NAPHTHOQUINONES: A BRIEF REVIEW
HTML Full TextNAPHTHOQUINONES: A BRIEF REVIEW
Radhika S. Vyavahare *, Virendrakumar M. Kamble, Shailaja B. Jadhav and Rupali A. Jinturkar
Department of Pharmaceutical Chemistry, P. E. S Modern College of Pharmacy, Nigdi, Pune, Maharashtra, India.
ABSTRACT: Quinones are a class of extremely reactive naturally occurring chemical entities including an extensive variety of biological functions, & their reactive nature as well as structural composition is an intriguing topic that is being investigated around the globe. The group of organic quinones which include naphthoquinone. There isan ongoing theme extending across the activities, and numerous research have been developed to better understand the details of these activities. The naphthoquinones & their analogues offer several pharmacological actions, involving an antioxidant, antibiotic, antiviral, cancer prevention. antimalarial, as well as antifungal capabilities. The objectives of this study aim provide recent information from the literature on the chemistry and methods for preparation of Naphthoquinone and their pharmacological activities. All the information has been collated, set up, and arranged in rational areas to offer a recent overview of the area of naphthoquinone chemistry. The present article reveals naphthoquinones' compositional variation and fascinating biological roles. This present article reveals the understanding of the synthetic approach for several derivative, their activity and medicinal uses.
Keywords: Naphthoquinone (NQ), Review, quinone
INTRODUCTION: The biosynthetic origin and structural characteristics of secondary metabolites in plant species are the key categories for classification. Citing as pertinent examples flavonoids, alkaloids, terpenes, coumarins, phenolic acids, and quinones 1. There are around 1200 naturally occurring chemicals that fall under the category of quinones, which have an ubiquitous quinoid structure 2. The existence of substituted molecules either inside the quinonic or on the neighbouring rings greatly impacts how quinones function chemically.
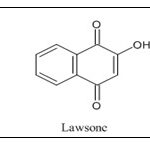
Different medicinal properties can be seen when quinone analogues containing hydroxyl groups are used. There are various quinones in nature that have a direct connection to a quinone structure by multiple hydroxyl groups. The quantity, structural diversity, and vast array of potent pharmacological benefits of quinone derivatives, such as vitamin K, plumbagin, juglone, lawsone, and shikonin, interested specialists across all over globe 3.
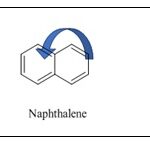
To create 1,4-naphthoquinone 4, a chemical in which the 1,4-quinoid nucleus is annulated with an aromatic (benzene) ring, two benzene ring atoms in the -position of the naphthalene nucleus must be oxidised. The quinone ring has a system of double bonds conjugated to carbonyl groups, making it easily reacted with by O-, N-, and Snucleophiles. By using a variety of agents, 1,4-naphthoquinone can be quickly reduced and changed into 1,4-dihydroxynaphthalene. Lawson and juglone 5, two naturally occurring hydroxyl derivatives of 1,4-naphthoquinone with hydroxyl groups in the α- and β-αpositions of the naphthalene core, are employed as dyes 6.
Structural Diversities of Naphthoquinone Entities: From a structural perspective, naphthoquinones are bicyclic compounds because they have two carbonyl groups in either position 1, 4 or 1, 2. Naphthoquinones' chemistry and biological function are significantly influenced by the location and chemical composition of the side groups that are attached to them. The naphthoquinone’s ring structure is coated with a variety of groups, including hydrogen, hydroxyl, methyl, nitrogen, sulphur, and halide. A hydroxyl and/or methyl group are often present in a quinone's structure in nature. There have been numerous recognised pharmacological uses for these substances 7.
Chemistry of Naphthoquinone: Naphthoquinones are chemical compounds that are structurally linked to naphthalene. They are known as 1,4-naphthoquinones because they have two carbonyl groups in the 1,4 positions. There is a small chance that carbonyl groups will also be present at the 1,2 locations 8. With hues ranging from yellow to red, naphthoquinones are highly reactive chemical molecules that are employed as natural or artificial dyes. These are α, β-unsaturated carbonyl compounds, together with their derivatives. 1,4-Naphthoquinone, which has a bright tint, is produced by the conjugation of double bonds with carbonyl groups 9.
In several traditional Asian remedies, naphthoquinones coloured chemical compounds that are naturally occuring secondary metabolites of plants are employed 10-13. Due to their large and diverse range of biological action, naphthoquinone nuclei have previously been thoroughly investigated by the field of medicinal chemistry.) Many natural and synthetic compounds of naphthoquinone have potent antibacterial, anti-inflammatory, neuroprotective, and cytotoxic properties 14–18.
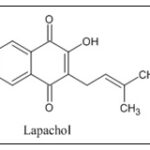
A wide range of chemical compounds, such as benzoic acid and its derivatives, benzaldehydes, cyclopentene dialdehydes, flavonoids, which furan, a naphthoquinone, quinones, which naphthoquinones and anthraquinones, found abundant in the inside peel of tree and duramen of red lapacho 19. Lapachol, known for its cancer-fighting properties, was the earliest naphthoquinone to be extracted using duramen of red lapacho. The formula of this compound represents C15H14O3 20.
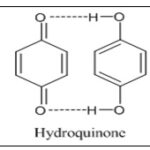
The photo chromic group known as quinones is well recognised for having two characteristic forms: a coloured quinone form and a colourless hydroquinone form. The two kinds can be combined through chemical or electrochemical stimulation. Seldom are photochromic quinones-based photo switchable fluorescence switches found. This redox mechanism is used in numerous biological electron transport systems. It is a reversible process in which two protons and two electrons are exchanged in a protic medium to convert quinone to hydroquinone. Hydroquinones are excellent electron donors and acceptors, while quinones have demonstrated to be good electron acceptors 21–24.
The molecular composition of the monomeric naphthoquinones C1 and C4 (1,4-naphthoquinones) or C1 and C2 (1,2-naphthoquinones) depends upon the Naphthalene framework with carbonyl groups placed at locations. Whenever combined, monomeric naphthoquinones can form dimers, trimers and less frequently, tetramers with a variety of possible an alternative class 25.
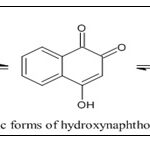
Structural Properties of Naphthoquinone: A typical C9-C13 chain hydroxynaphthoquinone is a crystalline yellow solid with a melting point of between 70 and 130 degrees. Somewhat soluble in alcohol or lignin, more soluble in acetic acid, benzene, or ether and only very marginally soluble in water 26. Lawson has the chemical formula C10H6O3 and a melting point of 190°C. There are three tautomeric variants of it. The most stable tautomeric form is the 1,4-naphthoquinone structure, followed by the 1,2-naphthoquinone and 1,2,4-naphthotrione. The trione system is the least stable but is likely in equilibrium in solution with the other two tautomeric forms. The 1,4 isomer's stability results from the cancelling of carbonyl groups' dipolar moments in conjunction with intramolecular hydrogen bonds 27.
Quinones, semiquinones (the one-electron reduced form of quinone) 28 and catechol (the two-electron reduced form) are equivalent materials that tend to link to the ions of metals in three distinct modes of oxidation, and it is suggested that these molecules have an essential function in biological processes partly because of their capacity to adhere 29. The catalytic process for oxidation is initiated by the metal focused which may sustain the coordinated semiquinone (II) and catechol (III) forms of the naphthoquinone 30.

Synthetic Approaches of Naphthoquinone: There are many methods to synthesize naphthoquinone and its derivatives. In which they are listed below.
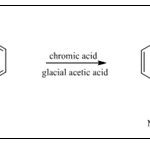
Synthesis from Naphthalene: For the synthesis of 1,4-napthoquinone firstly the naphthalene was mixed with the chromic acid and the glacial acetic acid 31-32.
Synthesis from Hydroquinone: To synthesizenaphthoquinone from hydroquinone by using organic solvent 1,4-dioxane and Heteropoly acid (HPA). Then it is washed with water and it is dried over by using v2o5.
The formed product is benzoquinone then this reacts with the isoprene &the formation of substituted tetrahydro-1,4-naphthoquinone (THNQ). Treatment of THNQ with heteropoly acid solution the formation ofsubstituted dihydro-1,4-naphthoquinone (DHNQ) and again treated with heteropoly acid the reduction reaction gets occurred and formation of desired substituted 1,4-naphthoquinonethe way displayed in the parts that follow schematic 33.
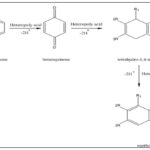
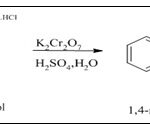
Synthesis from 1, 4-aminonaphthol hydro-chloride: 1,4-napthoquinone can be prepared by oxidation of 1,4-aminonaphthol with the help of potassium dichromate or ferric chloride and concentrated sulphuric acid 31.
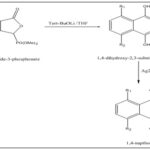
Synthesis from dimethyl phthalide-3-phosphonate: Dimethyl phthalide-3-phosphonate was mixed with tetrahydrofuran solution and with tert-BuOLi at -78°C for 30 minutes after work-up will gave 1,4-dihydroxy-2,3-substituted naphthalene by annulation process.
Some of substituted naphthalene-1,4-diol can be easily reacted with the oxidizing agent Ag2O in the presence of ether which will form 1,4-napthoquinone 34.
Synthesis from Phenylacetic Acid Derivative: The ketone with a good yield is obtained by acylating the phenylacetic acid derivative with the corresponding amounts of hexanoic anhydride in toluene that contains catalytic perchloric acid. by cyclization process ketone gets converted into 1,3,6,8- tetraoxygenated-2-alkyl-naphthalene with the major product as a naphthoquinone 35.
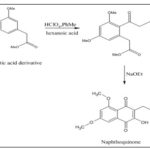
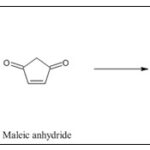
Synthesis from 1,4-dimethoxybenzene: Synthesis of naphthoquinone from the reaction in between 1,4-dimethoxybenzene and maleic anhydride by using AlCl3 and NaCl were put together to generate naphthazarin, a precursor of a naphthoquinone 36-37.
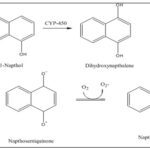
Synthesis from 1-napthol: The generation of a polyol by 1-naphthol's hydrogenation via OH' and/or Cytochrome P-450 occurs within oxidation process of a polyol to 1,4-napthoquinone 38.
Therapeutic Activities of Naphthoquinone: Since, they have biological attributes, industrial uses, and their capacity to function an intermediary in the biosynthesis of heterocyclic compounds, naphthoquinones are one synthetic group that has maintained of constant fascination over the past few decades 39-40.
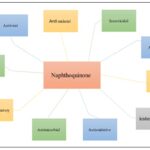
This is worthwhile to point out both cellular biological as well as pharmacological features have also been recorded for such molecules in either a diversity of research, involving tissue regeneration, anti - fungal, anti-inflammatory, anti - oxidative, leishmanicidal, as well as antineoplastic activity 41–46. The several treatments, notably streptonigrin1, actinomycins 2, mitomycins 3, as well as others, are documented to be containing the 1,4-naphtoquinone subunit, which provides antitumor potential 47-49.
Naphthoquinone-containing composites exhibit favourable efficacy towards human cervical carcinoma, breast cancer, liver cancer, and Gastric cancer 48-51.
Numerous organic and chemical substances do have 1,4-naphthoquinone framework, as well as the dual attached carbonyl compounds at C1& C4 is what really give this complex their biological function 52. According to research detailing to creation, manipulation, or investigation of a biological functions of organic products with a 1,4-naphthoquinone nucleus 53.
FIG. 1: NAPHTHOQUINONE SHOWING DIFFERENT KINDS OF ACTIVITIES
SUMMARY: A wide range of a novel 1,4-naphthoquinones have been created identified via natural sources all throughout the previous ten years.the many kinds of biological functions performed by naphthoquinones indicate that they are an appealing class of synthetic substances. 1,4-naphthoquinones represent a category of chemical compounds that possesses an enormous variety of biological roles and have considerable possibilities for usage in medication. The scientific study of naphthoquinone is expected to be promising in the coming decades.
The enormous quantity of literature that has been constantly expanding over time will make sure that the chemistry of naphthoquinone maintains an appropriate study in the decades afterward. However, the combinatorial properties of Naphthoquinones & their analogues positioned them to be an emerging substance in the field of therapeutic chemistry.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Bernards MA: Plant natural products: a primer. Canadian Journal of Zoology 2010; 88: 601- 614.
- Thomson RH: Naturally Occurring Quinones. London: Chapman & Hall 1987 111.
- Tandon VK, Yadav DB, Singh RV, Chaturvedi AK and Shukla PK: Bioorg. Med Chem Lett 2005; 15: 5324–5328.
- Thomson RH: “Naturally occurring quinones,” 4th ed., Blackie Academic and Professional, London-New York, 1997.
- Tomaszkiewicz-Potępa A, Vogt O, Wiadom. Chem 2004; 58: 881–894.
- Hook I, Mills C and Sheridan H: “Bioactive naphthoquinones from higher plants,” Studies in Natural Product Chemistry, Vol. 41, ed. by Atta-ur-Rahman, Elsevier Science Publisher, Amsterdam 2014; 119–160.
- Sánchez-Calvo JM, Barbero GR, Guerrero-Vásquez G, Durán AG, Macías M, Rodríguez-Iglesias, MA, Molinillo JM and Macías FA: Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: A structure–activity relationship study. Med Chem Res 2016; 25: 1274–1285.
- Kumagai Y, Shinkai Y, Miura T and Cho AK: The chemical biology of naphthoquinones and its environmental implications. Annu Rev Pharmacol Toxicol 2012; 52: 221-247.
- López Ll, Leyva E and García R: Las naftoquinonas: más que pig- mentos naturales. Rev Mex Cienc Farm 2011; 42(1): 6-17.
- López López LI, Nery Flores SD, Silva Belmares SY and Sáenz Galindo A: Naphthoquinones: Biological properties and synthesis of lawsone and derivatives A structured review. Vitae 2014; 21: 248–258.
- Vukic MD, Vukovic NL, Djelic GT, Popovic SL, Zaric MM, Baskic DD, Krstic GB, Tesevic VV and Kacaniova MM: Antibacterial and cytotoxic activities of naphthoquinone pigments from onosma Visianii clem. EXCLI J 2017; 16: 73–88.
- Tandon VK, Chhor RB, Singh RV, Rai S and Yadav DB: Design, synthesis, and evaluation of novel 1,4-naphthoquinone derivatives as antifungal and anticancer agents. Bioorganic Med Chem Lett 2004; 14: 1079–1083. [CrossRef] [PubMed]
- Kim BH, Yoo J, Park SH, Jung JK, Cho H and Chung Y: Synthesis, and evaluation of antitumor activity of novel 1,4- naphthoquinone derivatives (IV). Arch Pharm Res 2006; 29: 123–130.
- Linzner N, Fritsch VN, Busche T, Tung QN, Van Loi V, Bernhardt J, Kalinowski J and Antelmann H: The plant-derived naphthoquinone lapachol causes an oxidative stress response in Staphylococcus aureus. Free Radic Biol Med 2020; 158: 126–136.
- Song R, Yu B, Friedrich D, Li J, Shen H, Krautscheid H, Huang SD and Kim MH: Naphthoquinone-derivative as a synthetic compound to overcome the antibiotic resistance of methicillin-resistant aureus. Commun Biol 2020; 24: 3.
- Novais JS, Carvalho MF, Ramundo MS, Beltrame CO, Geraldo RB, Jordão AK, Ferreira VF, Castro HC and Figueiredo AMS: Antibiofilm effects of N, O-acetals derived from 2-amino-1,4-naphthoquinone are associated with down- regulation of important global virulence regulators in methicillin-resistant Staphylococcus aureus. Sci Rep 2020; 10: 19631.
- Campora M, Canale C, Gatta E, Tasso B, Laurini E, Relini A, Pricl S, Catto M and Tonelli M: Multitarget Biological Profiling of New Naphthoquinone and Anthraquinone-Based Derivatives for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci 2021; 12: 447–461.
- Oliveira V, Dantas E, Queiroz A, Oliveira J, Silva M, Ferreira P, Siva F, Ferreira V and Lima Á: Novel Solid Dispersions of Naphthoquinone Using Different Polymers for Improvement of Antichagasic Activity. Pharmaceutics 2020; 12: 1136.
- Castellanos JRG, Prieto JM and Heinrich M: Red Lapacho (Tabebuia impetiginosa) - a global ethnopharmacological commodity? J. Ethnopharmacol 2009; 121: 1–13. https://doi.org/10.1016/j.jep.2008.10.004.
- da Silva MN, Ferreira VF, Souza MCB and de V: Um panorama atual da química e da farmacologia de naftoquinonas, com ˆenfase na β-lapachona e derivados. Quim. Nova 2003; 26: 407–416. https://doi.org/10.1590/S0100- 40422003000300019.
- Illos RA, Shamir D, Shimon LJW, Zilbermann I and Bittner S: N-Dansyl-carbazoloquinone; a chemical and electrochemical flu- orescent switch, Tetrahedron Lett 2006; 47: 5543–5546.
- A. Illos, E and Harlev S: Bittner, A novel all-organic chemical and electrochemical fluorescent switch, Tetrahedron Lett 2005; 46: 8427–8430.
- Kutzki O and Montforts FP: Synthesis of a chlorine-quinone dyad and a benzochlorin derivative, Synlett 1 2001; 53–56.
- Ravichandiran P, Santhoshkumar P and Vasanthkumar S: Synthe- sis of chemical and electrochemical ‘off–on–off’ fluorescent switches of new 5H-benzo[b]carbazole-6,11-dione deriva- tives, J Saudi Chem Soc 2013; http://dx.doi.org/10.1016/ j. jscs.2013.08.002.
- Babula P, Adam V, Havel L & Kizek R: Noteworthy secondary metabolites naphthoquinones– their occurrence, pharmacological properties, and analysis. Current Pharmaceutical Analysis 2009; 5: 47-67.
- Fieser and Fieser: This Journal 1934; 56: 1565.
- Lamoureux G, Perez A, Araya M and Agüero C: Reactivity and struc- ture of derivatives of 2-hydroxy-1,4-naphthoquinone (lawsone). J Phys Org Chem 2008; 21(12): 1022-1028.
- Salunke-Gawali, L. Kathawate, Y. Shinde, V. G. Puranik and T. Weyhermüller: J Mol Struct 2012; 1010, 38-45.
- Valle-Bourrouet, V. M. Ugalde-Saldívar, M. Gómez, L. A. Ortiz-Frade, I. González and C. Frontana, Electrochim. Acta 2010; 55: 9042-9050.
- M. El-Hendawy, Polyhedron 1991; 10: 2511-2518.
- Conant and Fieser, J. Am. Chem. Soc., 46, 1862 (1924); Fieser and Fieser, J. Am. Chem. Soc., 57, 491 (1935); Org. Syntheses Coll. Vol. 1, 383 (1946).
- Miller J. Russ. Phys. Chem. Soc., 16, 414 (1884); Japp and Miller, J. Chem. Soc., 39, 220 (1881).
- Gogin, L.L., Zhizhina, E.G., Pai, Z.P. and Parmon, V.N. (2015) Prospects of Using Мо-Vphosphoric Heteropolyacid Solutions as Bifunctional Catalysts for Syntheses of Anthraquinones and Their Substituted Derivatives. Russian Chemical Bulletin, 64, 2069-2075. https://doi.org/10.1007/s11172-015-1119-8.
- Watanabe M, Morimoto H, Nogami K, Ijichi S & Furukawa S: An Annulation Reaction to Naphthalene-1,4-diols Using Dimethyl Phthalide-3-phosphonates. Chemical & Pharmaceutical Bulletin 1993; 41: 968-970.
- McCulloch MW & Barrow RA: Towards a synthesis of naphthalene derived natural products. Molecules (Basel, Switzerland), 2005; 10(10): 1272–1278. https://doi.org/10.3390/10101272.
- Shen GN, Choi JH, Gajulapati K, Lee JH, Kim YK, Rho MC and Choi Y: 2-Substitiuted Thio- and Amino-5,8-dimethoxy-1,4- naphthoquinones as a novel class of acyl-CoA: Cholestrol acyltransferase inhibitors. Bulletin of the Korean Chemical Society 2009; 30(5): 1088–1092.
- Sun HN, Shen GN, Jin YZ, Jin Y, Han YH, Feng L and Jin CH: 2-cyclohexylamino-5,8-dimethoxy-1,4-naphthoquinone inhibits LPS- induced BV2 microglial activation through MAPK/NF-kB signal- ing pathways. Heliyon 2016; 2(7): e00132. https://doi.org/10.1016/j.heliyon. 2016.e00132.
- Fluck D, Rappaport SM, Eastmond DA & Smith MT: Conversion of 1-naphthol to naphthoquinone metabolites by rat liver microsomes: demonstration by high-performance liquid chromatography with reductive electrochemical detection. Archives of Biochemistry and Biophysics 1984; 235 2: 351-8.
- For some recent references on biological properties of naphthoquinones see: (a) Wurm, G.; Schwandt, S. Phar- mazie 1999,547, 487; (b) Wurm, G.; Probst, R.; Bruemmer,U. Sci. Pharm. 1998, 66, 279; (c) Lin, T.-S.; Xu, A.-P.; Zhu, L.-Y.; Cosby, L.; Sartonelli, A. J. Med. Chem. 1989, 32, 1467; (d) Kappe, T.; Wildpanner, H. Monatsch. Chem. 1988; 119: 727; (e) Lin, T.-S.; Xu, A.-P.; Zhu, L.-Y.; Divo, A.; Sartonelli, A. J. Med. Chem. 1981, 34, 1634; (f) Jacobsen, N.; Wengel, A. Pestic. Sci. 1986, 17, 686; (g) Hudson, A. T.; Pether, M. J.; Randall, A. W.; Fry, M.; Latter, V. S.; McHardy, N. Eur. J. Med. Chem. 1986, 21, 271; (h) Ikushima, H.; Takase, S.; Kawai, Y.; Itoh, Y.; Okamoto, M.; Tanaka, H.; Imanaka, H. Agric. Biol. Chem. 1983, 47, 2231; (i) Boehm, P.; Cooper, K.; Hudson, A. T.; Elphick, J. P.; McHardy, N. J. Med. Chem. 1981, 24, 295; (j) Ikushima, H.; Okamoto, M.; Tanaka, H.; Ohe, O.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. Antibiot. 1980, 33, 1107.
- For industrial uses of naphthoquinones see: (a) For appli- cations in color chemistry, see: Yoshida, K.; Yamanaka, Y.; Euno, Y. Chem. Lett. 1994, 2051; Takagi, K.; Mat- suoka, M.; Hamano, K.; Kitao, T. Dyes Pigments 1984, 5, 241. (b) For applications in hair dying, see: Kikuchi, M.; Komatsu, K.; Nakano, M. Dyes Pigments 1990, 12, 107. (c) For applications as photostabilizers, see: Escolas- tico, C.; Santa Maria, M. D.; Claramunt, R. M.; Jimero, M. L.; Alkorta, I.; Foces-Foces, C.; Cano, F. H.; Elguero, J. Tetrahedron 1994, 50, 12489.
- Sánchez-Calvo JM, Barbero GR, Guerrero-Vásquez G, Durán AG, Macías M and Rodríguez-Iglesias MA: Synthesis, antibacterial and antifungal activities of naphthoquinone derivatives: a structure–activity relationship study. Med Chem Res 2016; 25: 1274–1285.
- Durán AG, Chinchilla N, Molinillo JM and Macías FA: Influence of lipophilicity in O-acyl and O-alkyl derivatives of juglone and lawsone: a structure-activity relationship study in the search for natural herbicide models. Pest Manag Sci 2018; 74: 682–694.
- Kawamura M, Kuriyama I, Maruo S, Kuramochi K, Tsubaki K and Yoshida H: Anti-tumor effects of novel 5-O-acyl plumbagins based on the inhibition of mammalian DNA replicative polymerase activity. PLoS One 9: e88736 (2014).
- de Araújo M, de Souza P, de Queiroz A, da Matta C, Leite A and DaSilva A: Synthesis, leishmanicidal activity and theoretical evaluations of a series of substituted bis-2-hydroxy-1,4-naphthoquinones. Molecules 2014; 19: 15180–15195.
- McBride TJ, Oleson JJ and Woolf D: Cancer Res 1966; 26: 727.
- Reich E, Goldberg IH and Rabinowitz M: Nature 1962; 196: 743.
- Keyes SR, Loomis R, DiGiovanna MP, Pritsos CA and Rockwell S: Sartorelli, A. C. Cancer Commun 1991; 3: 51.
- Du W, Hao X and Yuan Z: Shikonin potentiates paclitaxel antitumor efficacy in esophageal cancer cells via the apop- totic pathway. Oncol Lett 2019; 18: 3195–201.
- Liu B, Jin J and Zhang Z: Shikonin exerts antitumor activity by causing mitochondrial dysfunction in hepatocellular car- cinoma through PKM2–AMPK–PGC1a signaling pathway. Biochem Cell Biol 2019; 97: 397–405.
- Li B, Yuan Z and Jiang J: Anti-tumor activity of Shikonin against afatinib resistant non-small cell lung cancer via negative regulation of PI3K/Akt signaling pathway. Biosci Rep 2018; 38: 1–9.
- Liu JP, Liu D and Gu JF: Shikonin inhibits the cell viability, adhesion, invasion, and migration of the human gastric can- cer cell line MGC-803 via the Toll-like receptor 2/nuclear fac- tor-kappa B pathway. J Pharm Pharmacol 2015; 67: 1143–55.
- Tonholo J, Freitas LR, de Abreu FC, Azeved DC, Zani C L. de Oliveira AB and Goulart MOF: Electrochemical Properties of Biologically Active Heterocyclic Naphthoquinones. J Braz Chem Soc 1998; 2; 163-169.
- Tandon VK and Kumar S: Expert Opin Ther Patents 2013; 23: 1087–1108.
How to cite this article:
Vyavahare RS, Kamble VM, Jadhav SB and Jinturkar RA: Naphthoquinones: a brief review. Int J Pharmacognosy 2023; 10(8): 454-62. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(8).454-62.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
3
454-462
674 KB
1107
English
IJP
Radhika S. Vyavahare *, Virendrakumar M. Kamble, Shailaja B. Jadhav and Rupali A. Jinturkar
Department of Pharmaceutical Chemistry, P. E. S Modern College of Pharmacy, Nigdi, Pune, Maharashtra, India.
radhikavyavahare2@gmail.com
08 August 2023
26 August 2023
30 August 2023
10.13040/IJPSR.0975-8232.IJP.10(8).454-62
31 August 2023