
5 |
Formulation and characterization of Aloe vera cosmetic herbal hydrogel |
Cosmetic herbal hydrogel |
International Journal of Pharmacy and Pharmaceutical Sciences |
Aloe vera liquid was prepared by heating at low temperature and the hydrogel was prepared by simple dissolving method of other ingredients in a specific manner. |
Cosmetic purpose |
pH of all the formulations were adjusted 6±0.
Next day pH was again observed which was found to be between 6. 2 to 6. 4. All the formulations contained 1% w/v preservatives e. potassium sorbate and sodium benzoate. Studies were performed for microbial growth using nutrient agar and none of the petriplates showed microbial colony even two weeks incubation. |
Yogesh pounikar* and et al, 2012 |
||
4. Product Name:
Safranal Biological Source: Is a spice derived from the flower of Crocus sativus, commonly known as the "saffron crocus".
IUPAC Names: 2, 6, 6-trimethyl-1, 3-cyclohexadiene-1-carboxaldehyde.
Natural Sources:
- Microcystis (Cyanobacterium)
- Aspalathus linearis (Rooibos)
- Camellia sinensis (Tealeaf)
- Crocus sativus (Saffron)
- Ficus carica (Fig leaf)
- Lycium chinense (Wolfberry)
- Cuminum cyminum (Cumin Seed)
- Centaurea sibthorpii
- Centaurea amanicola
- Centaurea consanguinea
- Erodium cicutarium (common stork's-bill or pinweed)
- Calycopteris floribunda (Ukshi)
- Sambucus nigra (elderberry)
- Citrus limon (lemon)
| Properties | |
| Chemical formula | C10H14O |
| Molar mass | 150. 21 g/mole |
| Density | 0. 9734 g/cm3 |
| Boiling point | 70 °C (158 °F; 343 K) at 1 mmhg |
Safranal
TABLE 6: FORMULATIONS OF SAFRANAL 44-48
| S. no. | Title of Paper | Formulation | Journal Name | Materials and Methods | Therpeutic Activity | Conclusion | Authors and Year of Publication |
|
1 |
Safranal-loaded solid lipid nanoparticle |
Sunscreen lotion |
Iranian journal of basic medical sciences |
Glyceryl monostearate, tween 80 and different amt of safrana with high shear homogenizers |
Skin protection |
The Sun protection factor of SLN-safranal formulations was increased when the amount of safranal increased. Mean particle size for all formulas was approximately 106 nm by probe sonication and 233 nm using High pressure homogenization method. The encapsulation efficiency of
safranal was around 70% for all SLN-safranal formulations |
Bahman Khameneh, Vahid Halimi and et al, 2015 |
|
2 |
Development of safranalniosomal in-situ nasal gel formulation |
Niosomal in-situ nasal gel |
World journal of pharmaceutical research | Niosomes are prepared usind surfactants in different ratio with chlorestero. l nasal gel was formulated using surface response
factorial method |
Nasal decongestion |
Then to increase resident time of formulation in the nasal cavity optimized niosomal formulation further formulated in to in-situ nasal gel using surface response factorial Method, gel concentration (pluronic F127: gelrite, (17. 3:0. 07) | Dr. Chaudhari shilpa p., BhandurgeNitin, and et al, 2015. |
|
3 |
Preparation, characterization & evaluation of sun protective &moisturizing
effects of nanoliposomes containing safranal |
Nanoliposome containingsafranal sunscreen lotion |
Iranian journal of basic medical sciences | Nanoliposomes were prepared using 0. 25, 0. 5, 1, 2, 4, 8% of safranal and nl were prepared using fusion method and homogenization. | Sun protective & moisturizing effect | The SPF of liposomes containing 8% safranal (Lip-Safranal 8%) was significantly higher than 8% homosalate reference. These results showed that in equal concentrations, Lip-Safranal
could act as a better antisolar agent compared to homosalate and have no moisturizing effect in 1 and 4% concentrations. |
ShivaGolmohamm ad
zadeh et al 2011 |
|
4 |
Microencapsulation of saffron (crocus sativus l.) Extract in copolymer complexes using extrusion
method. |
Microencapsulation |
Chiang mai university journal of natural
sciences |
Copolymers such as chitosan and alginate were used. Extrusion method was employed for microencapsulation |
Preserving saffron components |
The results clearly indicated that, in combination with alginate- chitosan was a better copolymer than gelatin for encapsulating saffron components. |
Pooriashakoori and WunwisaKrasaeko opt*
2015. |
|
5 |
Characterization & anti- tumor activity of pegylated nanoliposomes containing safranal in mice bearing c26
colon carcinoma |
Nanoliposome of safranal |
International journal of pharmaceutical sciences and
research |
They were prepared by using homogenization process |
Anti-tumor activity |
Results indicated that the current safranal liposomes could increase the in vitro cytotoxicity, however did not enhance the antitumor activity at a dose of 50 mg/kg, due to the physicochemical properties and dose dependent effects of
safranal molecules, And low encapsulation in liposomes. |
Mahmoud R. Jaafari* et al, 2016 |
β- Carotene
5. Product Name:
Beta-carotene Natural Sources:
- Yellow-orange, Green leafy fruits Vegetables (such as carrots, spinach, lettuce, tomatoes, sweet potatoes, broccoli, Cantaloupe, and winter squash).
- In general, the more intense the color of the fruit or vegetable the more beta-carotene it has.
IUPAC Name: 1, 3, 3-Trimethyl-2-[3, 7, 12, 16-tetramethyl-18-(2, 6, 6-trimethylcyclohex-1-en-1-yl) octadeca-1, 3, 5, 7, 9, 11, 13, 15, 17-nonaen-1-yl] cyclohex-1-ene
Uses: Beta-carotene is an antioxidant.
Therapeutic Uses: Prevention of heart disease or cancer.
- Treatment of Sun Sensitivity,
- Age-related Macular Degeneration,
- Metabolic Syndrome,
- Oral leukoplakia,
- Scleroderma
| Properties | |
| Chemical formula | C40H56 |
| Molar mass | 536. 89 g·mol−1 |
| Appearance | Dark orange crystals |
| Density | 0. 941 g/cm3[2] |
| Melting point | 176–184 °C (349–363 °F; 449–457 K)
decomposes[2][4] |
| Boiling point | 654. 7 °C (1, 210. 5 °F; 927. 9 K) at 760 mmhg |
| Solubility in water | Insoluble |
| Solubility | Soluble in CS2, benzene, CHCl3, ethanol
Insoluble in glycerin |
TABLE 7: FORMULATIONS OF β-CAROTENE 49-53
| S.
no. |
Title of the paper | Type of
formulation |
Journal name | Materials &
methods |
Therapeutic
effect proposed |
Conclusion | Authors and year
of publications |
|
|
1 |
Development of slow release formulations of β- carotene employing amphiphilic polymers and their release kinetics study in water and different ph
conditions |
Nanosphere |
Journal of food science and technology | Analysis of β- carotene by hplc Synthesis of amphiphilic copolymers | Potent Antioxidant | The release kinetics of β-carotene from developed formulations in water revealed increased solubility and prolonged stability of β- carotene. the release of β-carotene was high at pH 7. 8 and slightly
higher at pH 6. 8. |
Braj Bhushan Singh, and et al, 2015. |
|
|
2 |
Characterization and chemical stability evaluation of β-carotene microemulsions prepared by spontaneous emulsification method using vco and palm oil as oil phase |
Microemulsions |
International food research journal |
Characterization of β- carotene microemulsions Chemical stability evaluation of β- carotene loaded microemulsions |
Prevention of cardiovascular diseases, cancer, and immune system enhancer |
β-carotene loaded in palm oil microemulsions were more stable toward chemical degradation during storage rather than those loaded in VCO microemulsions. In order to minimize β-carotene degradation, the VCO microemulsions must be stored at temperature not more than 4ºC, whereas the palm oil microemulsions could be stored at
15ºC. |
Ariviani, S., Anggrahini, S., Naruki, S. And *Raharjo, S., (2015) |
|
|
3 |
-bSNEDDS (self- nanoemulsifying drug delivery system) formulation of carotene in olive oil (olea europaea) |
Self nanoemulsifying drug delivery system |
International journal of advanced research |
Optimization by simplex lattice design, |
Prevent degenerative diseases such as cardiovascular, cancer, neurodegenerative, autoimmune
diseases, |
-carotene with concentration of 3 mg/g can be formulated with ratio of 9. 860 %: 80. 280 %:bSNEDDS
of 9. 860 % or 1:8, 1:1 olive oil, Tween 80 and PEG 400, respectively. SNEDDS can produce nanoemulsion in 24. 47 ± 0. 906 seconds after contacting |
Erna Wulandari, Adella Clara Alverina and et al, 2016. |
|
| rheumatoid arthritis, cataract and aging | with artificial gastric fluid with 91. 17 ± 0. 45 % transmittance, sufficient stability at gastric fluid for 4 hours, average droplet size
42. 6 nm with a polydispersity index 0. 608 and zeta potential value -38. 7 mV |
|||||||
|
4 |
Efficacy of beta-carotene topical application in melasma – an
open clinical tria |
Topical cream |
Indian journal of dermatology, venereology, and leprology | Open clinical trial
By topical application in melasma |
Effective and safe for treatment of melasma. |
To conclude, beta-carotene in nanothalospheres appears to be an effective drug added to armamentorium of fight against melasma with minimal side effects. Long duration of treatment
is associated with better result. |
Kar hk 2003 |
|
|
5 |
Assessment and degradation study of total carotenoid and ß-carotene in bitter yellow cassava (manihot esculenta crantz) varieties |
- |
African journal of food science |
The assessment of the variability of total carotenoid, ß- carotene, all-e, and 13 and 9-z-ß-carotene isomers in twelve bitter yellow cassava was carried out,
Hplc and uv/ visible spectrophotometry were used in sample analyses |
Potential antioxident |
On account of the results we may presume that other factors influenced the total carotenoid degradation such as package permeability to oxygen since the samples had not been wrapped up under vacuum, maintenance of the samples under refrigeration, and temperature of the storage room. The total carotenoid degradation in yellow bitter cassava flour was completed between the 12th and 19th days of storage in four of the
five analyzed varieties. |
R. G. Alcides oliveira1, m. J. Lucia de carvalho1 *, and et al, 2010 |
|
6. Product Name:
Lutein Biological Source: Lutein is synthesized only by plants, and, like other xanthophylls is found in high quantities in green leafy vegetables such as spinach, kale, and yellow carrots.
IUPAC Name: βε-carotene-3, 3'-diol.
Uses: Many people think of lutein as “the eye vitamin. They use it to prevent eye diseases, including age-related macular degeneration (AMD), cataracts, and retinitis pigmentosa.
Formulations Still Now:
- Lutein nanosuspension converted into pellets and filled into hard gelatin capsules.
- Matrix beadlet
- Cream
- Nanoemulsion
- Lutein softgel
- Self-emulsifying phospholipid suspension
Lutein
| Properties | |
| Chemical formula | C40H56O2 |
| Molar mass | 568. 871 g/mol |
| Appearance | Red-orange crystalline solid |
| Melting point | 190 °C (374 °F; 463 K) |
| Solubility in water | Insoluble |
| Solubility in fats | Soluble |
TABLE 8: FORMULATIONS OF LUTEIN 54-58
| S.
no. |
Title of the Paper |
Type of Formulation |
Journal Name |
Materials & Methods | Therapeutic Effect Proposed | Conclusion | Authors and Year of
Publications |
| 1 | Lutein nanocrystals as antioxidant formulation for oral and dermal delivery | Lutein nanosuspension converted into pellets and filled into hard gelatin capsules | International journal of pharmaceutics | Saturation solubility, Dissolution velocity, Dermal penetration | Antioxidant | A pronounced increase in saturation solubility by 26. 3 fold was obtained for lutein nanocrystals compared to a coarse powder. The lutein nanosuspension was converted into pellets and filled into hard gelatin capsules for nutraceutical use, showed a
superior in vitro release |
Khalil
Mitri Ranjita Shegokar, And et al, 2011 |
|
2 |
Effects of formulation on the bioavailability of lutein And zeaxanthin: a randomized, double-blind, cross-over, Comparative, single-dose study in healthy subjects |
Matrix beadlet |
European journal of nutrition |
Healthy volunteers were randomized Into double-
blind, cross-over study investigating the Plasma kinetics of lutein provided as two different beadlet Formulations. |
Antioxidant |
The current study was designed to assess the effect of different formulation technologies on the bioavailability profile of lutein and zeaxanthin after single oral doses of two comparative test articles, both of which contained lutein and zeaxanthin, specifically in a
starch-based or in an alginate-based matrix. Starch matrix beadlet demonstrated greater bioavailability than Alginate matrix beadlet. |
Malkanthi Evans, and et al 2013. |
|
3 |
Formulation and in vitro evaluation for sun protection factor of lutein ester extracted from tagetes erecta linn flower (family- asteraceae) sunscreen
creams |
Cream |
Research journal of pharmaceutical, biological and chemical sciences |
Lutein ester(flowers of tagetes erecta) In vitro sun protection factor (spf) by colipa method |
Sunscreen activity |
This method has thus helped to determine the SPF value of a novel drug-like Tagetes erecta L. (Asteraceae) and stating that it has good sunscreen activity and can be considered as active sunscreen agent or can be incorporated into other sunscreen formulations as an
additive to enhance the activity |
Shantanu kale*, Snehal Bhandare, Megha Gaikwad 2011 |
|
4 |
Lutein absorption is facilitated with cosupplementation of ascorbic acid in young adults |
Lutein softgel |
Journal of the american dietetic association |
Evaluate the bioavailability of crystalline lutein supplements and compare lutein uptake and clearance in humans
simultaneously |
Antioxidant |
lutein is absorbed faster with simultaneous supplementation of vitamin C ( P </=. 026). In conclusion, the bioavailability of crystalline lutein from supplements varies greatly both within and between subjects and therefore reformulation should be
considered. |
Sherry S. Tanumihardjo, and et al 2005, |
|
5 |
Enhanced bioavailability and retinal accumulation of lutein from self- emulsifying phospholipid suspension (seps) |
. Self-emulsifying phospholipid suspension |
International journal of pharmaceutics |
--- |
Prevention of ocular diseases |
This enhancement was about 16. 1 folds and 4. 27 folds compared to placebo and CF, respectively. The relative BA study in dogs and retinal accumulation study in rats demonstrated the excellent ability of SEPS to enhance the BA of
lutein |
Srinivasan Shanmugam, and et al, 2011, |
7. Product Name:
Caryophyllene Biological Source: Caryophyllene or (−)-β-caryophyllene, is a natural bicyclic sesquiterpene that is a constituent of many essential oils, especially clove oil, the oil from the stems and flowers of Syzygium aromaticum (cloves), the essential oil of Cannabis sativa, rosemary.
IUPAC Name: (1R, 4E, 9S)-4, 11, 11-Trimethyl-8-methylidenebicyclo [7. 2. 0] undec-4-ene.
Natural Sources:
- Cannabis, hemp, marijuana (Cannabis sativa)
- Black caraway (Carum nigrum)
- Cloves (Syzygium aromaticum)
- Hops (Humulus lupulus) Oregano (Origanum vulgare)
- Black pepper (Piper nigrum) Lavender (Lavandula angustifolia)
- Rosemary (Rosmarinus officinalis)
- Malabathrum (Cinnamomum tamala)
- Ylang-ylang (Cananga odorata)
- Copaiba oil (Copaifera spp.)
Caryophyllene
| Properties | |
| Chemical formula | C15H24 |
| Molar mass | 204. 36 g·mol−1 |
| Density | 0. 9052 g/cm3 (17 °C) |
| Boiling point | 254–257 °C (489–495 °F; 527–530 K) |
TABLE 9: FORMULATIONS OF CARYOPHYLLENE 59-61
| S. no. | Title of Paper | Formulation Type | Journal Name | Materials & Methods | Therapeutic Effect | Conclusion | Authors and Year of Publication | |
| 1 | A Semiochemical Slow- release Formulation in a Biological Control Approach to Attract Hoverflies | Alginate gel beads | Journal of Environment and Ecology | Semiochemical diffusion from beads was studied in the laboratory according to abiotic parameters | Potential biological control tool to attract aphid predators. | Alginate beads proved their effectiveness as semiochemical slow-release systems on field experiments despite their limitation of use
at high relative humidity. |
Stephanie Heuskin, Stéphanie Lorge, Georges Lognay, and et al,
(2012) |
|
|
2 |
Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems |
Microemulsion |
International Journal of Cosmetic Science |
The solubilization behaviour of a number of essential oils (EOs) containing volatile phenolic constituents was investigated in five different micellar solutions. |
stability of some phenolic-bearing essential oils formulated in different microemulsion systems |
Results showed that Tween 20 (T20) was more suitable to solubilize these oils compared with Tween 80 (T80). Clove EO was found to be easily microemulsifiable compared with the other EOs, whereas oregano showed the least
tendency to form a microemulsion. |
A. E. Edris,
C. F. R. Malone 2012 |
|
|
3 |
Use of β-caryophyllene to combat bacterial dental plaque formation in dogs |
Topical solution |
BMC Veterinary Research |
To evaluate the antimicrobial activity of β- caryophyllene against bacteria from dog’s dental plaque in vitro and in vivo agar microdilution assay, the induction or inhibition of bacterial adherence by sub- inhibitory concentrations in
96-well plates |
antimicrobial activity |
β-caryophyllene has antimicrobial activity against the proliferation of dog’s dental plaque-forming bacteria representing a suitable alternative to the use of chlorhexidine in prophylaxis and treatment of periodontal disease of dogs. |
Fábio Alessandro Pieri and etal (2016) |
|
|
4 |
Essential Oil Composition and Antibacterial Studies of Vitex negundo Linn.
Extracts |
Essential Oil |
Indian Journal of Pharmaceutical Sciences |
GC-FID and GC/MS techniques |
Antibacterial potential |
Fruits and leaves oil were found to be most active against E. coli and S. aureus, respectively. Only flowers
oil was found to be active against P. aeruginosa. |
S. L. Khokra*, and et al., 2008. |
|
8. Product Name:
Pinene Biological Source: Alpha-pinene appears in conifers and numerous other plants. Pinene is a major component of the essential oils of Sideritis spp. (ironwort) and Salvia spp. (sage). Cannabis also contains alpha-pinene. Resin from Pistacia terebinthus is rich in pinene.
IUPAC Name: (1S, 5S)-2, 6, 6-trimethylbicyclo 3, 1, 1 hept-2-ene.
Uses:
- Anaesthetic, antifungal, antiseptic and antibacterial.
- In the chemical industry, selective oxidation of pinene with some catalysts gives many compounds for perfumery
- Pinenes are the primary constituents of turpentine.
- Pinene has also been used as an anti-cancer agent in Traditional Chinese medicine,
- Also for its anti-inflammatory, antiseptic, expectorant and bronchodilator properties.
Formulations till Now:
- Unani formulation zinda tilismath.
- Essential oil.
- Conventional insecticide.
| Properties | |
| Chemical formula | C10H61 |
| Molar mass | 136. 24 g/mol |
| Appearance | Liquid |
| Density | 0. 86 g. cm-3 (alpha, 15 degree c) |
| Melting point | -62 to -55 degree c |
| Boiling point | 155 to 156-degree c |
| Solubility in water | Practically insoluble in water |
| Vapor pressure | 1. 0 kPa |
Pinene
TABLE 10: FORMULATIONS OF PINENE 62-67
| S.
no. |
Title of the Paper | Type of Formulation | Journal Name | Materials & Methods | Therapeutic Effect Proposed | Conclusion | Authors and Year of Ppublications | ||
| 1 | Determination of chemical composition of essential oil portion of reputed marketed unani formulation
zinda tilismath |
Unani formulation zinda tilismath | International journal of pharmacy and pharmaceutical sciences |
Chemical analysis by gc/ms |
Antibacterial, antifungal, analgesic, anti‐inflammator y | Eight compounds constituting about 90. 58% of the essential oil were identified. The main components were L‐limonene, Tetradecane, Decane, Isoborneal, camphor, Terpane, Cymol & Alpha‐pinene | K. Ashok kumar*, and et al, 2011. | ||
|
2 |
Biological activities of
- pinene and β-pinene enantiomers |
-- |
molecular diversity preservation international (mdpi). | Inhibition of microbial phospholipase and esterase activities In vitro biofilm susceptibility assay Time-kill curves | Antimicrobial | The potential of (+)-α-pinene and (+)-β- pinene to inhibit phospholipase and esterase activities was also evaluated, and the best inhibition results were obtained with Cryptococcus neoformans. C. albicans biofilm formation was prevented with the MIC concentration of (+)-α-pinene and twice the MIC value of (+)-β-pinene. | Daniela sales alviano * & etal
2012 |
||
| 3 | Chemical composition, antioxidant and antimicrobial activities of essential oil from wedelia prostrate | Essential oil | experimental and clinical sciences, international online journal | Gc–ms analysis, Antioxidant activity determination, Inhibitory effect via the disc diffusion method | Antioxidant activity, antimicrobial activity | The activities of limonene and α-pinene were also determined as main components of the oil. α-Pinene showed higher antimicrobial activity than the essential oil with a diameter of zones of inhibition (20. 7 to 22. 3 mm) and MIC values (62. 5 to 125 µg/ml). The antioxidant and antimicrobial properties of the essential oil
may be attributed to the synergistic effects of its diverse major and minor components. |
Jiali dai, liang zhu*, li yang, jun qiu.
2013 |
||
| 4 | Pharmacognosy of
pinus roxburghii: a review |
-- |
Journal of
pharmacognosy and phytochemistry |
-- |
Stimulant, diaphoretic | The recent evidences show an effective role
of P. roxburghii in the development of formulations used for curing skin diseases. |
Mohd shuaib, Mohd ali1*, and et al, 2013 | ||
| 5 | Essential oils: a perfect solution for headlice. | conventional insecticide | Research journal of pharmaceutical, biological and chemical sciences | Review of pediculosis study | Ovicidal | In the present study, it is observed that from literature survey it is given that eucalyptus have higher toxicity than clove but practically clove oil have higherMtoxicity as compare to eucalyptus oil to head lice | T Dhumal, and JS Waghmare* 2014, | ||
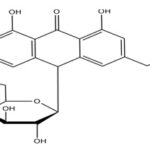
9. Product Name:
Sylamarine Biological Source: Silybum marianum has other common names include Cardus marianus, milk thistle, blessed milk thistle, Marian thistle, Mary thistle, Saint Mary's thistle, Mediterranean milk thistle, variegated thistle, and Scotch thistle Asteraceae family.
IUPAC Name: (2R, 3R)-3, 5, 7-trihydroxy-2-[(2R, 3R) - 3 - (4-hydroxy-3- methoxyphenyl) - 2 (hydroxymethyl) - 2, 3-dihydrobenzo[b] [1, 4] dioxin-6-yl] chroman-4-one.
Uses:
- Milk thistle has also been known to be used as food.
- Silibinin is under investigation to see whether it may have a role in cancer treatment (e.g. Due to its inhibition of STAT3 signaling).
- Silibinin also has a number of potential mechanisms that could benefit the skin. These include chemoprotective effects from environ-mental toxins, anti-inflammatory effects, protection from UV induced photocarcino-genesis, protection from sunburn, protection from UVB-induced epidermal hyperplasia and DNA repair for UV induced DNA damage.
| Properties | |
| Formula | C25H22O10 |
| Molar mass | 482. 44 g/mol |
Formulations till Now:
Sylimarine:
- Gel
- Floating tablet
- Solid dispersion tablets
- Floating microspheres
Sylimarine
TABLE 11: FORMULATIONS OF SYLIMARINE 68-72
| S.
no. |
Title of Paper | Formulation | Journal Name | Materials & Methods | Therpeutic Effect | Conclusion | Authors and Year of Publication | |
|
1 |
Formulation development and evaluation of silymarin gel for psoriasis treatment | Gel | Journal of innovations in pharmaceuticals and biological sciences. | Silymarin, methyl paraben, propyl paraben, glycerin | Silymarin gel and check antipsoriasis Activity | Silymarin gel shows the good viscosity which shows the pseudoplastic flow property. Gel shows good spreadability and pH lie in the range of skin pH. It has been observed that gel shows good antifungal activity like Flucanazole. Formulation shows the stability up to two month at the temp. 400c And it shows no skin irritation in human
Volunteers. |
Pathanazhar Khan, Rahul Thube, Rukhsana A.
, 2014 |
|
|
2. |
Formulation and in vitro evaluation of silymarin floating matrix tablet |
Floating tablet |
International journal of pharmacy and pharmaceutical sciences. |
Silymarin, (hpmc k4m) and Eudragit rs100, polyvinyl pyrrolidone (pvp k30)
Evaluation of floating tablets Drug release kinetics (curve fitting analysis) |
Protecting liver cells from toxic chemicals and drugs and enhance the effects of estrogen. |
Floating matrix tablets based on combination of three polymers namely; hydroxypropylmethylcellulose K4M, carbopol 934P and sodium alginate exhibited desired floating and prolonged drug release for 24 h. Carbopol loading showed negative effect on floating properties but were found helpful to control the release rate of
drug. |
R. B. Desi Reddy, 2012 |
|
|
3 |
Design and evaluation of sylamarin hp-beta- cyclodextrin solid dispersion tablets |
Solid dispersion tablets |
Indian journal of pharmaceutical science |
Invitro dissolution profiles,
Beta cyclodextrin improves oral bioavailability of sylamarin |
Hepatoprotective & hepatogenrative |
The attributes for these findings are dispersion of silymarin in HP-β-CD which increases the solubility and the superdisintegrants which cause swelling leading to sufficient hydrodynamic pressure to induce complete disintegration. |
P. d. nakhat & et al 2007. |
|
|
4 |
Hepatoprotective activity of silymarin floating drug delivery system against anti tuberculosis drug |
Floating tablet |
International journal of pharmacy&technolog |
Evaluation of floating tablets Tablets were prepared by direct compression method using a single punch-tableting machine (minipress-i) |
Hepatoprotective |
The preformulation studies and tablet evaluation
tests were performed and results were within the limits. Tablets remained buoyant over 20 hours in the release medium and the amount of sodium bicarbonate found to be significant for not only to remaining buoyant without causing a disintegration of the tablet. |
Vinay kumar d * et al. 2010. |
|
|
5 |
Gastroretentive floating microspheres of silymarin: preparation and in vitro evaluation |
Floating microspheres |
Tropical Journal of pharmaceutical research |
Emulsion-solvent evaporation method, Evaluate physicochemical properties |
Antioxidant, scavenger and regulator of the intracellular content of glutathione, cell membrane stabiliser and permeability regulator to prevent hepatotoxic agents from entering hepatocytes | The developed floating microspheres of silymarin exhibited prolonged drug release in simulated gastric fluid for at least 12 h, and, therefore, could potentially improve the bioavailability of the drug as well as patient compliance. |
Rajeev Garg and G D Gupta*, 2010. |
|
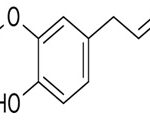
Geraniol
10. Product Name: Geraniol: Biological source: is a monoterpenoid and an alcohol. It is the primary part of rose oil, palmarosa oil, and citronella oil (Java type). It also occurs in small quantities in geranium, lemon, and many other essential oils.
IUPAC Name: (Z)-3, 7-Dimethyl-2, 6-octadien-1-o.
Uses: Research has shown geraniol to be an effective plant-based mosquito repellent.
Formulations till Now:
- Essential oil
- Carbopol
- gels
| Properties | |
| Chemical formula | C10H18O |
| Molar mass | 154. 25 g·mol−1 |
| Density | 0. 889 g/cm3 |
| Melting point | −15 °C (5 °F; 258 K)[2] |
| Boiling point | 230 °C (446 °F; 503 K)[2] |
| Solubility in water | 686 mg/L (20 °C)[2] |
TABLE 12: FORMULATIONS OF GERANIOL 73-75
| S.
no. |
Title of the Paper | Type of Formulation | Journal Name | Materials & Methods | Therapeutic Effect Proposed | Conclusion | Authors and Year of Publications |
|
1 |
Cymbopogon
martinii essential oil and geraniol at noncytotoxic concentrations exerted immunomodulatory/anti -inflammatory effects in human monocytes |
Cymbopogon martinii essential oil |
Journal of Pharmacy and Pharmacology |
Monocyte cultures were incubated with EO or geraniol, cytokine production was determined by ELISA. |
pro- and anti-inflammatory cytokines |
Data showed that noncytotoxic concentrations of EO and geraniol exerted an anti-inflammatory action by increasing IL-10 production; moreover, geraniol seemed to be probably responsible for EO immunomodulatory
activity in our assay condition. |
Bruna Fernanda Murbach Teles Andradem & et al, 2014 |
| 2 | Geraniol, a component of plant essential oils–a review of its pharmacological activities | Essential oil | International Journal of Pharmacy and Pharmaceutical Sciences | Male Wistar rats were subjected to carcinogen 4nitroquinoline-1-oxide and protective nature of GOH (200mg/kg. b. w) was investigated with reference to lipid peroxidation, membrane bound atpases (Na+ /K+ atpase, Ca2+ atpase and Mg2+ atpase) and protein bound carbohydrate
components |
Anti-inflammatory |
The present review reports the diverse pharmacological potentials which are explored by different researchers. However, more biological potentials are still untapped. The geraniol and related metabolites are used in the traditional system of medicine for various diseases related to the human race. |
Madankumar Arumugam& et al, 2013 |
|
3 |
Enhancing effect of terpenes on the in vitro percutaneous absorption of diclofenac sodium |
Carbopol gels |
International Journal of Pharmaceutics |
in vitro percutaneous absorption of diclofenac sodium from carbopol gels containing propylene glycol was investigated, Permeation experiments were performed on excised abdominal rat skin |
Permeation enhancer |
Acyclic alcohols were found to be the best enhancers for DFS, being geraniol, with an almost 20-fold increase, the most outstanding penetration enhancer. However, although the addition of terpenes increased DFS flux, diffusional lag times were longer than
For the control gel. |
A. Arellao S. Santoyo. C. Martina, . P. Ygartua, 1996. |
11. Product Name:
Eugenol IUPAC Name: 1 - Methyl - 4 - (1 -methylethyl) - 1, 4 - cyclohexadiene.
Uses: Eugenol is used in perfumes, flavorings, and essential oils. It is also used as a local antiseptic and anesthetic.
Formulations till Now:
- Permeation enhancer.
- Antibacterial, local analgesic and anaesthetic
Eugenol
| Properties | |
| Chemical formula | C10H12O2 |
| Molar mass | 164. 20 g·mol−1 |
| Density | 1. 06 g/cm3 |
| Melting point | −7. 5 °C (18. 5 °F; 265. 6 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Acidity (pKa) | 10. 19 at 25 °C |
| Magnetic susceptibility (χ) | -102. 1·10−6 cm3/mol |
TABLE 13: FORMULATIONS OF EUGENOL 76-79
| S.
no. |
Title of the Paper | Formulation Type | Journal Name | Materials & Methods | Therapeutic Effect proposed | Conclusion | Authors and Year of Publications |
| 1 | Formulation and evaluation of nutraceutical tablet using herbal drugs by direct compression method | nutraceutical tablet
|
Journal of drug delivery & therapeutics | The nutraceutical tablet containing lactose and mannitol as a diluent and containing natural drugs like clove and cinnamon which was prepared by direct compression method | Analgesic | The results of all evaluation parameters of the nutraceutical tablet were within the acceptable limit. Pre-compression studies of nutraceutical tablets show satisfactory results. The thickness, hardness, weight variation, and friability of nutraceutical tablet were found to in acceptable range. The in-vitro drug release of eugenol from optimised nutraceutical formulation was
found to be 90. 23%. |
Upendra nagaich, *Ashok Kumar Pal, and et al, 2014. |
|
2 |
Formulation and evaluation of transdermal patches and to study the permeation enhancement effect of eugenol |
transdermal patches |
Journal of applied pharmaceutical science | study the effect of polymers on transdermal release of the drugs In vitro permeation studies were performed using rat abdominal skin as the permeating membrane in
Franz diffusion cell. |
Permeation enhancer | Optimized batch was evaluated for permeation enhancement through rat skin using the natural permeation enhancer Eugenol, and it was concluded that permeation enhancement through Eugenol was comparable to the commercially available permeation enhancer
Dimethyl sulfoxide 1% (DMSO) |
Nirav S Sheth, Rajan B Mistry 2011 |
|
3 |
Protective effect of clove oil and eugenol microemulsions on fatty liver and dyslipidemia as components of metabolic syndrome |
Microemulsions |
Journal of medicinal food |
Clove oil dispersed in water as conventional cloudy emulsion was also subjected to the same biological evaluations for comparison
with the microemulsified form of this oil |
Protective |
The study concluded that administration of clove oil conventional emulsion, clove oil emulsion, or eugenol microemulsion produced significant improvement in fatty liver and dyslipidemia with consequent expected
protection from cardiovascular diseases and other complications of fatty liver. |
Al-okbi Sahar Y. and et al, 2014. |
|
4 |
Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases |
mucoadhesive tablets |
Journal drug development and industrial pharmacy |
Development and evaluation of controlled-release mucoadhesive tablets for gingival application, containing eugenol, which are prepared by taking carbopol 934 p and (hpmc) k4m in the ratio of 1:2, 1:1,
and 2:1. |
Antibacterial, local analgesic, and anesthetic treatment |
The release study indicates that an increase in carbopol increases the release rate of eugenol from the formulation whereas HPMC retards it. Increased in vitro bioadhesion is related to HPMC content of the formulation. The release kinetics of eugenol in vitro correlates with the in vivo results. This indicates the increased potential of eugenol as antibacterial, local
analgesic, and anaesthetic treatment. |
Bhimrao K. Jadhav*, and et al, 2004 |
Limonene
12. Product Name:
Limonene Biological Source: Limonin is enriched in citrus fruits and is often found at higher concentrations in seeds, for example, orange and lemon seeds. Limonin is also present in plants such as those of the Dictamnus genus.
IUPAC Name: 1- Methyl - 4 - (1 - methylethenyl) - cyclohexene.
Uses:
- Limonene is common in cosmetic products
- D-limonene is used in food manufacturing and some medicines
- It is also used as a botanical insecticide
- Limonene is increasingly being used as a solvent for cleaning purposes.
- As it is combustible, limonene has also been considered as a biofuel
Formulations till Now:
- Invasomes
- Transdermal patch
| Properties | |
| Chemical formula | C10H16 |
| Molar mass | 136. 24 g·mol−1 |
| Appearance | colorless to pale yellow liquid |
| Odor | Orange |
| Density | 0. 8411 g/cm3 |
| Melting point | −74. 35 °C (−101. 83 °F; 198. 80 K) |
| Boiling point | 176 °C (349 °F; 449 K) |
| Solubility in water | Insoluble |
TABLE 14: FORMULATIONS OF LIMONENE 80-84
| S.
no. |
Title of Paper | Formulations Type | Journal Name | Material and Method | Therapeutic Effect | Conclusion | Author with Year of Published |
|
1 |
Design and development of optimal invasomes for transdermal drug delivery using computer program | Invasomes | Asian journal of pharmaceutical sciences | phosphatidylcholine, cholesterol and capsaicin, and various percentages of d- limonene and Comperlan, optimization | Penitration enhancer | The skin permeability of the optimal invasomes was significantly higher than conventional liposomes and commercial product (0. 15% capsaicin in ethanolic solution). The response surfaces estimated by the computer program were helpful for the development of optimal invasomes for transdermal drug
delivery |
Sureewan Duangjit a, c, *, and et al, 2016 |
|
2 |
Trandermal delivery of Ketorolac |
Trandermal Patch |
The pharmaceutical society of Japan |
Preparation of ketorolac gel system & fabrication of reservoier type patch |
Penetration enhancer |
Permeation enhancement of ketorolac with different enhancers followed the order eucalyptus oil> transcutol> DMSO> d-limonene. Cyclic terpene containing eucalyptus oil was found to be the most promising chemical permeation
enhancer for transdermal delivery of ketorolac. |
Charndra Amrish*, Sharma Pramod Kumar. 2009 |
|
3 |
Penetration enhancer: a novel strategy or enhancing
transdermal drug delivery |
Transdermal drug delivery | International research journal
of pharmacy |
Bioavailability study by use of penetration
enhancers |
Penetration enhancer | Terpines can be used potential penetration enhancers for low or no
skin irritating potential |
Singla Vikas*, and et al, 2011. |
|
4 |
Transdermal permeation enhancement of Tolterodine Tartrate through invasomes and iontophoresis |
Invasomes |
Scholars Research Library journal |
Tolterodine Tartrate invasomes and iontophoresis Transdermal permeation enhancement techniques. |
Penetration enhancer |
Findings from this study demonstrate that transdermal delivery of invasomes encapsulating drug molecules in combination with iontophoresis may be applicable to
various drugs in order to increase the permeation through the skin |
Kalpana B and Lakshmi P K* 2013. |
REGULATORY AGENCIES FOR NUTRACEUTICALS 85-89
| Name of Country | Regulatory Authority | Description |
| Japan
Dietary supplements and natural nutraceuticals preferred as: “Foods with Health Claims” |
Food Safety Commission Pharmaceutical Affairs and Food Sanitation Council, The Ministry of Health, Labor and Welfare Consumer Affairs Agency Food of Special Health Uses (FOSHU) Act Japan Health Food Association (JHFA) Japan Health Food and Nutrition Food Association (JHNFA) | For regulatory purposes, nutraceuticals are divided into two groups.
1. “Foods with Nutrient Function Claims, ” contains twelve vitamins and five minerals. “Foods for Specified Health Uses,” or FOSHU. |
| China | China Health Care Association (CHCA) China’s State Food and Drug Administration (SFDA) US-China Health Products Association (USCHPA) Ministry of Health (MOH Administration of Quality Supervision Inspection and Quarantine (AQSIQ) | SFDA: In harge of dietary supplements and issue registration Ministry of Health (MOH): approval of new novel food ingredients Administration of Quality Supervision Inspection and Quarantine (AQSIQ): controls over imports and exports |
| Israel
Innovation hub for the nutraceutical industry |
1. Ministry of Health (MoH) | The industry is driven by ingredient companies such as Solbar Industries, LycoRed Natural Ingredients, Adumim Food Ingredients, Enzymotec, Algatechnologies and Frutarom etc. |
| India | Food Safety and Standards Act (FSSA) Indian Pharmacopoeia
1. Federation of Indian Chambers of Commerce and Industry (FICCI) Centre for Food Safety and Applied Nutrition (CFSAN) HADSA ( Health Food and Dietary Supplements Association NIN ( National Institute of Nutrition) FDTRC ( food and Drug Toxicology Research Centre) 2. NNMB (National Nutrition Monitoring Bureau) 3. Indian Health Foods and Dietary Supplements Association (INHADSA). Indian Council of Medical Research (ICMR) |
1. FSSA: food and nutraceutical safety and standards. Also regulates manufacture, storage, distribution, sale and import.
2. Indian Pharmacopoeia: Standards for safety and quality like for plant extracts and phytochemicals 3. Federation of Indian Chambers of Commerce and Industry (FICCI): Improved regulatory framework to validate product claims which meets consumer demand 4. CFSAN: Diverse process of New Dietary Ingredient (NDI) 5. NIN: Focused studies on protein energy malnutrition, nutrition situation, methods of management and prevention of nutritional problems, . NIN is working under the aegis of 6. FDTRC: Study drug nutrient interactions (drug metabolism, toxicity, |
| 11. The Food Safety & Standards Authority of India (FSSAI). | valuate, identify naturally occurring food ingredients which are rich in
antioxidants hypoglycemic hypolipidemic and cancer prevention) |
|
|
Brazil |
1. Brazilian Association of Foods for Special Purposes and Congeners (ABIAD).
1. Committee for Scientific and Technical Assessment of Functional and New Foods (CTCAF) National Health Surveillance Agency (ANVISA) 4.Ministério da Agricultura, Pecuária e Abastecimento (MAPA) |
ANVISA: Registration and regulation of new products
2. National Policy of Integrative and Complementary Practice (PNPIC) in the Unified Health System (SUS):research and use of medicinal plant s and herbal medicines according quality, safety and efficacy statements. |
| Mexico | 1. National Association of Food Supplements Industry (ANAISA)
2.The Federal Commission for Protection against Health Risks (COFEPRIS) |
General Health Act defines dietary supplements as “herbal products, plant extracts, traditional foods, dehydrated or concentrated fruit added or not, vitamins or minerals that may arise in a pharmaceutical form and intended use is to increase total dietary intake, supplement it or replace
Some component of one’s diet.” |
|
United States |
FDA United
States Department of Agriculture (USDA) DSHEA Federal Trade Commission (FTC) |
Dietary supplement contain: a herb or other botanical or a concentrate, metabolite, constituent, extract or combination of any ingredient
from the other categories. Regulatory bodies evaluate, investigate, regulate, inspect and sanction. |
| European Union | 1. European Food and Safety Authority (EFSA). | Food supplements are defined as concentrated sources of nutrients and
Other substances with a beneficial nutritional effect. |
| UK | Food Standards Agency (FSA)
Medicines and Healthcare products Regulatory Agency (MHRA) |
Guidelines for safe levels of intake for vitamins and minerals. |
| Malaysia | 1. National Pharmaceutical Control Board (NPCB Drug Control Authority ( DCA) | All claims are product specific and are subject to a pre-market approval
of the National Pharmaceutical Control Bureau (NPCB) |
| Canada | Food and Drug Authority Natural Health Product Regulations Canadian Food inspection agency | Natural Health Product Regulations: set requirements for efficacy, safety and quality reviews and provide Natural Product Number (NPN)
Products regulated under the Food and Drug Regulations (FDRs) Canadian Food Inspection Agency: Regulate labelling and advertising National Health Products Directorate (NPHD): evaluates product licence applications |
|
Russia |
1.Ministry of Health and Social Development
2.Federal Service on Supervision in Sphere of Public Health Services and Social Development (Roszdravnadzor) |
1. Nutraceuticals are regulated under the term Biologically Active Dietary Supplements (BADS). They are recommended prophylactically and for the prevention of pharmaceutical therapy induced side-effects and the achievement of complete remission.
2.Roszdravnadzor: register and issues Registration Certificate |
| Canadian Food Inspection Agency: Regulate labelling and advertising
National Health Products Directorate (NPHD): evaluates product licence applications |
||
| Australia | Department of Health and ageing | |
| Australia New
Zealand |
Australia New Zealand Therapeutic Products Authority (ANZTPA) | 1. ANZTPA: Authority over complementary and alternative medicines,
including dietary supplements (nutraceuticals) |
| Republic of Korea | Korean Food and Drug Administration (KFDA) | KFDA: Evaluates toxicity tests, efficacy, human studies, safe use of
product |
|
Singapore |
Sale of Food Act and the Food regulations. Agri-food and Veterinary Authority (AVA). Health Sciences Authority
1. Health Promotion Board 2002 |
1. The health supplements in Singapore are regulated by the Medicines Act 1975.
2. Various claims for regulation of nutraceuticals like Functional health claims, Permissible health claims, Health claims and Nutritional claims, nutrition function claims, Nutrient function claims, nutrient content claims |
| Taiwan | 1. Health Department in Health Food Control Act | 1. Health Food Control Act: regulate the production and health claims of health foods and health food labelling
2.Food Administration Act: Regulate Conventional food labelling |
| Philippines | The Bureau of Foods and Drugs of the Health Department in The
Philippines |
|
| Thailand | Thai Food and Drug Administration | The nutrition claims and labelling standards follow the guidelines of
Codex Alimentarius |
| Global food and nutrition bodies are:
1. WHO (World Health Organisation) 2. FAO (Food and Agriculture Organisation 3. WTO (World Trade Organisation) 4. CODEX (Codex Alimentarius)
|
||
VARIOUS SCHEDULES FOR FOOD AND NUTRACEUTICALS 89
| Schedule | Ingredients | Examples with dose |
| I. Schedule I | 1. Vitamins
2. Minerals |
1. Vitamin A: 30 %
2. Vitamin B: B1 /B2/B6/B12: 25%; B3:10% 3. Vitamin C: 20 % 4. Vitamin D: 30 % 5. Vitamin E: 10 % 6. Vitamin K1: 30 % 7. Pantothenic acid: 10% 8. Folic acid: 25% 9. Minerals: 10% Iodine: 20% |
| II. Schedule II | Essential amino acids Non-essential Amino acids Nucleotides | 1. Vitamin A: 35-100µg/100kcal Vitamin B:
2. B1: 0. 06-0. 5 mg/kcal 3. B2: 0. 08-0. 5 mg/kcal 4. B6: 0. 08-0. 5 mg/kcal 5. B12: 0. 07-0. 7 mg/kcal 6. B3: 0. 9-3mg/100kcal 7. Vitamin C: 2. 25-22 mg/100kcal 8. Vitamin D: 0. 5-2. 5µg/100kcal 9. Vitamin E: 0. 5-3mg/100kcal 10. Vitamin K: 3. 5-20µg/100kcal 11. Pantothenic acid: 0. 15-1. 5mg/100kcal 12. Folic acid: 10-50µg/100kcal 13. Minerals: 14. Sodium:30-175 mg / 100kcal 15. Chloride: 30-175mg / 100kcal 16. Potassium: 80-295mg / 100kcal 17. Phosphorous: 80-295mg / 100kcal 18. Iron: 0. 5-2 mg / 100kcal 6. Zinc: 0. 5-1. 5mg / 100kcal 19. Copper: 60-500µg / 100kcal 20. Iodine: 6. 5-35µg / 100kcal 21. Selenium: 2. 5-10µg / 100kcal 22. Manganese: 0. 05-0. 5mg / 100kcal 23. Chromium: 1. 25-15µg / 100kcal 24. Molybdenum: 3. 5-18 µg / 100kcal |
| III. Schedule III | These elements allowed to be used for special dietary use or medical purpose (other than those intented for use in infant formula)
Vitamins Minerals Trace elements |
1. Vitamins Vitamin A: 35-180 µg / 100kcal
2. Vitamin D: 0. 5-2. 5µg / 100kcal 3. Vitamin K: 3. 5-20µg / 100kcal 4. Vitamin C: 2. 25-22µg / 100kcal 5. Vitamin B6 or Riboflavin: 0. 08-0. 05µg / 100kcal 6. Vitamin B12: 0. 07-0. 7µg / 100kcalFolic acid: 10-50µg / 100kcal 7. Biotin:. 75-7. 5µg / 100kcal Minerals |
| IV. Schedule | Ingredients | Examples with dose |
|
V. Schedule I |
Vitamins
Minerals |
1. Vitamin A: 30 %
2. Vitamin B: B1 /B2/B6/B12: 25%; B3:10% 3. Vitamin C: 20 % 4. Vitamin D: 30 % 5. Vitamin E: 10 % 6. Vitamin K1: 30 % 7. Pantothenic acid: 10% 8. Folic acid: 25% 9. Minerals: 10% 10. Iodine: 20% |
| VI. ScheduleII | Essential amino acids
Non-essential Amino acids Nucleotides |
1. Vitamin A: 35-100µg/100kcal
2. Vitamin B: 3. B1: 0. 06-0. 5 mg/kcal 4. B2: 0. 08-0. 5 mg/kcal 5. B6: 0. 08-0. 5 mg/kcal 6. B12: 0. 07-0. 7 mg/kcal 7. B3: 0. 9-3mg/100kcal 8. Vitamin C: 2. 25-22 mg/100kcal 9. Vitamin D: 0. 5-2. 5µg/100kcal 10. Vitamin E: 0. 5-3mg/100kcal 11. Vitamin K: 3. 5-20µg/100kcal 12. Pantothenic acid: 0. 15-1. 5mg/100kcal 13. Folic acid: 10-50µg/100kcal 14. Minerals: 15. Sodium:30-175 mg / 100kcal 16. Chloride: 30-175mg / 100kcal 17. Potassium: 80-295mg / 100kcal 18. Phosphorous: 80-295mg / 100kcal 19. Iron: 0. 5-2 mg / 100kcal 6. Zinc: 0. 5-1. 5mg / 100kcal 20. Copper: 60-500µg / 100kcal 21. Iodine: 6. 5-35µg / 100kcal 22. Selenium: 2. 5-10µg / 100kcal 23. Manganese: 0. 05-0. 5mg / 100kcal 24. Chromium: 1. 25-15µg / 100kcal 25. Molybdenum: 3. 5-18 µg / 100kcal |
| VII. Schedule III | These elements allowed to be used for special dietary use or medical purpose (other than those intented for use in infant formula)
Vitamins Minerals Trace elements |
1. Vitamins
2. Vitamin A: 35-180 µg / 100kcal 3. Vitamin D: 0. 5-2. 5µg / 100kcal 4. Vitamin K: 3. 5-20µg / 100kcal 5. Vitamin C: 2. 25-22µg / 100kcal 6. Vitamin B6 or Riboflavin: 0. 08-0. 05µg / 100kcal 7. 6Vitamin B12: 0. 07-0. 7µg / 100kcal Folic acid: 10-50µg / 100kcal 8. Biotin:. 75-7. 5µg / 100kcal Minerals |
| 5. Beet red: Colour | ||
| VIII. Schedule VF | Food addative use in tablet, capsule and syrup for special medical purpose food other than infant food, special medical purpose food, food with Probiotics / prebiotics, food as heath supplements, nutraceuticals, food containing
plant ingredients. |
1. Maximum permitted level in percentage
2. Ascorbic acid/ esters: 0. 5 % 3. Benzoic acid: 0. 5 % 4. Calcium stearate: 1 % 5. Citric acid: 2 % 6. Methyl paraben: 0. 2 % |
| IX. Schedule VI | Ingredients as a nutraceuticals | 1. Maximum permitted level
2. Citrus bioflavonoids: 150-600 mg/day 3. Lactase / Beta galactosidase: 3000- 9000IU/day 4. Piper nigrum/longa extract: 15mg/day 5. Siberian ginseng: 100-450 mg/day 6. Vaccinium myrstillus extract/ bilberry extract: 50-600 mg/day |
| X. Schedule VII | List of microorganism as a probiotics
These microoraganism use as asingle or in combination but must declare on label with information about Non-GMO. |
Lactobacillus acidophilus
1. Bacillus coagulans 2. Bifidobacterium bifidum 3. Streptococcus thermophilus 4. Saccharomyces cerevisiae |
| XI. Schedule VIII | List of prebiotic compounds | 1. Polydextrose
2. Inulin 3. Lactulose 4. Lactoferrin 5. Sugar alcohols |
CONCLUSION: Nutraceuticals provide all the essential substances that should be present in a healthy diet for the human. From the above study, it can be concluded that various chemical constituents from natural sources can be obtained and prepared into various optimized, safe, stable formulations for the treatment and diagnosis of diseases. Nutraceuticals are widely used in the food and pharmaceutical industries. Most of the nutraceuticals are from either mineral origin, animal origin or vegetable origin like gamma terpinenes, beta carotene, curcumine, limonene, eugenol, pinene, safranal, geraniol, aloine, caryophylline, licopine and sylimarine.
These constituents are prepared into dosage forms as topical, oral, etc. viz. creams, lotions, ointments, emulsions, unani formulations, aromatic oils, microemulsions, SMEDDS, beads, tablets, emul-gels, herbal formulations etc. used in various categories as antidiabetic, antibiotic, antimicrobial, anti-inflamatory, anti cancer, protective, etc. results of study indicate that demand and consumption of nutraceuticals are now going on increasing due to safety, therapeutic efficacy, stability of formu-lations.
ACKNOWLEDGEMENT: Nil
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Singh J and Sinha S: Classification, regulatory acts and applications of nutraceuticals for health: a review. International Journal of Pharmacy and Biological Sciences 2012; 2(1): 177-87.
- Smarta RB: Paradigm shift from pharmaceuticals to nutraceuticals, Nuffoods Spectrum. 2017http://www. nuffoodsspectrum.in/inner_view_single_details. php?page=4&content_type=&vrtcl_panel_nm=&ele_id=NOR_589314edba5a36. 92526952&contentPage=3.
- Maxwell J: Denis Smith, Mike Brewster, Susan Eggleton, Food as pharma as wellness products evolve, the distinction between food and medicine blurs. R&C worlds express 2012. http://www. pwc. Com/gx/en/retail-consumer/pdf/rc-worlds-newsletter-foods-final. Pdf.
- Biotech for Wellness: Driving Successful R & D and Licensing in Nutraceuticals through New Business Models and Collaboration, Research and Markets, May 27, 2010, http://www.businesswire.Com/news/home/20100527005898/en/Research-Markets-Biotech-Wellness-Driving-Successful-Licensing.
- Kumar P, Kuma Nr and Omer T: Nutraceuticals- critical supplement for building a healthy India, World Journal of Pharmacy and Pharmaceutic Sciences 2016; 5(3): 579-94.
- Olaiya CO, Soetan KO and Esan A: The role of nutraceuticals, functional foods and value added food products in the prevention and treatment of chronic diseases M. 1, African Journal of Food Science 2016; 10(10): 185-93.
- Shinde N, Bangar B, Deshmukh S and Kumbhar P: Nutraceuticals: a review on current status. Research J Pharm and Tech 2014; 7(1): 110-13.
- Kharb S and Singh V: Nutriceuticals in health and disease prevention. Indian J Clin Biochem 2004; 19(1): 50-53.
- Vouloumanou EK, Makris GC and Karageorgopoulos DE: Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Age 2009; 34: 1-10.
- http://probiotics. org/l-casei/
- Bennett A: List of Probiotic Bacteria 2015, http: //www. livestrong.com.
- Sanders EM: Food Technology 1999; 53(11): 67-78.
- Soccol CR: Luciana porto de souza vandenberghe, michele rigon spier, adriane bianchi pedroni medeiros, caroline tiemi yamaguishi, juliano de dea lindner, ashok pandey and vanete thomaz-soccol the potential of probiotics: a review. Food Technol Biotechnol 2010; 48(4): 413-34.
- Parvez S, Malik KA, Ah Kang S and Kim HY: Probiotics and their fermented food products are beneficial for health, Journal of Applied Microbiology 2006; 100(6): 1171-85.
- Guerra PN, Bernárdez FP, Méndez J, Cachaldora P and Castro CL: Roduction of four potentially probiotic lactic acid bacteria and their evaluation as feed additives for weaned piglets. Anim Feed Sci Technol 2007; 134: 89-07.
- Wu ZJ, DU X and Zheng J: Role of Lactobacillus in the prevention of Clostridium difficile-associated diarrhea: A meta-analysis of randomized controlled trials. Chin Med J (Engl.) 2013; 126: 4154–61.
- Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S and O'Brien M: Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in-vitro and in-vivo and protects against Clostridium difficile toxin A-induced enteritis, J Biol Chem 2006; 281: 24449-54.
- Maia OB, Duarte R, Silva AM, Cara DC and Nicoli JR: Evaluation of the components of a commercial probiotic in gnotobiotic mice experimentally challenged with Salmonella enterica subsp. enterica ser. Typhimurium, Vet. Microbiol 2001; 79(2): 183-89.
- Montrose DC and Floch MH: Probiotics used in human studies. J Clin Gastroenterol 2005; 39(6): 469-84.
- Hugenholtz J, Smid JE, Ladero V and Hols P: Metabolic engineering of lactic acid bacteria for the production of nutraceuticals. Antonie Van Leeuwenho 2002; 82: 217-35.
- Borkar N, Saurabh SS, Rathore KS, Pandit A and Khandelwal KR: An insight on nutraceuticals. Pharma Tutor 2015; 3(8): 13-23.
- Zou L, Zhang R, Salvia-Trujillo L, Kumosani T and Xiao H, Enhancing Nutraceutical Performance Using Excipient Foods: Designing Food Structures and Compositions to Increase Bioavailability David Julian McClements. Comprehensive Reviewsin Food Science and Food Safety 2015; 14: 824-47.
- International Food Information Council. Functional Foods Fact Sheet: Omega-3 Fatty Acids http://www.foodinsight. org/Functional_Foods_Fact_Sheet_Omega_3_Fatty_Acids
- Dewick, P. M. Medicinal Natural Products: A Biosynthetic Approach. United Kingdom: John Wiley & Sons, 2006: 187-97.
- https://www. Nutraceuticalbusinessreview.
- com/technical/article_page/Overcoming_problematic_production_issues_in_nutraceutical s/98237
- Zaki NM (2014) Progress and Problems in Nutraceuticals Delivery. J Bioequiv Availab 6: 075-077. doi: 10. 4172/jbb. 10000183
- Moghimipour E, Aghel N, Mahmoudabadi AZ, Ramezani Z and Handali S: “Preparation and Characterization of liposomes containing essential oil of eucalyptus camaldulensis leaf, Jundishapur Journal of Natural Pharma Products 2012; 7(3): 117-22.
- Athikomkulcha S, Rith W Panida T: The development of anti-acne products from Eucalyptus globulus and Psidium guajava Journal Health Resource 2008; 22(3): 109-13.
- Seth NW:The Use of two new formulations of Ocimum canum Sims and Cymbopogon schoenanthus In the control of amitermes evuncifer silvestri (termitidae: termitinae), in togo. International Journal of Natural Sciences Research 2014; 2(10): 195-05.
- Kumar KA and Choudhary RK: “Determination of antibacterial, antifungal activity and chemical composition of essential oil portion of unani formulation kulzam”, International J of Green Pharmacy 2011; 5(1): 28-33.
- Desai H, Sav A, Amin P: Formulation and evaluation of mucoadhesive anti-infective solution containing solubilised tea tree oil for vaginal infections. International Journal of Advances in Pharmacy Biology and Chemistry, 2013; 2(2): 385-89.
- Chalamaiah M and Sharma K: Novel encapsulation of Lycopene in noisome & assessment of its anticancer activity. Journal of Bioequivalence and Bioavailability 2016; 8(5):224-32.
- Kumari, Singh A, Saurabh SS, Rathore KS and Israni R: Formulation and evalution of Lycopene emulgel”, Indo Ameri J of Pharmaceutical Sciences 2015; 2(6): 1013-27.
- Jain N, Sareen R, Mahindroo N and Dhar KL: Development and optimization of osmotically controlled asymmetric membrane capsules for delivery of solid dispersion of lycopene. The Scientific World Journal 2014; 438528, 7 pages, 2014. doi:10.1155/2014/438528.
- Soma S: Development & evaluation if the antioxidant activity of tomato based confectionary. Internationa Food Journal 2013; 20(6): 3167-70.
- Luciana B, Vande HL, Venugopal V and Stanay: Topical delivery of Lycopene using microemulsion. Willay Science Journal 2010; 99(3): 1346-57.
- Divyen S, Gaud RS, Mishra A and Nparkin R: Formulation of water soluble mucoadhesive film of lycopene. International Journal of Pharmaceutical Science and Research 2010; 2(1): 06-10.
- Aslani A and Ghannadi A: Design formulation and evaluation of Aloe vera chewing gum. Journal of Advanced Biomedical Research 2015; 4: 175-82.
- Ahmad JF, Barkata MA and Javedahmada D: Formulation design of micronized silver sulfadiazine containing aloe vera gel for wound healing. Current Bioactive Compounds 2016; 12(2): 63-68.
- Suseem SR, Ojhakhyati and Shenoyvranda: Formulation and evaluation of hydrogel with ascorbic acid using Aloe vera gel powder as a drug carrier. Innovare journal of science 2013; 1(1): 18-20.
- Tarkase KN and Danve AV: Formulation evaluation and in-vitro drug release characteristics of Aloe vera herbal suppositories. Scholars Resear Library 2015; 7(2): 310-16.
- Pounikar Y, Jain P, Khurana NK, Omray and Patil S: Formulation and characterization of Aloe vera cosmetic herbal hydrogel. International Journal of Pharmacy and Pharmaceutical Sciences 2012; 4(4): 85-86.
- Khameneh B, Halimi V, Jaafari MR and Shiva Golmohammadzadeh S: Safranal-loaded solid lipid nanoparticle. Iranian Journal of Basic Medical Sciences 2015; 18(1): 58-63.
- Shilpa CP, Nitin B, Sadhanas K and Mukesh RP: Development of safranal niosomal in-situ nasal gel formulation. World Journal of Pharmaceutical Research 2015; 7(1): 13-21.
- Zadeh SG: Preparation, characterization & evaluation of sun protective &moisturizing effects of nanoliposomes containing safranal. Iranian Journal of Basic Medical Sciences 2011; 14(6): 521-33.
- Pooriashakoori, Wunwisakrasaekoopt: Microencapsulation of saffron (Crocus sativus) Extract in copolymer complexes using extrusion method. Chiang mai University Journal of Natural Sciences 2015; 14(1): 53-71.
- Jaafari RM: Characterization & anti-tumor activity of pegylated nanoliposomes containing safranal in mice bearing c26 colon carcinoma. International J of Pharmaceut Sciences and Research 2016; 7(11): 4379-86.
- Singh BB, Shakil NA, Kumar J, Walia S and Kar A: Development of slow release formulations of β-carotene employing amphiphilic polymersand their release kinetics study in water and different ph conditions. Journal of Food Science and Technology 2015; 52(12): 8068-76.
- Ariviani S, Anggrahini S, Naruki S and Raharjo S: Characterization and chemical stability evaluation of β-carotene microemulsions prepared by spontaneous emulsification method using vco and palm oil as oil phase. International Food Research Journal 2015; 22(6): 2432-39.
- Erna Wulandari, Adella Clara Alverina, Ronny Martien: snedds (self- nanoemulsifying drug delivery system) formulation of carotene in olive oil (Olea europaea)”, International J of Advanced Resear 2016; 4(11): 1031-43.
- Kar HK: Efficiency of beta-carotene topical application in Melasma-an open clinical trial. Indian J Dermatol venereal Leprol [serial online] 2003 [cited2017 Jul 7]; 69: 92-4. Available from: http://ijdvl. Com / tex. Asp. 2003/69/2/92/5884.
- Oliveira RGA, Lucia De Carvalho MJ, R. Marília Nutti L, De Carvalho JV and Fukuda WG: Assessment and degradation study of total carotenoid and ß-carotene in bitter yellow cassava (Manihot esculenta crantz) varieties. African Journal of Food Science 2010; 4(4): 148-55.
- Shegokar KMR, Gohla S, Snselmi C and Müller HR: Lutein nanocrystals as antioxidant formulation for oral and dermal delivery”, International Journal of Pharmaceutics 2011; 420(1): 141-46.
- Evans M, Beck M, Elliott J, Stephaneetheve, Richard R: Effects of formulation on the bioavailability of lutein and zeaxanthin: a randomized, double-blind, cross-over, Comparative, single-dose study in healthy subjects. European Journal of Nutrition 2013; 52(4): 1381-91.
- Kale S, Bhandare S and Gaikwad M: Formulation and in-vitro evaluation for sun protection factor of lutein ester extracted from Tagetes erecta Linn flower (family asteraceae) sunscreen creams. Research Journal of Pharmaceutical Biological and Chemical Sciences 2011; 2(3): 947-55.
- Mitri K, Shegokar R, Gohla S, Anselmi C, Rainer H: Müller, lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. International Journal of Pharmaceutics 2011; 414(1–2): 29, 267-75.
- Tanumihardjo SA, Jialiang L, Dosti MP: Lutein absorption is facilitated with cosupplementation of ascorbic acid in young adults. Journal of the American Dietetic Association 2005; 105(1): 114-18.
- Shanmugam S, Park J, Kim KS, Piao ZZ, Yong CS, Choi GH and Woo JS: Enhanced bioavailability and retinal accumulation of lutein from self-emulsifying phospholipid suspension (SEPS). International Journal of Pharmaceutics 2011; 412(1-2): 99-05.
- Schmitt D, Levy R and Carroll B: Toxicological evaluation of β-Caryophyllene oil. International Journal of Toxicology 2016; 35(5): 558-67.
- Heuskin S, Lorge S, Lognay G, Wathelet J, s Béra F, Leroy P, Haubruge E, Brostaux Y: A Semiochemical Slow-release formulation in a biological control approach to attract hoverflies. Journal of Environment and Ecology 2012; 3(1): 72-85.
Edris E and Malone CFR: Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems. International Journal of Cosmetic Science 2012; 34(5): 441-50.
-
- Pieri: Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Veterinary Research 2016; 12(216): 1-8.
- Khokra SL, Prakash O, Jain1 S, Aneja KR, Dhingra Y: Essential oil composition and antibacterial studies of Vitex negundo Extracts.Indian Journal of Pharmaceutical Sciences 2008; 70 (4): 522-26.
- Kumar KA, Choudhary KR, Anand DY, Vidya B and Solomon R: Determination of chemical composition of essential oil portion of reputed marketed unani formulation zinda tilismath. International Journal of Pharmacy and Pharmaceutical Sciences 2011; 3(3): 67-68.
- Alviano DS: Biological activities of -pinene and β-pinene enantiomers. Molecular Diversity Preservation International 2012; 17(6): 6305-16.
- Dai J, Zhu L, Yang L and Qiu J: Chemical composition, antioxidant and antimicrobial activities of essential oil from wedelia prostrate. Experimental and Clinical Sciences International Online Journal 2013; 12: 479-90.
- Shuaib M, Ali M, Ahamad J, Naquvi JK, Ahmad MI: Pharmacognosy of pinus roxburghii: a review.Journal of Pharmacognosy and Phytochemistry 2013; 2(1): 262-68.
- Dhumal T and Waghmare J: Essential oils: a perfect solution for headlice. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014; 5(3): 1486-04.
- Khan P, Thube R and Arab R: Formulation development and evaluation of silymarin gel for psoriasis treatment. Journal of Innovations in Pharmaceuticals and Biological Sciences 2014; 1(1): 21-26.
- Reddy RBD, Malleswari K and Narayana L: Formulation and in-vitro evaluation of silymarin floating matrix tablet. International Journal of Pharmacy and Pharmaceutical Science 2012; 4(5): 468-72.
- Nakhat PD: Design and evaluation of sylamarin hp-beta-cyclodextrin solid dispersion tablets. Indian Journal of Pharmaceutical Science 2007; 69(2): 287-89.
- Kumar VD: Hepatoprotective activity of silymarin floating drug delivery system against anti tuberculosis drug. International Journal of Pharmacy & Technology 2010; 2(2): 233-44.
- Garg R and Gupta GD: Gastroretentive floating microspheres of silymarin: preparation and in vitro evaluation. Tropical Journal of Pharmaceutical Research 2010; 9(1): 59-66.
- Bruna Fernanda Murbach Teles Andradem: Cymbopogon martinii essential oil and geraniol at noncytotoxic concentrations exerted immunomodulatory/anti-inflammatory effects in human monocytes. Journal of Pharmacy and Pharmacology 2014; 66(10): 1491-96.
- Arumugam MK: Geraniol, a component of plant essential oils a review of its pharmacological activities. International Journal of Pharmacy and Pharmaceutical Sciences 2013; 1(5): 416-20.
- Arellao, Santoyo SC, Martina P, Ygartua: Enhancing effect of terpenes on the in-vitro percutaneous absorption of diclofenac sodium. International Journal of Pharmaceutics 1996; 130(1): 141-45.
- Nagaich U, Pal AK, Bharti C and Gulat N: Formulation and evaluation of nutraceutical tablet using herbal drugs by direct compression method. Journal of Drug Delivery & Therapeutics 2014; 4(2): 47-51.
- Sheth SN and Mistry RB: Formulation and evaluation of transdermal patches and to study permeation enhancement effect of Eugenol. Journal of Applied Pharmaceutical Science 2011; 01(03): 96-101.
- Al-okbi Sahar Y, Doha M, Thanaa EH, Edris EA: Protective effect of clove oil and eugenol microemulsions on fatty liver and dyslipidemia as components of metabolic syndrome. Journal of Medicinal Food 2014; 17(7): 764-71.
- Jendresen MP and Phillips WR: A comparative study of four zinc oxide and eugenol formulations as restorative materials. The J of Prosthet Dentistry 1969; 21(3): 300-09.
- Jadhav KB, Khandelwal RK, Ketkar RA and Pisal SS: Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases. Drug Development and Industrial Pharmacy 2004; 30(2): 195-203.
- Duangjit ACS, Nimcharoenwan BT, Chomya N, Locharoenrat BN and Ngawhirunpat T: Design and development of optimal invasomes for transdermal drug delivery using computer program. Asian Journal of Pharmaceutical Sciences 2016; 52-53.
- Amrish C and Kumar SP: Transdermal delivery of Ketorolac. The Pharmaceutical Society of Japan 2009; 129(3): 373-79.
- Vikas S, Seema S, Gurpreet S, Rana AC and Baibhav AJ: Penetration enhancer: a novel strategy or enhancing transdermal drug delivery. International Research Journal of Pharmacy 2011; 2(12): 32-36.
- Kalpana B and Lakshmi PK: Transdermal permeation enhancement of Tolterodine Tartrate through invasomes and iontophoresis. Scholars Research Library Journal 2013; 5(6): 119-26.
- Regulatory environment for nutraceuticals and functional foods. Valerie Baker Brenda Brady Mary Velin NRC-CISTI National Research Council of Canada 2012; 6
- http://www. Inspection. gc. ca/
- Malla S, Hobbs EJ and Sogah EK: Functional foods and natural health products regulations in canada and around the world. Nutrition Labels and Health Claims. Report prepared for the Canadian Agricultural Innovation and Regulation Network (CAIRN) 2013.
- Hasler MC: Regulation of functional foods and nutraceuticals. A Global Perspective Blackwell Publishing State Avenue Ames Iowa 50014 USA 2007; 37: 89.
- http://old.fssai.gov.in/Portals/0/pdf/Direction_Operationalisation_HS_SMP_NF_Nutra_24_11_2016. pdf.
- Dillard CJ and German JB: Phytochemicals Nutraceuticals and human health. J Sci Food Agric 2000; 80: 1744-56
How to cite this article:
Patel S: Nutraceutical: a review. Int J Pharmacognosy 2020; 7(11): 272-00. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP. 7(11).272-00.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
272-300
1752
1425
English
IJP
S. Patel
Department of Pharmacognosy, Nootan Pharmacy College, Sankalchand Patel University, Visnagar, Gujarat, India.
sejupatel04@gmail.com
05 May 2020
23 September 2020
28 September 2020
10.13040/IJPSR.0975-8232.IJP.7(11).272-00
01 November 2020