MAHUA OIL CONTAINING SUPPOSITORY BASE EXHIBITED HIGHER DRUG RELEASE AS COMPARED TO COCOA BUTTER BASE
HTML Full TextMAHUA OIL CONTAINING SUPPOSITORY BASE EXHIBITED HIGHER DRUG RELEASE AS COMPARED TO COCOA BUTTER BASE
Ujwala N. Mahajan *, Debarshi Kar Mahapatra, Nilesh M. Mahajan, Fahimuddin S. Kazi and Nupoor Baghel
Department of Pharmaceutics, Dadasaheb Balpande College of Pharmacy, Nagpur - 440037, Maharashtra, India.
ABSTRACT: The present research aimed at scrutinizing the role of Mahua Oil (MO) as a suppository base which can enhance the release of drug in the site of administration. Three formulations were designed with erythromycin as the drug utilizing; F1 (MO and Beeswax in the ratio of 3:1), F2 (MO and PEG 6000 in the ratio of 1:1), and F3 (cocoa butter - 100%) as the base material. The suppositories were prepared using pour molding method after suitable calibration of molds. All the formulations were characterized in terms of breaking strength, disintegration time, drug content, in-vitro dissolution, and accelerated stability studies. The MO containing formulations displayed a good breaking strength, optimized disintegration time, and higher drug content as compared to the cocoa butter formulation. The optimized formulation F2 represented the optimized release of 81.35% over the range of 1 h. A little difference in drug content (1.78%), breaking strength (0.2 Kg/cm2), and disintegrating time (0.6 min) was observed in the accelerated stability study. The study concluded that MO has a noteworthy effect on drug release. The study emphasized the application of MO for release modification in suppositories since it is a non-toxic, chemically inert, economic, wide availability, and biodegradable component.
| Keywords: |
Mahua, Madhuca longifolia, Oil, Suppository, Formulation, Base
INTRODUCTION: In modern pharmaceutics, the emergence of natural products as excipients was widely perceived among the researchers, because synthetically derived products do have several complications 1. Once upon a time, the excipients were believed bulk inactive stuff which only complements the API. But now, with the advancement in knowledge and proofs, they are now being considered as an imperative factor in pharmacodynamics 2.
These components elevate the activity of API by promoting drug absorption or solubility and play a vital role in formulation development 3. Recently, several nature derived or ethnopharmacologically reported components are utilized for their miscellaneous applications in pharmaceutical formulation development 4.
They are acceptable among the population owing to their social acceptance in traditional usage, ethnobotanical aspects, and diverse, safe pharmacological applications. Of them, Mahua Oil (MO), an oily component, which is extracted from the seeds of Madhuca longifolia (family: Sapotaceae), also known as the Mahua or butternut tree, present through the Indian subcontinent to South Asian nations 5. MO is a well-known nutraceutical product containing several natural ingredients with high nutritional value along with long shelf life. MO is a non-toxic, cheap, non-reactive, and easy extraction procedure that finds traditional use by the low socio-economic status population for decades as cooking oil in rural areas.
The medicinal applications like headache, emetic, hemorrhoids, laxative, skin disease, constipation, emollient, rheumatism, piles, etc. are also reasons for its popularity among a section of the society 6. From the survey of traditional databases, it has been evidenced that the utilization of MO in modern pharmaceutics is not yet done. Mahajan and co-workers have recently reported the use of MO as an emulsifier in cream formulations and permeation enhancer in gel formulations 7-8.
The present research aimed at scrutinizing the role of Mahua Oil (MO) as a suppository base which can enhance the release of drug in the site of administration. Three formulations were designed with erythromycin as the drug utilizing; F1 (MO and Beeswax in the ratio of 3:1), F2 (MO and PEG 6000 in the ratio of 1:1), and F3 (cocoa butter - 100%) as the base material.
All the formulations were characterized in terms of breaking strength, disintegration time, drug content, in-vitro dissolution, and accelerated stability studies.
MATERIALS AND METHODS:
Chemicals: A gift sample of Erythromycin Stearate was received from Flamingo Pharmaceutical Ltd., Mumbai. Gondwana Herbs, Gadchiroli, Maharashtra, India remained the supplier for Mahua oil. Beeswax and cocoa butter were obtained from SD Fine Chemicals Ltd., Mumbai. PEG 6000 was purchased from HiMedia Ltd., Mumbai.
Instruments: The ingredients were weighed using a Shimadzu® electronic balance (Model AUW220D, Kyoto, Japan). The spectroscopic analysis was performed using double-beam Shimadzu® Ultraviolet-Visible Spectrophotometer (Model UV-1800, Kyoto, Japan). The accelerated stability study was carried out using a stability chamber (Bio-Technics, India). Transonic Digital S (Sonicator) was employed for sonication. Electrotab TDT-6N instrument was employed for studying the dissolution of the prepared suppository.
Preformulation and Standardization:
Determination of Acid Value: 10 gm of MO was dissolved in a 50 mL mixture of ethanol (95%) and ether, previously neutralized with 0.1 M KOH. In the presence of 1 mL of phenolphthalein solution, the content was titrated with 0.1 M KOH until the solution becomes faintly pink permanently. The acid value was calculated as per the formula: Acid Value = 5.61 n/w; where, n = volume of KOH required, and w = weight of the sample (in g) 9.
Determination of Saponification Value: 2 g of weighed MO was taken in a flask fitted with a reflux condenser. 25 mL of 0.5 M ethanolic KOH solution and little pumice powder were added, and the content was refluxed for 30 min. 1 mL of phenolphthalein solution was added, and titration was performed immediately with 0.5 M HCl. Alongside, a blank titration was carried out omitting the MO. The saponification value was calculated as per the formula: Saponification Value = 28.05 (b- a) / w; where, w = weight (in g) of MO, b = volume of HCl utilized in blank titration, and a = volume of HCl consumed 9.
Determination of Iodine Value: An accurately weighed quantity of MO was placed in a dry iodine flask. To it, 10 mL of CCl4 and 20 mL of iodine monochloride solution were added. The content was allowed to stand in the dark at a temperature between 15 °C - 25 °C for 30 min. 15 mL KI solution was placed in the cup top, and the stopper was carefully removed. From the side of the flask, 100 mL water was added and titrated with 0.1 M sodium thiosulphate using starch solution indicator. The amount required was noted. A blank titration was also performed sidewise. The iodine value was calculated as per the formula: Iodine Value = 1.269 (b - a) / w; where, w = weight (in g) of the substance, b = volume of titrant utilized in blank titration, and a = volume of titrant consumed 9.
Preparation of Formulations: The suppositories were prepared by pour molding method. The formulation 1 and formulation 2 were prepared where MO was added to the beeswax / PEG 6000 in the desired ratio and the content was heated to 70 – 80 °C in the presence of water containing accurately weighed erythromycin drug in dispersed form, to produce homogenous content. The molds were previously calibrated (1 g capacity) using the molten bases (not drug), followed by weighing the product. The process was performed for each base. The mold capacities were found to be in the range of 0.991 - 1.021 g. The formulation 3 was produced using cocoa butter and drug in a similar manner. Table 1 portrays the formulation of batch information.
TABLE 1: FORMULATION OF SUPPOSITORIES
| Formulation no. | Ingredients | Ratio | Melting range |
| F1 | Mahua Oil : Bees wax | 3:1 | 20 - 25 min |
| F2 | Mahua Oil : PEG 6000 | 1:1 | 20 - 25 min |
| F3 | Cocoa Butter | 100% | 20 - 25 min |
Evaluations of Suppository Formulations:
Determination of Drug Content: A suppository formulation was accurately weighed and dissolved in phosphate buffer (pH 7.2). The content was further sonicated for a period of 10 - 15 min and volume was made up to 100 mL. 10 mL of the content was pipetted out and diluted further to 100 mL with phosphate buffer (pH 7.2), and the final dilution was made to get a concentration within Beer-Lambert’s range. The absorbance was measured spectrophotometrically at 225 nm against blank suppository treated in the same manner as the sample.
Determination of Breaking Strength: Breaking strength was carried out to determine the tensile strength of suppositories to reveals the ability to withstand the hazards of packing and transportation. The hardness of the formulated suppositories was tested using Pfizer hardness tester 10.
Determination of Disintegration Time: The disintegration time of the suppositories was determined by using USP disintegration test apparatus IP. The time taken for the disintegration of entire suppository was recorded in phosphate buffer (pH 7.2) maintained at 37 ± 0.5 °C 11.
Determination of in-vitro Drug Release: The in-vitro drug diffusion study of suppository formulation was carried out using a USP dissolution test apparatus-II (basket type). The dissolution medium was 900 mL of phosphate buffer pH 7.4. The drug release study was performed at 37 ± 0.5 °C, with a rotation speed of 50 rpm. 5 mL of the samples were withdrawn at predetermined time intervals by a syringe fitted with prefilter and replaced with fresh dissolution medium to maintain the sink conditions. The samples were analyzed by UV spectrophotometer at 225 nm after appropriate dilution. The cumulative drug release was calculated and plotted against time 12. The study was performed in triplicate manner.
Accelerated Stability Study: The suppository formulation was put in a PVC container and covered with an aluminum foil. The accelerated stability study was performed for the optimized suppository formulation (F2) under accelerated conditions of temperature (40 °C ± 2 °C) and moisture (75% ± 5% RH) for 90 days. After the preferred time duration, the suppository was evaluated for physical appearance, drug content, disintegration time, and breaking strength 13.
RESULT AND DISCUSSION: The physico-chemical parameters of the MO like iodine value, saponification value, and acid value were studied comprehensively to determine the authenticity and purity of the base. The parameters were found to be held by the pharmacopoeial range, and the purity was ascertained accordingly Table 2.
TABLE 2: OBSERVED STANDARDIZATION RESULTS FOR MAHUA OIL
| Parameters | Standard value | Observed value |
| Acid value | 20 | 26.92 |
| Saponification value | 187 – 197 | 190 |
| Iodine vale | 55 - 70 | 63.45 |
The fabricated suppository formulations come into view as a firm, solid content cast according to the mold. All the formulations were free from grittiness, quite uniform, and smooth. The MO containing formulations had dark coloration and a characteristic odor. After getting satisfactory organoleptic properties, the formulations were further studied for breaking strength, disintegration time, in-vitro drug release, and drug content. The MO containing formulations displayed a good breaking strength as compared to the cocoa butter formulation. It has been noticed that as the concentration of MO was increased, the breaking strength increases significantly.
The formulation F1 containing the highest concentration (3:1) had a breaking strength of 5.4 Kg/cm2 which signifies that a good polymer structure was formed by MO which imparted the desired strength. The drug content was also found to be highest for F1 formulation which may be explained that MO has good polymeric characteristics that influenced better drug loading as compared to F3 (cocoa butter) formulation.
However, the formulation F2 exhibited the lowest drug content since it contained the lowest amount of MO (1:1). Thus, it might be established that the drug entrapment characteristic is a function of the concentration of MO. The disintegration time was found to be directly proportional to that of breaking strength. The formulation F1 had that highest disintegration time of 8.9 min whereas the formulation F3 had the lowest time of 8.1 min Table 3. The evaluation parameters signified that the concentration of MO imparted a desirable strength which leads to enhanced disintegration time. The increased disintegration time may be an added clinical advantage regarding the site of administration to the necessary therapeutic action.
TABLE 3: EVALUATION OF SUPPOSITORY FORMULATIONS
| Formulations | Breaking strength (Kg/cm2) | Disintegration time (min) | Drug Content (%) |
| F1 | 5.4 | 8.9 | 86.21 |
| F2 | 3.3 | 8.3 | 82.96 |
| F3 | 2.9 | 8.1 | 85.74 |
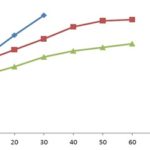
The in-vitro drug dissolution studies of the MO containing formulations were executed in phosphate buffer pH 7.4. The formulation F1 containing the highest ratio of MO released 84.41% of the drug in just 30 minutes Fig. 1. The formulation F2 represented the optimized release of 81.35% over the range of 1 h. In contrast, F3 presented the lowest cumulative drug release of 74.04% at 60 minutes Table 4.
The lowest drug release from cocoa butter formulation was attributed due to the high molecular weight and the intrinsic structure which increases the polymer density and provides significant diffusional resistance 14. It has been perceived that as the concentration of MO gets increased, the drug release increased significantly. Therefore, a ratio of 1:1 provided the best control drug release for 1 h duration. Additionally, the MO has a property of permeation enhancement which will further assist clinical absorption of the drug from the administration route.
TABLE 4: IN-VITRO DRUG RELEASE PROFILES OF SUPPOSITORY FORMULATIONS
| Time (min) | F1 | F2 | F3 |
| 10 | 54.52 | 49.89 | 51.54 |
| 20 | 69.23 | 56.52 | 57.60 |
| 30 | 84.41 | 64.47 | 64.52 |
| 40 | - | 74.36 | 68.85 |
| 50 | - | 80.48 | 71.44 |
| 60 | - | 81.35 | 74.04 |
The accelerated stability conditions for 90 days revealed that the formulation F2 did not show any such changes at 40 °C ± 2 °C and 75% ± 5% RH. A little difference in drug content (1.78%), breaking strength (0.2 Kg/cm2), and disintegrating time (0.6 min) was observed. The reduction in physical parameters may be attributed due to the polymorphism or temperature-induced loss of polymeric structural changes, which leads to reduction in the strength of the formulation, which eventually results in faster disintegration and loss of drug content 15. The accelerated stability results are mentioned in Table 5.
TABLE 5: ACCELERATED STABILITY STUDIES OF OPTIMIZED FORMULATION (F2)
| Duration | Breaking strength (Kg/cm2) | Disintegra-tion time (min) | Drug Content (%) |
| 0 day | 3.3 | 8.3 | 82.96 |
| 90th day | 3.1 | 7.7 | 81.18 |
FIG. 1: IN-VITRO DRUG RELEASE FROM SUPPOSITORY FORMULATIONS
CONCLUSION: This research outcome indicated that the combination of MO, a key ingredient obtain from Madhuca longifolia and PEG 6000 (F2) demonstrated the highest drug release of 81.35% at the end of 1 h which was far better than the cocoa butter based formulation (F3) which exhibited a release of 74.04%. The ratio of 1:1 of MO was also found to be better than the ratio of 3:1 which demonstrated the highest release of 84.41% in 30 min.
The study also concluded that MO has a noteworthy effect on drug release. Based on the concentration, MO expressed the drug release from the formulation. Therefore, the study emphasized the application of MO for release modification in suppositories, since it is a non-toxic, chemically inert, economic, wide availability, and biodegradable component.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Raymond CR, Sheskey PJ and Owen SC: Handbook of excipients. An imprint of RPS Publishing 2009: 158-00.
- Kibbe HA: Hand Book of Pharmaceutical Excipients. London, England: American Pharmaceutical Association.
- Karsa DR and Stephenson RA: Excipients and delivery systems for pharmaceutical formulations. Royal Society of Chemistry 1995.
- Zhang J, Onakpoya IJ, Posadzki P and Eddouks M: The safety of herbal medicine: from prejudice to evidence. Evid Compl Alt Med 2015.
- Patel M: Biochemical investigations of fresh Mahua (Madhuca indica) flowers for nutraceuticals http://eprint. iitd.ac.in/bitstream/2074/3230/1/TH-3692.pdf. 2017.
- Sunita M and Sarojini P: Madhuca lonigfolia (Sapotaceae): A review of its traditional uses and nutritional properties. Int J Human Social Sci Invent 2013; 2(5): 30-6.
- Mahajan UN, Mahapatra DK, Mahajan NM, Kazi FS and Baghel N: Exploring the role of Mahua oil as a potent emulsifier in cream formulations. Int J Herb Med 2017; 5(3): 93-97.
- Mahajan UN, Mahapatra DK, Mahajan NM, Kazi FS and Baghel N: Mahua Oil, an Ayurvedic product demonstrated permeation enhancing attribute in topical gel formulations. J Nat Prod Plant Resour 2017; 7(3): 8-14.
- Mahapatra DK and Bharti SK: Handbook of Research in Medicinal Chemistry. Ontario: Apple Academic Press 2017.
- Patil MD, Mahapatra DK and Dangre PV: Formulation and in-vitro evaluation of once-daily sustained release matrix tablet of nifedipine using rate retardant polymers. Inventi Pharm Tech 2016; (4): 1-7.
- Dangre PV, Godbole MD, Ingale PV and Mahapatra DK: Improved dissolution and bioavailability of eprosartan mesylate formulated as solid dispersions using conventional methods. Indian J Pharm Edu Res 2016; 50(3): S209-S217.
- Kazi FS, Mahajan RK, Mahapatra DK and Mahajan UN: Formulation development of innovator equivalent extended-release tablets of gliclazide: A way ahead to Generic medicines. J Pharm Sci Pharm 2017.
- Sonkusre N, Dhabarde DM and Mahapatra DK: Formulation and development of mirtazapine self-emulsifying drug delivery system (SEDDS) for enhancement of dissolution profile. Inventi NDDS 2016; (4): 155-63.
- Swamy PV, Farhana L, Shrisand SB, Ali MY and Patil A: Design and Evaluation of rectal suppositories of carvedilol. Int J Pharm Sci Nanotechnol 2009; 2(3).
- Swamy PV, Farhana L, Ali M and Varma MM: Design and evaluation of sustained release suppositories of carvedilol. Adv Sci Eng Med 2014; 6(4): 417-20.
How to cite this article:
Mahajan UN, Mahapatra DK, Mahajan NM, Kazi FS and Baghel N: Mahua oil containing suppository base exhibited higher drug release as compared to cocoa butter base. Int J Pharmacognosy 2018; 5(5): 308-12. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.5(5).308-12.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
9
308-312
491
1650
English
IJP
U. N. Mahajan *, D. K. Mahapatra, N.M. Mahajan, F. S. Kazi and N. Baghel
Department of Pharmaceutics, Dadasaheb Balpande College of Pharmacy, Nagpur, Maharashtra, India.
ujwalat5@gmail.com
05 January 2018
03 February 2018
13 February 2018
10.13040/IJPSR.0975-8232.IJP.5(5).308-12
01 May 2018