MEDICINAL POTENTIAL OF PHYLLANTHUS EMBLICA (LINN.) FRUITS EXTRACTS: BIOLOGICAL AND PHARMACOLOGICAL ACTIVITIES
HTML Full TextMEDICINAL POTENTIAL OF PHYLLANTHUS EMBLICA (LINN.) FRUITS EXTRACTS: BIOLOGICAL AND PHARMACOLOGICAL ACTIVITIES
S. M. Moazzem Hossen * 1, Mohi Uddin 1 and Md. Raihan Sarkar 2
Department of Pharmacy 1, University of Chittagong, Chittagong - 4331, Bangladesh.
Department of Pharmacy 2, Jagannath University, Dhaka, Bangladesh.
ABSTRACT: The objective of the present study was to investigate the scientific basis of the traditional use of the fruit of Phyllanthus emblica (Linn.). The selected pharmacological investigations are antimicrobial screening, analgesic activity, antidiarrheal activity screening and the brine shrimp lethality test for cytotoxic activity. Phytochemical analysis of ethanolic fruits extracts Phyllanthus emblica (Linn.) showed the presence of flavonoids, alkaloids, tannin, steroids, reducing sugar and gum. The fruit extract produced 19.07% and 38.67% protection or writhing inhibition at the oral dose of 250 and 500 mg/kg-body weights respectively. From the observations, it is obvious that the ethanol extract of the fruit of Phyllanthus emblica (Linn.) has analgesic activity. The ethanolic fruit extract of Phyllanthus emblica (Linn.) significantly inhibited ear edema formation in xylene-induced ear edema model mice. This inhibition can be considered as direct evidence that is supporting the anti-inflammatory activity of ethanolic fruit extract of Phyllanthus emblica (Linn.) The ethanolic fruit extract of Phyllanthus emblica (Linn.) performing as a remedy for different bacterial diseases is supported by the antibacterial screening tests. During the antidiarrheal activity screening at the dose of 500 mg/kg body weight, the extract of Phyllanthus emblica (Linn.) compared to the control group, offered about 0.5389 of the mean latent period for the diarrheal episode to ensure and the result was significant (P<0.2). The mean numbers of stool at the 1st, 2nd, 3rd and 4th hour of the study were 8.2, 9.6, 7.8 and 4.4 respectively. Phyllanthus emblica (Linn.) showed moderate antidiarrheal activity in castor oil induced test in mice and caused an increase in the latent period, i.e., delayed the onset of a diarrheal episode and decreased the frequency of defecation. The decreased frequency of defecation and increase mean latent period of test group than the control group and comparison with positive control group can claim that Phyllanthus emblica (Linn.) might possess antidiarrheal activity. A t-test of these responses showed that the result is significant throughout the observation period. The ethanolic fruit's extract have cytotoxic activity, and test sample showed a different mortality rate at different concentrations. The LC50 values were found to be 60µg/ml for the crude extract. The 90% mortality (LC90) values were 100µg/ml respectively. The approximate significance value for Chi-Square Tests (Linear by linear association) is equal to 0.02, and also significance value for Pearson’s R is 0.001 which are significant at 5% level of significant. According to the results of the present investigation, it can be concluded that the ethanolic fruits extract of Phyllanthus emblica (Linn.) has significant analgesic, anti-inflammatory, antimicrobial, anti-diarrheal and cytotoxic effects that support to the traditional use of this plant for the treatment of related diseases. This study also suggests for the further detail investigation of mechanisms of the pharmacological effects and also to isolate the active compound(s) responsible for those properties.
| Keywords: |
Phyllanthus emblica (Linn.), Antimicrobial, Analgesic, Antidiarrheal, anti-Inflammatory, Bioassay
Introduction: Phyllanthus emblica (Linn.) or Emblica Officinalis (Gaertn) belongs to the Family Euphorbiaceae.
Bengali/vernacular names are Amloki, Amla; Aila (Sylhet) and tribal names are Ambari (Garo); Amloti (Chakma); Soi sha (Marma); Sowan Lu (Bawm); Khulu (Murong). English names are Emblic Myrobalan, Indian Gooseberry 1. Fruit a globose drupe, about 2.5 cm across, obscurely 6-lobed. Occurs in the dry forests of Chittagong, Chittagong Hill Tracts, Cox’s Bazar, Sylhet, Dhaka-Tangail (Sal forest) and Dinajpur; also cultivated elsewhere 1. Fruits are diuretic, refrigerant, carminative, astringent, tonic, stomachic, laxative, antacid and rich in Vitamin C, improves appetite, useful in vomiting and burning urination, diseases of the heart and liver, piles, stops the nasal hemorrhage. It promotes children’s resistance to a cough and cold; used as a hair tonic. Dried fruits are useful in hemorrhoids, diarrhea, dysentery, anemia, jaundice, and dyspepsia. The fruits are also said to be beneficial in insomnia, skin problems, gall pain, leucorrhoea, and tympanites. Sherbet prepared from the fruit along with lemon juice is used for arresting acute bacillary dysentery. Fruits are a valuable component of “Triphala” used in different Ayurvedic preparations.
Flowers are cooling and aperient. The bark is astringent 1. Ethanolic extract of the leaves possesses good antibacterial properties and mild antifungal properties 2, 3. Phyllambin isolated from fruit potentiates pharmacological action of adrenaline, has mild depressant action on the central nervous system and possesses spasmolytic action 1. Water extract of the fruit causes moderate relaxation of the isolated guinea-pig ileum, but it does not interact with the activity of Acetylcholine 4.
Fruit is a rich natural source of vitamin C. It also contains tannins and colloidal substances, phyllembic acid, lipids, gallic acid, ellagic acid, trigalloylglucose, terchebin, corilagin, and emblicol. Phyllembin and mucic acid have been isolated from the fruit pulp. Seeds contain fixed oil, phosphatides, tannins, and essential oil. Bark, fruits, and leaves are rich in tannin. They also contain lupeol, β-sitosterol, and ellagic acid. Bark also contains leucodelphinidin. Seed oil also contains linoleic acid (64.8%), closely resembled linseed oil 1, 5, 6.
The selected pharmacological investigations are antimicrobial screening, evaluation of analgesic activity, antidiarrhoeal activity screening and the brine shrimp lethality test by using the ethanolic fruit extract of Phyllanthus emblica (Linn.). The present study investigates the phytoconstituents to correlate the folkloric claims with the bioactive compounds present in the plant and investigates the cytotoxicity potential of the plant on brine shrimp (Artemia salina) larvae. Since, toxicological evaluation of plant extracts seeks to determine its possible collateral effects to ensure the safety of its use, brine shrimp larvae being sensitive to toxic substances are commonly used for toxicity assays in pharmacology 7, 8.
MATERIAL AND METHODS:
Plant Material Collection and Identification: Fruits of Phyllanthus emblica (Linn.) were collected from the district of Chittagong and were identified by the experts and preserved in the herbarium, department of botany, University of Chittagong. (Acc. No. SBU1521).
Plant Extracts Preparation: The collected fruits were separated from undesirable plant materials or plant parts. They were air-dried for one week. The fruits were ground into a coarse powder. The powder was stored in an airtight container and kept in a cool, dark and dry place until analysis commenced. Cold extraction was performed. About 350 gm of powdered material was taken in a clean, flat bottomed glass container and soaked in 900 ml of 80% ethanol. The container with its contents was sealed and kept for 10 days accompanying occasional shaking and stirring.
The whole mixture then underwent a coarse filtration by a piece of clean, white cotton material. Then it was filtered through Whatmann filter paper [Bibby RE200, Sterilin Ltd., UK]. The filtrate (ethanol extract) obtained was evaporated under the ceiling fan and in a water- bath until dried and % yield of fruit calculated 4.57% 9. The extract was stored at 4oC until use.
Experimental Animals: Young Swiss-albino mice aged 4-5 weeks, average weight 20-25 gm were used for the experiment. The mice were purchased from the Animal Research Branch of the International Centre for Diarrhoeal Disease and Research, Bangladesh (ICDDR, B). They were kept in a standard environmental condition (RH 55% to 60%, room temperature 25 ± 2 ºC and 12 h light/ dark cycle) for one week for adaptation after their purchase and fed ICDDR, B formulated rodent food and water. The experimental study was performed under the guidelines of the Institutional Animal Ethics Committee 10.
Chemicals and Drugs: Drug Diclofenac Sodium, Kanamycin, Loperamide were from square pharmaceuticals limited and Vincristine Sulphate (VINCRIRST ®) from Techno Drugs Ltd. Used other reagents and analytical kits were laboratory reagent grade and from Merck Specialities Private Limited, India.
Phytochemical Screening: Preliminary phyto-chemical studies were performed to identify different phytochemical constituents in the extract as well as powdered drug adapting standard methods as described 11-14.
Analgesic activity of the ethanolic extract of Phyllanthus emblica (Linn.) was tested using the model of acetic acid-induced writhing in mice 15-20. Young Swiss-albino mice, the experimental animals were randomly selected and divided into four groups denoted as group-I, group-II, group III and group-IV, consisting of 5 mice in each group. Group I received 1% tween 80 in water (10 ml/kg) as control, group II received standard drug Diclofenac sodium (25 mg/kg) while group III and IV received 250 mg/kg and 500 mg/kg extract of sample respectively. Each mouse was weighed properly, and the dose of the test samples and control materials were adjusted accordingly. Test samples and diclofenac sodium were given orally using a feeding needle, and the control was given intraperitoneally. After 30 min, the writhing inducing chemical, an acetic acid solution (0.7%, 10 ml/kg) was administered intraperitoneally to each of the animal's group. After five minutes, the number of squirms (writhing) was counted for 15 min. The mice didn’t always perform full writhing. The incomplete writhing was taken as a half writhing, so two half writhing were taken as one full writhing.
Anti-inflammatory Activity: Xylene induced air edema model mice were used to assess the anti-inflammatory activity of the plant extract following the method described by Dev et al. 21 Experimental mice were divided as the previous test. Group I received 1% tween 80 in water (10 ml/kg) as control, group II received standard drug Diclofenac sodium (25 mg/kg) while group III and IV received 250 mg/kg and 500 mg/kg extract of sample respectively. One hour after administration of the above dose, 0.01 ml of xylene was injected to the anterior and posterior surfaces of the right ear of each mouse. One hour after xylene injection mice were sacrificed and both treated and untreated ears were cut down by using a 7 mm diameter cork borer as circular sections and weighed. The weight difference between untreated and treated ear sections was calculated 19 - 22.
Antimicrobial Activity: The antimicrobial screening was performed using the disc-diffusion method. Sample disc 500 µg/disc and Standard Kanamycin (30 µg/disc) discs were used as positive control and blank discs were used as negative controls. The sample discs, standard antibiotic discs, and control discs were placed gently on marked zones in the agar plate’s pre-inoculated with test bacteria, protozoa, and fungi. The plates were then kept in a refrigerator at 4 ºC for about 24 h to allow sufficient diffusion of materials from discs to surrounding agar medium. The plates were then inverted and kept in an incubator at 37 ºC for 24 h. All organisms are listed in Table 1. Antibacterial activity of the crude ethanolic extract was determined by disc diffusion method 23 - 25. The selected organisms were Gram-negative bacteria (Salmonella typi, Shigella dysenteriae, Shigella sonnei, Vibrio cholera, Hafnia, Plesiomonas), Gram-positive bacteria (Staphylococcus aureus, Staphylococcus epidermis, Staphylococcus saprophyticus, Staphylococcus pyogenic) and fungi (Candida albicans, Fusarium solanii)
Antidiarrheal Activity: Young Swiss-albino mice aged 4-5 weeks, average weight 20-25 gm were used for the experiment. The mice were all screened initially by giving 0.3 ml of castor oil and only those showing diarrhea were selected for the final experiment. The test animals were randomly chosen and divided into three groups having five mice in each; they were accurately weighed & properly marked of the experimental groups, group-I or the control received only distilled water containing 1% Tween-80 (10 ml/kg). Group-II or the positive control received the standard anti-motility drug, Loperamide (3mg/kg) as an oral suspension. The test group III was treated with a suspension of fruits extract of Phyllanthus emblica (Linn.) at the oral dose of 500 mg/kg body weight. The method, described by Chatterjee was followed for this study 26. Test samples, control, and Loperamide were given orally using a feeding needle. The mice were fed with the samples, control and Loperamide 1 hr. prior to the oral administration of castor oil at a dose of 0.5 ml per mouse. Individual animals of each group were placed in separate cages having adsorbent paper beneath and examined for the presence of diarrhea every hour in four hours study after the castor oil administration. A number of stools or any fluid material that stained the adsorbent paper were counted at each successive hour and were noted for each mouse. The latent period of each mouse also counted. At the beginning of each hour, old papers were replaced for the new ones. During an observation period, the total number of fecal output including diarrheic faces excreted by the animals was recorded. A numerical score based on stool consistency was assigned as follows: normal stool=1 and watery stool=2 27 - 29.
Brine Shrimp Lethality Bioassay: This bioassay indicates cytotoxicity as well as a wide range of pharmacological activities such as antimicrobial, anti-viral, pesticidal and anti-tumor, etc. of the compounds. For the cytotoxic activity of the ethanolic extracts of Phyllanthus emblica (Linn.) stock solution was prepared to have a concentration of 5μg/μl. Simulated sea water was prepared by dissolving 38 gm of sodium chloride per liter of distilled water and filtered. This simulated sea water was used for hatching of brine shrimp. Test solutions of different concentration (5, 10, 20, 40, 60, 80 and 160 µg/ml) were prepared from the stock solution of plant extract, and living shrimps were kept to each of the solutions. After 24 h the test tubes were observed, and the number of survived nauplii in each test tube was counted, and the results were noted. From this, the percentage of lethality of brine shrimp nauplii was calculated at each concentration for each sample 30 - 34.
% mortality = No. of dead nauplii × 100% / Initial No. of live nauplii
RESULTS:
Phytochemical Screening: Phytochemical analysis of Phyllanthus emblica (Linn.) showed the presence of flavonoids, alkaloids, tannin, steroids, reducing sugar and gum. The results were given in Table 1.
TABLE 1: TEST RESULT FOR CHEMICAL GROUPS OF PHYLLANTHUS EMBLICA (LINN.)
| S. no. | Compound groups | Present/absent |
| 1 | Flavonoid | + |
| 2 | Alkaloid | + |
| 3 | Saponin | - |
| 4 | Tannin | + |
| 5 | Steroid | + |
| 6 | Reducing Sugar | + |
| 7 | Gum | + |
EE =Ethanolic Extract, + = Present, - = Absent
Analgesic Activity: Effect of ethanolic fruits extract of Phyllanthus emblica (Linn.) on acetic acid-induced writhing test on mice model showed mild analgesic activity.
TABLE 2: ANALGESIC ACTIVITY OF PHYLLANTHUS EMBLICA (LINN.) ON ACETIC ACID INDUCED MICE MODEL
| Animal Group
and Treatment |
Writhing
count |
%
Writhing |
% Writhing inhibition | (t-test)
p values |
| Group-I 1% tween-80 solution in water | 36.2 ± 2.035 | 100 | -- | -- |
| Group-II Diclofenac sodium 25 mg/kg | 7.8 ± 1.113 | 21.55 | 78.45 | (12.243) P<0.001 |
| Group-III Extract (250 mg/kg) | 29.3 ± 1.21 | 80.93 | 19.07 | (5.87) P<0.01 |
| Group-IV Extract (500 mg/kg) | 22.2 ± 2.395 | 61.33 | 38.67 | (4.454) P<0.01 |
Values are expressed as mean ± SEM, SEM= Standard error of the mean, n=No. of mice, %=Percentage
Anti-inflammatory Activity:
TABLE 3: ANTI-INFLAMMATORY EFFECT OF ETHANOLIC FRUITS EXTRACT OF PHYLLANTHUS EMBLICA (LINN.) ON XYLENE INDUCED EAR EDEMA MODEL
| Group | n | Increased weight | Inhibition rate % |
| Blank (Xylene 0.01mL. injection) | 5 | 10.5 ± 0.22 | 00 |
| Positive control (Diclofenac sodium 10 mg/Kg) | 5 | 6.5 ± 0.23* | 35 |
| Test 1, Extract (250 mg/kg) | 5 | 7.25 ± 0.25* | 18 |
| Test 2, Extract (500 mg/kg) | 5 | 6.75 ± 0.22* | 32.5 |
* P<0.01, vs. blank control group
Antimicrobial Activity: The fruit's extract of Phyllanthus emblica (Linn.) showed mild antimicrobial activity and may be the remedy for different bacterial diseases is supported by the antibacterial and antifungal screening tests.
TABLE 4: IN-VITRO ANTIMICROBIAL ACTIVITY OF ETHANOLIC FRUIT EXTRACT OF PHYLLANTHUS EMBLICA (LINN.) BY FOLLOWING DISC DIFFUSION METHOD
| Strains | The diameter of the zone of inhibition in mm | |
| Ethanol extract (500 μg/disc) | Kanamycin (30 μg/disc) | |
| Bacterial Gram-negative | ||
| Salmonella typhi | 9.3 | 25.5 |
| Vibrio cholera | R* | 24.3 |
| Shigella dysenteriae | 7.1 | 28 |
| Plesiomonas | R* | 27.1 |
| Sheigella sonnie | 10.24 | 25 |
| Hafnia | 8.4 | 29.6 |
| Bacterial Gram-positive | ||
| Bacillus subtilis | 11.2 | 25.6 |
| Bacillus megaterium | 8.5 | 24.8 |
| Staphylococcus aureus | R* | 30 |
| Staphylococcus epidermis | 7.1 | 22.2 |
| Staphylococcus saprophyticus | R* | 23.8 |
| Staphylococcus pyogenas | 9.7 | 29.4 |
| Fungal Strain | ||
| Candida albicans | 11.0 | 27.35 |
| Fusarium solanii | 9.5 | 26.8 |
R= Resistant or No growth. The antibacterial screening tests support the fruit of Phyllanthus emblica (Linn.) performing as a remedy for different bacterial diseases.
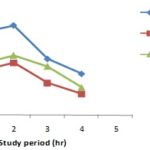
Antidiarrheal Activity: Phyllanthus emblica (Linn.) showed moderate antidiarrheal activity in castor oil induced test in mice model. Antidiarrheal activity results are summarized in Table 5 and Fig. 1.
TABLE 5: EFFECT OF PHYLLANTHUS EMBLICA (LINN.) ON CASTOR OIL INDUCED DIARRHEA IN MICE
|
Groups |
Period
of study (hr) |
Mean latent
Period ± S.E. |
Mean no.
of stools |
S.E. | t-test
(p-value)
|
|
Control
|
C1 |
0.399 ± 0.0396 |
13.2 | 0.583 | -- |
| C2 | 14.4 | 0.678 | -- | ||
| C3 | 9 | 0.447 | -- | ||
| C4 | 6.6 | 1.077 | -- | ||
| Positive
Control (Loperamide) |
P1 |
0.929 ± 0.0475 |
7 | 1 | 5.356a |
| P2 | 8.4 | 0.748 | 5.887a | ||
| P3 | 5.2 | 2.154 | 1.727c | ||
| P4 | 3.4 | 1.777 | 1.540c | ||
| Extract of Phyllanthus emblica Linn. 500 mg/kg | E1 |
0.497 ± 0.0419 |
8.2 | 1.281 | 3.5549b |
| E2 | 9.6 | 1.077 | 3.7605 b | ||
| E3 | 7.8 | 0.860 | 1.5328c | ||
| E4 | 4.4 | 0.696 | 1.7156 c |
Values are t-test. (n=5), cp<0.2, a p<0.001, b p<0.01 vs. control. Student’s t-test.
FIG. 1: EFFECT OF LOPERAMIDE AND PHYLLANTHUS EMBLICA (LINN.) ON CASTOR OIL INDUCED DIARRHOEA IN MICE THROUGH THE OBSERVATION PERIOD (4HR).
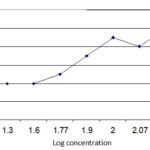
Cytotoxic Activity: Test sample showed a different mortality rate at different concentrations. The mortality rate of brine shrimp increased with the increase in the concentration of the sample and plot of percent mortality versus log concentration on the graph paper produced an approximately linear correlation between them. From the graph Fig. 2 the concentrations at which 50% mortality (LC50) of brine shrimp nauplii occurred were obtained by extrapolation. The values were found to be 60 µg/ml for the crude extract. The 90% mortality (LC90) values were 100 µg/ml respectively.
TABLE 6A: RESULT OF BRINE SHRIMP LETHALITY BIOASSAY OF ETHANOLIC EXTRACT OF FRUIT OF PHYLLANTHUS EMBLICA (LINN.)
|
Test sample |
Conc.
(µg/ml) |
Log
(Conc.) |
No. of alive shrimp | % mortality | LC50
(µg/ml) |
LC90
(µg/ml) |
|
Ethanolic fruits extract of Phyllanthus emblica (Linn.) |
20 | 1.30 | 6 | 40 |
60 |
100
|
| 40 | 1.60 | 6 | 40 | |||
| 60 | 1.77 | 5 | 50 | |||
| 80 | 1.90 | 3 | 70 | |||
| 100 | 2 | 1 | 90 | |||
| 120 | 2.07 | 2 | 80 | |||
| 140 | 2.14 | 0 | 100 |
TABLE 6B: TEST SIGNIFICANCE ANALYSIS OF BRINE SHRIMP LETHALITY BIOASSAY OF ETHANOLIC EXTRACT OF FRUIT OF PHYLLANTHUS EMBLICA (LINN.)
| Ethanolic Extract of Phyllanthus emblica (Linn.) | ||
| Name of applied test | Chi-value | Sig. |
| Linear-by-Linear Association | 5.44 | 0.02 |
| Pearson's R | 0.95 | 0.001 |
FIG. 2: LC50 AND LC90 OF PHYLLANTHUS EMBLICA (LINN.)
DISCUSSION: Phytochemical analysis of Phyllanthus emblica (Linn.) showed the presence of flavonoids, alkaloids, tannin, steroids, reducing sugar and gum. Further studies are suggested to isolate its active components by using bioactivity guided approach.
The ethanol extract of the fruit of Phyllanthus emblica (Linn.) was subjected to acetic acid induced writhing method in mice for preliminary analgesic activity screening. The results of analgesic activity screening Table 2 showed that the ethanol extract might possess analgesic activity depending upon the nature of their active ingredients in the extracts. The fruit extract produced 19.07% and 38.67% protection or writhing inhibition at the oral dose of 250 and 500 mg/kg-body weights respectively. Acetic acid causes pain and localized inflammation by the action of prostaglandins production [mainly, prostacyclins and prostaglandin-E (PG-E)] which have been reported to stimulate the Aδ-fibres that cause a sensation of sharp well-localized pain 34.
It has also been reported that acetic acid induces the increased level of PGE2 and PGF2α in the peritoneal fluid which is responsible for pain production 35-40. It is various peripherally acting analgesic drugs such as ibuprofen, aspirin, diclofenac sodium and indomethacin that have been reported to inhibit acid-induced writhing by inhibition of prostaglandin synthesis 41. Therefore it can be concluded that any agent that reduces the number of writhing will demonstrate analgesic effect by inhibition of prostaglandin synthesis, a peripheral mechanism of pain inhibition 42. The result of the plant extract in acetic acid-induced writhing method suggests that the reduction of pain might be occurred due to the presence of analgesic properties in the extract via inhibition of prostaglandin synthesis. However, further study should be done for its isolated, purified active components. From the above observations, it is obvious that the ethanol extract of the fruit of Phyllanthus emblica (Linn.) has analgesic activity.
Xylene is known to cause severe vasodilation and edematous changes of skin as signs of acute inflammation 43. These histopathological changes cause the increased thickness of the ear tissues. In the present investigation; the plant extract significantly inhibited the xylene-induced increases in ear weight in a dose-related manner. This inhibition capacity of the plant extract can be regarded as the evidence of anti-inflammatory efficacy through reducing vasodilation and so that improving the edematous condition.
In this study, the ethanolic fruit extract of Phyllanthus emblica (Linn.) significantly inhibited ear edema formation in xylene-induced ear edema model mice. This inhibition can be considered as direct evidence that was supporting the anti-inflammatory activity of ethanolic fruit extract of Phyllanthus emblica (Linn.).The antibacterial screening tests support the ethanolic fruit extract of Phyllanthus emblica (Linn.) performing as a remedy for different bacterial diseases.
During the antidiarrhoeal activity screening at the dose of 500 mg/kg body weight, the extract of Phyllanthus emblica (Linn.) compared to the control group, offered about 0.5389 of the mean latent period for the diarrhoeal episode to ensure and the result was significant (P<0.2). The mean numbers of stool at the 1st, 2nd, 3rd and 4th h of the study were 8.2., 9.6, 7.8 and 4.4 respectively.
Phyllanthus emblica (Linn.) showed moderate antidiarrhoeal activity in castor oil induced test in mice at the dose of 500 mg/kg body weight as compared to the standard antidiarrhoeal agent loperamide. Phyllanthus emblica (Linn.) caused an increase in latent period, i.e. delayed the onset of a diarrhoeal episode and decreased the frequency of defecation. The decreased frequency of defecation and increase mean latent period of test group than the control group and comparison with positive control group can claim that Phyllanthus emblica (Linn.) might possess antidiarrheal activity. T-test of these responses showed that the result is significant throughout the observation period.
Test sample showed a different mortality rate at different concentrations. The mortality rate of brine shrimp was found to be increased with the increase in the concentration of the sample and plot of percent mortality versus log concentration on the graph paper produced an approximately linear correlation between them. From the graph (figure) the concentrations at which 50% mortality (LC50) of brine shrimp nauplii occurred were obtained by extrapolation. The values were found to be 60µg/ml for the crude extract. The 90% mortality (LC90) values were 100 µg/ml respectively. The approximate significance value for Chi-Square Tests (Linear by linear association) is equal to 0.02, and also significance value for Pearson’s R is 0.001 which are significant at 5% level of significance.
Therefore, mortality rate of shrimp due to Ethanolic extract concentration is not due to chance. So we can conclude there is a statistically significant relationship between ethanolic extract concentration and mortality rate of shrimp. The crude extracts Phyllanthus emblica (Linn.) were found to show high lethality against the brine shrimp nauplii. These results tend to suggest its possible antitumor, antibacterial or pesticidal activities. However, further researches are necessary particularly with its purified fraction.
The preliminary phytochemical analysis of the plant extract showed the presence of reducing sugars, alkaloids, flavonoids, tannins, steroids, gums and glycosides. The previous scientific studies have been reported that alkaloids, flavonoids, and tannins are known to inhibit prostaglandin synthetase that is responsible for its antinociceptive and anti-inflammatory effects 20, 44-50. Therefore the antinociceptive and anti-inflammatory effect of the extract may be due to the presence of flavonoids, tannins, and alkaloids either singly or in combination. Presence of tannins, alkaloids, flavonoids, sterol and reducing sugars in the medicinal plants have also been known to indicate antidiarrheal activity 51-52. In general antidiarrhoeal activity of tannins and flavonoids has been recognized for the inhibition of intestinal motility, antimicrobial action and antisecretory effects 48. Also, the astringent properties of tannins are known to cause anti-nociceptive, anti-inflammatory and antidiarrheal effects 20, 44, 53. Therefore the antinociceptive and anti-inflammatory effect of the extract may be due to the presence of flavonoids, tannins, and alkaloid either singly or in combination. Besides alkaloids, flavonoids or tannins may also be responsible for the anti-diarrheal potential of the plant extract.
CONCLUSION: According to the results of the present investigation, it can be concluded that the ethanolic fruits extract of Phyllanthus emblica (Linn) has significant analgesic, anti-inflammatory and anti-diarrheal effects that support to the traditional use of this plant for the treatment of related diseases. This study also suggests for the further detail investigation of mechanisms of the pharmacological effects and also to isolate the active compound(s) responsible for those properties.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Ghani A: Medicinal Plants of Bangladesh with chemical constituents and uses. 2nd edition, Asiatic Society of Bangladesh, 5 old Secretariate road, Nimtali, Dhaka, Bangladesh 2002.
- Anwar MN, Begum J, Dutta S, Khan S, Yusuf M and Chowdhury JU: Screening of thirty-eight medicinal plants of Bangladesh for antimicrobial activities. Asian Jr of Microbiol Biotech Env Sc 2007; 9(3): 703-707.
- Begum J, Yusuf M, Chowdhury JU, Khan S and Anwar MN: Antifungal activity of forty higher plants against phytopathogenic fungi. Bangladesh J. Microbiol 2007; 24(1): 76-78.
- Chakma TK, Choudhuri MSK, Jabbar S, Khan MTH, Alamgir M, Gafur MA, Ahmed K and Roy BK: Effect of some medicinal plants and plant parts used in Ayurvedic System of medicine on isolated guinea-pig ileum preparations. Hamdard Medicus 2001; X & IV (2): 70-73.
- Rastogi, Ram P and Mehrotra BN: Compendium of Indian Medicinal Plants. Vol. 1, Central Drug Research Institute, Lucknow and Publications & Information Directorate, New Delhi, India 1991.
- Rastogi, Ram P and Mehrotra BN: Compendium of Indian Medicinal Plants. Vol. 2, Central Drug Research Institute, Lucknow and Publications & Information Directorate, New Delhi, India 1993.
- Olajuyigbe OO and Afolayan AJ: Pharmacological assessment of the medicinal potential of Acacia mearnsii De Wild.: Antimicrobial and Toxicity activities. Int J Mol Sci 2012; 13: 4255-4267.
- Pelka M, Danzl C, Distler W and Petschelt A: A new screening test for toxicity testing of dental materials. J Dent 2000; 28: 341-345.
- Taylor RSL, Edel F, Manandhar NP and Towers GHN: Antimicrobial activity of Southern Nepalese medicinal plants. J Ethnopharmacol 1996; 50: 97-102.
- Zimmermann M: Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16(2): 109-110.
- Harborne JB: Phytochemical methods. London: Chapman and Hall, Ltd. 1973; 49-188.
- Trease GE and Evans WC: Pharmacognosy. 11th Brailliar Tiridel, Canada: Macmillian Publishers 1989.
- Sofowora A: Medicinal plants and traditional medicine in Africa. Ibadan, Nigeria, Spectrum Books Ltd 1993; 289.
- Edeoga HO, Okwu DE and Mbaebie BO: Phytochemical constituents of some Nigerian medicinal plants. Afri J Biotechnol 2005; 4: 685-688.
- Sadhu SK, Okayama E, Fujimoto H and Ishibashi M: Separation of Leucas aspera, a medicinal plant of Bangladesh, Guided by prostaglandin Inhibitory and Antioxidant activities. Chemical and Pharmaceutical Bulletin 2003; 51(5): 594-598.
- Whittle BA: The use of changes in capillary permeability in mice to distinguish between narcotic and non-narcotic analgesics. Br J Pharmacol Chemother 1964; 22: 246.
- Rang HP and Dale MM: Pharmacology. 2nd edition. Churchill Livingstone Publisher. UK 1993; 706-711.
- Saha A, Masud MA, Bachar SC, Kundu JK, Datta BK, Nahar L and Sarker SD: The analgesic and anti-inflammatory activities of the extracts of Phyllanthus reticulatus in Mice Model. Pharm Biol 2007; 45(5): 355-359.
- Ahmed F, Selim MS, Das AK and Choudhuri MS: Anti-inflammatory and antinociceptive activities of Lippia nodiflora Pharmazie 2004; 59(4): 329-30.
- Yaro AH, Magaji MG, Danjuma NM, Malami S and Isah A: Studies on analgesic and anti-inflammatory activities of Cissampelos mucronata in laboratory animals. Int J Pure App Sci 2008; 2: 111-117.
- Deb D, Dev S, Das AK, Khanam H, Banu M and Shahriar M: Antinociceptive, anti-inflammatory and anti-diarrheal activity of crude root extract of Lasia spinosa (Family- Araceae) Latin Am J Pharm 2010; 29: 1269-1276.
- Tang XC, Lin ZG, Cai W, Chen N, Shen L, Zhongguo Y and Bao LX: Anti-inflammatory effect of 3-acetylcarnitine 1984; 5(2): 85-9.
- Barry AL: Procedures for testing of antimicrobial agents in agar media. In: Antibiotics in laboratory medicine, (V. Lorian Edition), Williams and Wilkins Company, Baltimore, USA 1980; 1-23.
- Bauer AW, Kirby WMM, Sherris JC and Turck M: Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol 1966; 45: 493-496.
- Ahmed F, Islam MA and Rahman MM: Antibacterial activity of Leonurus sibiricus aerial parts. Fitoterapia 2006; 77(4): 316-7.
- Chatterjee TK: Handbook of laboratory Mice and Rats. 1st Jadavpur University, India 1993: 133-139.
- Jebunnessa, Uddin SB, Mahabub-Uz-Zaman M, Akter R and Ahmed NU: The antidiarrheal activity of ethanolic bark extract of Mitragyna diversifolia. Bangladesh J Pharm 2009; 4: 144-146.
- Nwodo OFC and Alumanah EO: Studies on Abrus precatorius II: Antidiarrheal activity. J Ethnopharmacol 1991; 31(3): 395-398.
- Atta AH and Mouneir SM: The antidiarrheal activity of some Egyptian medicinal plant extracts. J Ethnopharmacol 2004; 92: 303-09.
- Meyer BN, Ferrigin NR, Putnam JB, Jacobsen LB, Nichols DE and McLaughlin JL: Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med 1982; 45: 31-34.
- Goldstein AL and Kalkan SM: Principles of Drug Action, 2nd ed. Willy Biochemical Health Publications 1974; 376-381.
- Moshi MJ, Innocent E, Magadula JJ, Otieno DF, Weisheit A, Mbabazi PK and Nondo RSO: Brine shrimp of some plants used as traditional medicine in Kagera Region, North West Tanzania. Tanzan J Health Res 2010; 12: 63-67.
- Gupta MP, Monge A, Karitas G, Lopez de Cerain A, Solis PN, Leon E, de Trujilo M, Surez O, Wilson F, Montenegro G, Noriega Y, Santana AI, Correa M and Sanchez C: Screening of Panamanian medicinal plants for brine shrimp toxicity, crown gall tumor inhibition, cytotoxicity and DNA interaction. Intr J Pharmacol 1996; 34: 123-127.
- Ranolds J and Martindale EF: The extra pharmacopeia. 28th edition. The Pharmaceutical Press 1982; 245.
- Bose U, Gunasekaran K, Bala V and Rahman AA:. Evaluation of phytochemical and pharmacological properties of Dillenia indica leaves. J Pharm Toxicol 2010; 5: 222-228.
- Derardt R, Jougney S, Delevaliee F and Flahaut M: Release of prostaglandins E and F in an algogenic reaction and its inhibition. European J Pharmacol 1980; 61: 17-24.
- Zakaria ZA and Gani AZDF: Antinociceptive, anti-inflammatory and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J Nat Med 2008; 62: 179–187.
- Zulfiker AHM, Rahman MM, Hossain MK, Hamid K, Mazumder MEH and Rana MS: In-vivo analgesic activity of ethanolic extracts of two medicinal plants-Scoparia dulcis and Ficus racemosa Linn. Biol Med 2010; 2: 42-48.
- Bhalke RD, Anarthe SJ, Sasane KD, Satpute SN, Shinde SN and Sangle VS: Antinociceptive activity of Trigonella foenum-graecum leaves and seeds (Fabaceae) Iranian J Pharmacol Therapy 2009; 8: 57-59.
- Sulaiman MR, Moin S, Alias A and Zakaria ZA: Antinociceptive and anti-inflammatory effects of Sida rhombifolia in the various animal models. Res J Pharmacol 2008; 2: 13-16.
- Ishfaq AB, Dar A and Khan RA: Antinociceptive activity of methanolic extracts of St.John's wort (Hypericum perforatum) preparation. Pakistan J Pharm Sci 2004; 17: 13–19.
- Ferdous M, Rouf R, Shilpi JA and Uddin SJ: Antinociceptive activity of the ethanolic extract of Ficus racemosa (Moraceae) Oriental Pharm Exp Med 2008; 8: 93-96.
- Kim HD, Cho HR, Moon SB, Shin HD, Yang K and Park BR: Effects of beta-glucan from Aureobasidium pullulans on acute inflammation in mice. Arch Pharm Res 2007; 30: 323-328.
- Sabina EP, Chandel S and Rasool MK: Evaluation of analgesic, antipyretic and ulcerogenic effect of Withaferin A. Int J Integ Biol 2009; 6: 52-56.
- Kumar ABS, Lakshman K, Jayaveera KN, Sheshadri SD and Vivek C: Antinociceptive and antipyretic activities of Amaranthus viridis in different experimental models. Arch Biol Sci 2010; 62: 397-402.
- Citoglua GS, Ozbekb H and Severa B: Antinociceptive activity of Ballota glandulosissima -Mor & Patzak. Eastern J Med 2005; 10: 24-28.
- Chakraborthy GS and Ghorpade PM: Antinociceptive activity of Abutilon indicum (Linn) sweet stem extracts. Arch Pharm Sci Res 2010; 2: 241-245.
- Ahmadiani A, Hosseiny J, Semnanian S, Javan M, Saeedi F and Kamalinejad M: Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J Ethnopharmacol 2000; 72: 287-292.
- Owoyele BV, Nafiu AB and Soladoye OIA: Studies on the analgesic, anti-inflammatory and antipyretic effects of Parquetina nigrescens leaf extract. J Ethnopharmacol. 2008; 122: 86-90.
- Sharma P, Vidyasagar G, Bhandari G, Singh S, Bhadoriya U, Ghule U and Dubey U: Pharmacological evaluation of antidiarrhoeal activity of leaves extract of Murraya koenigii in experimentally induced diarrhoea in rats. Asian Pac J Trop Dis 2012; 2(3): 230-233.
- Ezenwali MO, Njoku OU and Okoli CO: Studies on the anti-diarrheal properties of seed extract of Monodora tenuifolia. Int J App Res Nat Prod 2010; 2: 20-26.
- Saralaya MG, Patel P, Patel M, Roy SP and Patel AN: The antidiarrheal activity of methanolic extract of Moringa oleifera Lam roots in an experimental animal model. Int J Pharm Res 2009; 2: 35-39.
- Ali MK, Ashraf A and Biswas NN:Antinociceptive, anti-inflammatory and anti-diarrhoeal activities of ethanolic calyx extract of Hibiscus sabdariffa Linn. (Malvaceae) in mice. J Chin Integr Med 2011; 9: 626-632.
How to cite this article:
Hossen SMM, Uddin M and Sarkar MR: Medicinal potential of Phyllanthus emblica (Linn.) fruits extracts: biological and pharmacological activities. Int J Pharmacognosy 2014; 1(5): 307-16. doi: 10.13040/IJPSR.0975-8232.1(5).307-16.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
307-316
655
2800
English
IJP
S. M. M. Hossen *, M. Uddin and M. R. Sarkar
Department of Pharmacy, University of Chittagong, Chittagong, Bangladesh.
hossen.pharmacy@cu.ac.bd
19 March 2014
22 April 2014
28 April 2014
http://dx.doi.org/10.13040/IJPSR.0975-8232.1(5).307-16
01 May 2014