NOVEL EXTRACTION PROCESS USE IN MEDICINAL PLANTS: A REVIEW
HTML Full TextNOVEL EXTRACTION PROCESS USE IN MEDICINAL PLANTS: A REVIEW
H. Vishal Thorat *, N. M. Harinath, A. Firoj Tamboli and A. Jadhav
Department of Pharmacognosy, Bharati Vidyapeeth College of Pharmacy, Kolhapur, Shivaji University, Kolhapur - 416012, Maharashtra, India.
ABSTRACT: The Plants having own biological potential to treat some major diseases. Medicinal plants are gaining much interest recently because their use in ethnomedicine treating common diseases such as cold, fever, and other medicinal claims are now supported with sound scientific evidence. The study on medicinal plants started with extraction procedures that play a critical role to the extraction outcomes (e.g., yield and phytochemicals content) and also to the consequent assays performed. A wide range of technologies with different methods of extraction is available nowadays. Hence, this review aims to describe and compare the most commonly used methods based on their principle, strength, and limitation to help to evaluate the suitability and economic feasibility of the methods.
| Keywords: |
Maceration, Percolation, Decoction, Soxhlet extraction, Microwave-assisted extraction, Ultrasound-assisted extraction, Accelerated solvent extraction, Supercritical-fluid extraction, Medicinal plants
INTRODUCTION: Medicinal plants are currently in considerable significant view due to their special attributes as a large source of therapeutic phyto-chemicals that may lead to the development of novel drugs. Most of the phytochemicals from plant sources such as phenolics and flavonoids have been reported to have a positive impact on health and cancer prevention 1. Modern Mediterranean and DASH (Dietary Approaches to Stop Hypertension) incorporate phytochemicals rich diet from fruit and vegetable sources as the plant-based diet has shown to extend life span in Okinawan people, that has the highest number of centenarians 2, 3.
Interest in utilizing natural sources in the development and formulation of skin products as an alternative to conventional drugs and synthetic products contribute to increasing interest in research and industrial application of medicinal plants 4. The high content of phenolic and flavonoids in medicinal plants have been associated with their antioxidant activities that play a role in the prevention of the development of age-related disease, particularly caused by oxidative stress. With regards to the beneficial phytochemicals in medicinal plants and the shift towards natural products in the pharmaceuticals and cosmeceuticals industry, the research on medicinal plants particularly is as important as the research on conventional drugs.
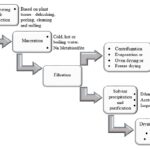
The study of medicinal plants starts with the pre-extraction and the extraction procedures, which is an important step in the processing of the bioactive constituents from plant materials. Traditional methods such as maceration and Soxhlet extraction are commonly used at the small research setting or at Small Manufacturing Enterprise (SME) level. Significance advances have been made in the processing of medicinal plants such as the modern extraction methods; microwave-assisted (MAE), ultrasound-assisted extraction (UAE), and supercritical fluid extraction (SFE), in which these advances are aimed to increase yield at a lower cost. Moreover, modifications to the methods are continuously developed. With such a variety of methods present, the selection of proper extraction methods needs meticulous evaluation. This review describes the principle, strength, and limitation of the commonly used methods with examples in recent years to help in the selection of proper methods. Pre-extraction preparation of plant samples.
The initial stage in studying medicinal plants in the preparation of plant samples to preserve the bio molecules in the plants prior to extraction. Plants samples such as leaves, barks, roots, fruits, and flowers can be extracted from fresh or dried plant material. Other pre-preparation of plant materials such as grinding and drying also influences the preservation of phytochemicals in the final extracts.
Fresh vs. dried samples: Both fresh and dried sample is used in medicinal plants studies. In most cases, a dried sample is preferred considering the time needed for experimental design. Sulaiman et al., limit the interval between harvest and experimental work at the maximum period of 3 h to maintain the freshness of samples, as fresh samples are fragile and tend to deteriorate faster than dried samples.
Comparison between fresh and dried Moringa oliefera leaves showed no significant effect in total phenolics but with higher flavonoids content in dried sample 5. Grinded vs. powdered samples: Lowering particle size increases surface contact between samples and extraction solvents. Grinding resulted in coarse smaller samples; meanwhile, powdered samples have a more homogenized and smaller particle, leading to better surface contact with extraction solvents. This particular pre-preparation is important, as for efficient extraction to occur, the solvent must make contact with the target analytes, and particle size smaller than 0.5 mm is ideal for efficient extraction 6. This particular size of particle was mentioned in Sulaiman et al., preparing vegetable samples that were ground to 400 µm (0.4 mm) in size. Conventional mortar and pestle or electric blenders and mills are commonly used to reduce the particle size of the sample.
Investigation of nano-particles powder of Centella asiatica produced by Planetary Ball Mill (PBM) showed 82.09% higher yield compared to micro powder using maceration technique in 90% methanol for 3 days 7. Particle size was a major factor when using enzyme-assisted extraction. Use of pectinlytic and cell wall polysaccharide degrading enzyme in sample preparation was influenced majorly by the particle size as smaller particle enhances enzyme action.
Air-Drying, Microwave-Drying, Oven-Drying and Freeze-Drying Lyophilisation of Plants Samples: Air-drying usually takes from 3-7 days to months and up to a year depending on the types of samples dried (e.g., leaves or seed). Plant samples usually plant leaves with stem were tied together and hang to expose the plant to air at ambient temperature. This drying method does not force dried plant materials using high temperature; hence, heat-labile compounds are preserved. However, air-drying take longer time in comparison to microwave dries and freeze-drying and may be subjected to contamination at unstable temperature condition. Microwave-drying uses electromagnetic radiation that possesses both electric and magnetic fields.
The electric field causes simultaneous heating through dipolar rotation, alignment on the electric field of the molecules possessing a permanent or induced dipole moment (e.g., solvents or samples), and ionic induction that produce oscillation of the molecules 8. Oscillation causes collisions between molecules and resulted in the fast heating of the samples simultaneously. This method can shorten drying time but sometimes causes degradation of phytochemicals. Oven-drying is another pre-extraction method that uses thermal energy to remove moisture from the samples. This sample preparation is considered as one of the easiest and rapid thermal processing that can preserved phytochemicals.
Oven-drying at 44.5 °C for 4 h using 80% methanol resulted in the highest antioxidants activities in Cosmos caudatus extracts, and the similar result was observed in optimized 80% methanol extracts at 44.12 °C for 4.05 h 9. A shorter period of extraction time was obtained using this method. However, the effect of drying on Orthosiphon stamineus showed no significant effect on the antioxidant activity, but the bioactive phytochemicals; such as sinensetin and rosmarinic acid content, were affected by the oven and sunlight-drying, suggesting the sensitivity of the compounds to temperature 10. Freeze-drying is a method base on the principle of sublimation. Sublimation is a process when a solid is changed into a gas phase without entering the liquid phase. A sample is frozen at -80 °C to -20 °C prior to lyophilization to solidify any liquid (eg. solvent, moisture) in the samples. After an overnight (12 h) freezing, the sample is immediately lyophilized to avoid the frozen liquid in the sample from melting. The mouth of the test tube or any container holding the sample is wrapped with needle-poked-parafilm to avoid loss of sample during the process. Most of the time, the sample was lost by splattering out into the freeze-flask Fig. 1A and 1B. Freeze-drying yielded to a higher level of phenolic contents compared to air-dying as most of the phyto-chemicals are preserved using this method. However, freeze-drying is a complex and expensive method of drying compared to regular air drying and microwave drying. Thus, the use is restricted to delicate, heat-sensitive materials of high value.
Microwave Dryer:
FIG. 1: FREEZE DRYER
Air Dryer:
FIG. 2: MICROWAVE DRYER
FIG. 3: AIR DRYER
Extraction Methods: Extraction is the separation of medicinally active portions of the plant using selective solvents through standard procedures 11. The purpose of all extraction is to separate the soluble plant metabolites, leaving behind the insoluble cellular marc (residue). The initial crude extracts using these methods contain a complex mixture of many plant metabolites, such as alkaloids, glycosides, phenolics, terpenoids, and flavonoids. Some of the initially obtained extracts may be ready for use as medicinal agents in the form of tinctures and fluid extracts but some need further processing. Several of the commonly used extraction methods are discussed below:
Maceration, Infusion Percolation and Decoction: Maceration is a technique used in winemaking and has been adopted and widely used in medicinal plant research. Maceration involved soaking plant materials (coarse or powdered) in a stoppered container with a solvent and allowed to stand at room temperature for a period of minimum 3 days with frequent agitation 11. The process intended to soften and break the plant’s cell wall to release the soluble phytochemicals. After 3 days, the mixture is pressed or strained by filtration. In this conventional method, heat is transferred through convection and conduction, and the choice of solvents will determine the type of compound extracted from the samples. Infusion and decoction use the same principle as maceration; both are soaked in cold or boiled water. However, the maceration period for infusion is shorter, and the sample is boiled in a specified volume of water (e.g. 1:4 or 1:16) for a defined time for decoction 11. A decoction is only suitable for extracting heat-stable compounds, hard plant materials (e.g., roots and barks) and usually resulted in more oil-soluble compounds compared to maceration and infusion. Unique equipment called percolator Fig. 1C and 1D is used in percolation, another method that shares a similar fundamental principle. Dried powdered samples are packed in the percolator, added with boiling water, and macerated for 2 hours. The percolation process is usually done at a moderate rate (e.g. 6 drops /min) until the extraction is completed before evaporation to get concentrated extracts 12.
FIG. 4: EXTRACTION PROCESS
Soxhlet Extraction or Hot Continuous Ex-traction: In this method, a finely ground sample is placed in a porous bag or “thimble” made from a strong filter paper or cellulose, which is a place, is in the thimble chamber of the Soxhlet apparatus. Extraction solvents are heated in the bottom flask, vaporize into the sample thimble, condense in the condenser, and drip back. When the liquid content reaches the siphon arm, the liquid contents are emptied into the bottom flask again, and the process is continued.
FIG. 5: SOXHLET EXTRACTION APPARATUS
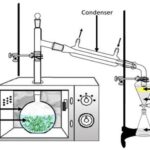
Microwave-Assisted Extraction (MAE): MAE utilizes microwave energy to facilitate the partition of analytes from the sample matrix into the solvent 21. Microwave radiation interacts with dipoles of polar and polarizable materials (e.g., solvents and sample) causes heating near the surface of the materials, and heat is transferred by conduction. Dipole rotation of the molecules induced by microwave electro-magnetic disrupts hydrogen bonding, enhancing the migration of dissolved ions and promotes solvent penetration into the matrix 8. In non-polar solvents, poor heating occurs as the energy is transferred by dielectric absorption only 11. MAE can be considered as selective methods that favour polar molecules and solvents with high dielectric constant. Strength and limitation: This technique reduced extraction time and solvent volume as compared to the conventional method (maceration & Soxhlet extraction). Improved recoveries of analytes and reproducibility were observed in MAE method but with caution of using proper conditions to avoid thermal degradation.
Accelerated Solvent Extraction (ASE): ASE is an efficient form of liquid solvent extraction compared to maceration and Soxhlet extraction as the method uses a minimal amount of solvent. A sample is packed with inert material such as sand in the stainless steel extraction cell Fig. 1E and 1G to prevent the sample from aggregating and block the system tubing [6,29]. Packed ASE cell includes layers of the sand-sample mixture in between cellulose filter paper and sand layers Fig. 1G.
This automated extraction technology is able to control temperature and pressure for each individual sample and requires less than an hour for extraction. Similar to other solvent techniques, ASE also critically depends on the solvent types. Cyclohexane acetone solution at the ratio of 6:4 v/v with 5 min heating (50 °C) showed to yield the highest bixin from Bixa orellana with 68.16% purity 29. High recoveries (~94%) of flavonoids from Rheum palmatun were observed using 80% aqueous methanol by ASE, suggesting the suitability of this method for quality control evaluation 30. Supercritical fluid extraction (SFE) Supercritical fluid (SF) or also called as dense-gas, is a substance that shares the physical properties of both gas and liquid at its critical point. Factors such as temperature and pressure are the determinants that push a substance into its critical region.
SF behaves more like gas but has the solvating characteristic of a liquid. An example of SF is CO2 that becomes SF at above 31.1 °C and 7380 kPa. Interest in SupercriticalCO2 (SC-CO2) extraction due to excellent solvent for nonpolar analytes and CO2 is readily available at low cost and has low toxicity. Even though SC-CO2 has poor solubility for polar compounds, modifications such as adding a small amount of ethanol and methanol enable it to extracts polar compounds. SC-CO2 also produces analytes at concentrate form as CO2 vaporizes at ambient temperature. SC-solvents strength can be easily altered by changing the temperature, pressure or by adding modifiers that lead to reduce extraction time. Optimization of SC-CO2 on Wadelia calendulacea achieved its optimum yield at 25 MPa, 25 ºC temperature, 10% modifier concentration, and 90 min extraction time 31. A major drawback of this method is the initial cost of the equipment is very high 17.
FIG. 6: MICROWAVE-ASSISTED EXTRACTION
FIG. 7: ACCELERATED SOLVENT EXTRACTION (ASE)
Supercritical Fluid Extraction (SFE): Super-critical fluid (SF) or also called as dense-gas, is a substance that shares the physical properties of both gas and liquid at its critical point. Factors such as temperature and pressure are the determinants that push a substance into its critical region. SF behaves more like gas but has the solvating characteristic of a liquid. An example of SF is CO2 that becomes SF at above 31.1 °C and 7380 kPa. Interest in Supercritical CO2 (SC-CO2) extraction due to excellent solvent for nonpolar analytes and CO2 is readily available at low cost and has low toxicity. Even though SC-CO2 has poor solubility for polar compounds, modification such as adding small amount of ethanol and methanol enable it to extracts polar compounds. SC-CO2 also produces analytes at concentrate form as CO2 vaporizes at ambient temperature. SC-solvents strength can be easily altered by changing the temperature, pressure or by adding modifiers that lead to reduce extraction time. Optimization of SC-CO2 on Wadelia calendulacea achieved its optimum yield at 25 MPa, 25 ºC temperature, 10% modifier concentration and 90 min extraction time 13. A major drawback of this method is the initial cost of the equipment is very high 14.
FIG. 8: SUPERCRITICAL FLUID EXTRACTION (SFE)
Ultrasound-Assisted Extraction (UAE) Or Sonication Extraction: UAE involves the use of ultrasound ranging from 20 kHz to 2000 kHz 11. The mechanic effect of acoustic cavitation from the ultrasound increases the surface contact between solvents and samples and permeability of cell walls. Physical and chemical properties of the materials subjected to ultrasound are altered and disrupt the plant cell wall; facilitating release of compounds and enhancing mass transport of the solvents into the plant cells 26. The procedure is simple and relatively low cost technology that can be used in both small and larger scale of phyto-chemical extraction.
Strength and Limitation: The benefits of UAE is mainly due reduction in extraction time and solvent consumption. However, use of ultrasound energy more than 20 kHz may have an effect on the active phyto-chemicals through the formation of free radicals.
FIG. 9: ULTRASOUND-ASSISTED EXTRACTION (UAE)
Studies: UAE was shown to be the most effective method in propolis extraction based on high yield, extraction time 10-30 min, and high selectivity 15. UAE was employed in the extraction of thermolabile compounds, such as anthocyanin from flower parts, to reduce extraction time and avoid exposure to high temperature 17] UAE of Withania somnifera by water solvent at 15 min showed maximum yield, 11.85% compared to ethanol and water-ethanol at different 5, 15 and 20 min extraction period 26. Higher efficacy on phenolics was observed in Cratoxylum formosum extraction by ultrasound at 45 kHz, 50.33% ethanol v/v, at 65 °C for 15 min 18. However, the formation of free radicals at irradiation higher than 20 kHz might need to be considered.
Other Extraction Methods: Other methods such as accelerated solvent extraction (ASE) and supercritical fluid extraction (SFE) are also being used in the extraction of plant materials. These methods are less popular due to the high cost despite the efficiency of the methods.
DISCUSSION: All the methods that employ solvents in the procedures (maceration, MAE, UAE and ASE) are critically influenced by the types of the solvent. However, no significant effect was caused by the solvent volume used using three methods (maceration, MAE and UAE) on the biologically active compounds in the poplar type propolis at ratio (1:10 w: v), suggesting the use of solvents at a greater ratio is unnecessary 19. However, the finding is limited to an assessment of phenolic, flavonoid content, and total yield as a comparison. Maceration has been suggested by Vongsak et al. as a more applicable, convenient, and less costly method for small and medium enterprises (SMEs) compared to other modern extraction methods. However, chemical waste is a major issue in maceration technique as compared to MAE and UAE, which is known as the “Green method 20. Although all these extraction methods resulted in crude extracts containing a mixture of metabolites, the efficacy of those crude extracts using nano-encapsulated processing in Centella asiatica showed to have similar efficacy as those purified 21. This particular fact suggests that further isolation and purification of extracts, which is rather complex and time-consuming, is not necessary if proper preparation and extraction are done.
Certain factors such as temperature and light need to be evaluated to extracts thermo-labile compounds. A slightly acidic solvent (0.1% HCl methanol v/v) was used to extract anthocyanin from the red and blue flowers, pointed the effect of pH in extraction procedures 22. Hydrochloric acid in the ethanol system was found to be more efficient than acetic acid in the extraction of anthocyanin 23. Among parameters such as solvent types, solvent strength, extraction time, agitation speed, sample-solvent ratio, and temperature investigated using factorial design experiment, solvent strength, which is 70% ethanol, is the most influential factor in Curcuma longa extraction 24. A similar observation of 70% ethanol as the most influential parameters was seen in triterpenoids extraction from Jatropha curcas leaves 25 and phenolic extractions from Moringa oliefera 5.
Among those optimization studies, the most influential parameter in almost all methods is solvent types and strength. However, solvent sample ratio is reported to have no significant effect, suggesting an unnecessary large volume of solvents can be avoided. Each optimized method is unique to the plants. All the influential factors (temperature, solvents, agitation speed and etc.) might have the ability to enhance extraction, but without proper judgment, may cause degradation of compounds. Thus, considering methods that have the least influential factors might be a wise selection step in choosing suitable methods. However, in the case of purity is a concern, advanced extraction technology such as ASE should be considered.
CONCLUSION: All stages of extractions, from the pre-extraction and extraction are equally important in the study of medicinal plants. The sample preparation such as grinding and drying affected the efficiency and phytochemical constituents of the final extractions, that eventually have an effect on the final extracts.
It can be concluded that no universal extraction method is the ideal method and each extraction procedure is unique to the plants. Previously optimized methods can be used to lead in the selection of suitable methods. However, the evaluation and selection of pre-extraction preparation and extraction methods depend on the study objectives, samples, and target compounds.
ACKNOWLEDGEMENT: Nil
CONFLICTS OF INTEREST: Nil
REFERENCES:
- Venugopal R and Liu RH: Phytochemicals in diets for breast cancer prevention: The importance of resveratrol and ursolic acid. Food Sci Hum Wellness 2012; 1: 1-13.
- Willcox BJ, Willcox DC, Todoriki H, Fujiyoshi A and Yano K: Caloric restriction, the traditional okinawan diet, and healthy aging the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann NY Acad Sci 2007; 114: 434-55.
- Willcox DC, Willcox BJ, Todoriki H and Suzuki M: The okinawan diet health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J Am Coll Nutr 28: 500-16.
- Mukherjee PK, Maity N, Nema NK and Sarkar BK: Bioactive compounds from natural resources against skin aging. Phyto Medicine 2011; 19: 64-73.
- Vongsak B, Sithisarn P, Mangmool S, Thong praditchote S and Wongkrajang Y: Maximizing total phenolics total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method Ind. Crops Prod 44: 566-71.
- Methods optimization in accelerated solvent extraction in. Technical Note 2013; 208: 1-4.
- Borhan MZ, Ahmad R, Rusop MM, Abdullah S: Impact of nano powders on extraction yield of Centella asiatica. Adv Mater Res 2013; 667: 246- 250.
- Kaufmann B and Christen P: Recent extraction techniques for natural products: microwave-assisted extraction and pressurized solvent extraction. Phytochem Anal 2002; 13: 105-13.
- Mediani FA, Khatib A and Tan CP: Cosmos caudatus as a potential source of polyphenolic compounds: optimisation of oven drying conditions and characterisation of its functional properties. Molecules 2013; 18: 10452-464.
- Abdullah S, Shaari AR and Azimi A: Effect of drying methods on metabolites composition of misaikucing orthosiphonst amineus APCBEE Procedia 2012; 2: 178-82.
- Handa SS, Khanuja SPS, Longo G and Rakesh DD: Extraction technologies for medicinal and aromatic plants, (1stedn), no. 66. italy: united nations industrial development organization and the. International Centre for Science and High Technology 2011.
- Rathi BS, Bodhankar SL and Baheti AM: Evaluation of aqueous leaves extract of Moringa oleifera Linn for wound healing in albino rats. Indian J Exp Biol 2012; 44: 898-901.
- Patil, Sachin BS, Wakte PS and Shinde DB: Optimization of supercritical fluid extraction and HPLC identification of wedelolactone from Wedelia calendulacea by orthogonal array design. J Adv Res 2013; 5: 629-635.
- Naudé Y, De Beer WHJ, Jooste S, Van Der Merwe L and Van Rensburg SJ: Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of DDT, DDD and DDE in sediment. Water SA 1998; 24: 205-14.
- Trusheva B, Trunkova D and Bankova V: Different extraction methods of biologically active components from propolis a preliminary study. Chem Cent J 2007; 13.
- Puttarak P and Panichayupakaranant P: A new method for preparing pentacyclic triterpene rich Centella asiatica Nat Prod Res 2013; 27: 7.
- Ebrahim N, Kershi M and Butnariub M: Anti oxidant activity and anthocyanin content in flower of Mirabilis jalab l collected from yemen. World Appl Scinces J 29: 247-51.
- Yingngam B, Monschein M and Brantner A: Ultrasound-assisted extraction of phenolic compounds from Cratoxylum formosum ssp. Form osum leaves using central composite design and evaluation of its protective ability against H2O2-induced cell death. Asian Pacific Journal of Tropical Medicine 2014; 7: 497-05.
- Trusheva B, Trunkova D and Bankova V: Different extraction methods of biologically active components from propolis a preliminary study. Journal Central Chemistry 2007; 13.
- Dhanani T, Shah S, Gajbhiye NA and Kumar: Effect of extraction methods on yield, phyto-chemical constituents and antioxidant activity of Withania somnifera. Arab J Chem 2013.
- Kwon MC, Choi WY, Seo YC, Kim JS and Yoon CS: Enhancement of the skin-protective activities of Centella asiatica l urban by a nano-encapsulation process. J Bio Technol 157: 100-06.
- Vankar PS and Srivastava J: Evaluation of anthocyanin content in red and blue flowers. Int J Food Eng 2010; 6: 4.
- Oancea S, Stoia M and Coman D: Effects of extraction conditions on bioactive anthocyanin content of Vaccinium corymbosum in the perspective of food applications. Procedia Eng 2012; 42: 489-495.
- Paulucci VP, Couto RO, Teixeira CCC and Freitas LAP: Optimization of the extraction of curcumin from curcuma longa rhizomes. Brazilian J Pharmacogn 2013; 23: 94-100.
- Wei L, Zhang W, Yin L, Yan F and Xu Y: Extraction optimization of total triterpenoids from Jatropha curcas leaves using response surface methodology and evaluations of their antimicrobial and antioxidant capacities. Electron J Bio Technol 2015; 18: 88-95.
How to cite this article:
Thorat VH, Harinath NM, Tamboli FA and Jadhav A: Novel extraction process uses in medicinal plants a review. Int J Pharmacognosy 2020; 8(4): 138-45. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.8(4).138-45.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
138-145
856
929
English
IJP
H. V. Thorat *, N. M. Harinath, A. F. Tamboli and A. Jadhav
Department of Pharmacognosy, Bharati Vidyapeeth College of Pharmacy, Kolhapur, Shivaji University, Kolhapur, Maharashtra, India.
Vishalthorat15@gmail.com
08 February 2021
25 April 2021
29 April 2021
10.13040/IJPSR.0975-8232.IJP.8(4).138-45
30 April 2021