INVESTIGATION ON NOVEL ROLE OF SOLANUM XANTHOCARPUM AND JUNIPERUS COMMUNIS EXTRACT AGAINST CCl4 INDUCED LIVER INJURY
HTML Full TextINVESTIGATION ON NOVEL ROLE OF SOLANUM XANTHOCARPUM AND JUNIPERUS COMMUNIS EXTRACT AGAINST CCl4 INDUCED LIVER INJURY
Bhuwan Chandra Joshi * 1, Sushmita Uniyal 1 and Sukanya 2
Department of Pharmacognosy 1, Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences and Research, Balawala, Dehradun - 248001, Uttarakhand, India.
Department of Pharmacy 2, Central University of Rajasthan Bandarsindri, Kishangarh - 305817, Rajasthan India.
ABSTRACT: Background: There are very less therapeutic and reliable liver protective drugs in modern medicine to prevent and treat the liver injury caused due to drugs. Traditionally Solanum xanthocarpum Schradt. (SX) and Wendl. and Juniperus communis Linn. (JC) have been used since long for their hepatoprotective effect. The current study was carried out to explore the hepatoprotective role of SX and JC against liver toxicity in rodents induced by carbon tetrachloride (CCl4). Materials and Methods: The antioxidant potential of hydroalcoholic extract of SX and JC were investigated by using established DPPH in-vitro assay method. The hydroalcoholic extract of SX and JC at doses (200 and 400 mg/kg b.w.) were studied for their liver protective activity against hepatic damage induced by CCl4 in Wistar rats. An assay of the oxidative stress parameters, alkaline phosphatase, transaminase, total bilirubin, albumin and liver histopathology were performed to evaluate the hepatoprotective activity of SX and JC. The assay results were introduced as the standard error ofthe mean (SEM) for each group. Results: The ethanolic extract of SX and JC has shown the potent antioxidant scavenging in-vitro activity viz. DPPH IC50 of 69.41 ± 0.76 µg/ml, 117 ± 0.34 µg/ml respectively. The hydroalcoholic extract of SX and JC showed a significant reduction in oxidative stress parameters and improved antioxidant and liver enzymes level as compare to toxicant treated rats. Moreover, histopathological studies also revealed the similar results which supported the liver protective activity of SX and JC herbal extracts. Conclusion: It is concluded that SX and JC showed significant hepatoprotective activity in CCl4 induced hepatoxicity in rodents. The promising mechanism for their therapeutic activity is due to their antioxidant and liver protective activity which scientifically supports their traditional use.
| Keywords: |
Solanum xanthocarpum, Juniperus communis, Hepatotoxicity
INTRODUCTION: Hepatic diseases are among the serious and common diseases occurring worldwide even with the advancement in modern medicine, their treatment and prevention options always have a scope.
Oxidative stress and inflammation are seen to be responsible for hepatic diseases. The liver is the important organ of the body involved in the elimination of drug and toxins. The major culprits of the liver toxicity are antibiotics, alcohol consumption, antitubercular drugs, malnutrition, infections, and other metabolic disorders.
Liver diseases progression is characterized from steatosis to chronic hepatitis, fibrosis, cirrhosis, and maybe hepatocellular carcinoma resulting in high morbidity and mortality rate 1.
The continuous researches on the management of the liver disease are required for the treatment in modern system of medicine 2. Drugs of natural origin with antioxidant properties are widely accepted and being used in developing a world for the prevention and treatment of hepatic disorders 3. It is considered to be inexpensive, safe and recommended for the treatment of liver disorder with very fewer side effects 4.
The experimental rodent’s model of hepatotoxicity can be developed by alcohol, paracetamol, CCl4, etc. For induction of liver fibrosis and hepatotoxicity experimentally, carbon tetrachloride CCl4 is the most commonly used hepatotoxic agent 5, 6. CCl4 is converted into metabolite trichloromethyl radical (⁰CCl3) and peroxy trichloromethyl radical (⁰OOCCl3) by cytochrome P4502E1. A free radical derivative of CCl4 induces and accelerates lipid peroxidation which ultimately causes liver injury 7, 8. In this study universally accepted CCl4 model for hepatotoxicity was selected to determine the hepatoprotective activity of SX and JC.
Solanum xanthocarpum Schrad. and Wendl. (Solanaceae) also known as Yellow Berried Nightshade (Kantkari), is a perennial herb found throughout India. This plant is reported to contain steroidal saponins in the form of sterols, glycoalkaloids, terpenoids, flavonoids, phenolic, tannins 10. The fruits are known to have anthelmintic, laxative, urinary stone treating, aphrodisiac 9, anti-inflammatory 11, antinociceptive 12, spasmolytic 13, antioxidant 14, hepatoprotective 15 and diuretic 16 activities.
Juniperus communis Linn. (Cupressaceae) is a coniferous shrub which is widely distributed across the Himalayas from Kumaon at an altitude of 1700-4200 m 17, 18. The plant is reported to contain various phytoconstituents like volatile oil, flavonoids, and coumarins 21. JC has been reported to be used traditionally for the cure of bronchitis 19 and tuberculosis 20 which is common lung disorders. JC is reported to have anti-inflammatory, antipyretic, analgesic 21 and antimicrobial 22 activities.
However, there is no any report yet demonstrated on comparative studies of Solanum xanthocarpum and Juniperus communis for effective liver protective potential. So, the present study was designed to evaluate the hepatoprotective potential of two medicinal plants against CCl4 induced hepatotoxicity in rats.
MATERIALS AND METHODS:
Chemicals and Reagents: L-ascorbic acid, 1, 1-diphenyl-2-picrylhydrazyl (DPPH) was procured from (Sigma-Aldrich Co., Mumbai). Carbon tetrachloride (CCl4), Silymarin, Trichloroacetic acid (TCA), Thiobarbituric acid (TBA), Ethylenediaminetetraacetic acid (EDTA), was purchased from Sigma Aldrich, Co., Mumbai. The diagnostic kits for Serum glutamate oxaloacetate transaminase (SGOT), Serum glutamate pyruvate transaminase (SGPT), alkaline phosphatase (ALP), total protein (TP) and total bilirubin (TB) were purchased from Calkine and coral private Limited.
Plant Material: Solanum xanthocarpum Schrad. and Wendl. (whole plant) was collected from (Dehradun) Uttarakhand, India. Juniperus communis Linn. (whole plant) was also collected from the local area of (Ranikhet) Uttarakhand, India in August to November 2016 and authenticated from Botanical Survey of India, Dehradun. A voucher specimen of the plants was deposited in the herbarium (115218A, 11521B).
Preparation of Plant Extract: The whole plant of SX and JC were dried and powdered. Powdered material (800g) was macerated with petroleum ether; the marc was extracted by a continuous hot extraction process using soxhlet apparatus using 80% v/v ethanol. The extract was separated by filtration and concentrated under reduced pressure and then dried in a lyophilizer (Labconco, USA). The yields obtained were 178.20 g and 198.10 g of solid residue (yield 22.27% w/w and 24.76% w/w respectively).
Phytochemical Screening: The hydroalcoholic extract of SX and JC were qualitatively tested for the presence of phytochemicals as per described standard methods 23-25.
In-vitro Free Radical Scavenging Activity:
DPPH Radical Scavenging Activity: The antioxidant activity of hydroalcoholic extract of SX and JC were assessed by determining its ability to scavenge free radicals. 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) is a stable free radical 26. The 0.1mM solution of DPPH was prepared in methanol. Then, 1ml of this solution was added to 2ml of test drug solution at different concentration (50-250 µg/ml). The mixture was agitated continuously further allowed to stand at room temperature for 30 min. Then, its absorbance was measured at 517 nm as standard Ascorbic acid was used. The percentage of scavenging activity was determined using the following formula:
Percentage of inhibition (%) = [(Acontrol – Asample / Acontrol)] ×100
Where, Acontrol - absorbance of DPPH, Asample -absorbance of DPPH with test sample 27.
In - vivo CCl4 Induced Hepatotoxicity in Rats:
Experimental Animals: Young Wistar rat (180-200g) breed in the Central Animal House, SBSPGI, Balawala, Dehradun, (India) was used in the study. Animals were acclimatized to laboratory conditions at room temperature before experimentation and kept under standard conditions of a 12 h light/dark cycle with food and water ad libitum in polyacrylic cages. All the experiments were performed between 09.00 and 16.00 h. The experimental protocol has been approved by the Institutional Animal Ethics Committee (IAEC) of college (IAEC/ CPCSEA/2016/101) and carried out as per the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA), Government of India on animal experimentation.
Experimental Protocol and Procedure: The Rats were divided into seven experimental groups consisting of six animals (n=6) in each group.
Group I received distilled water containing 0.5% Sodium Carboxymethyl cellulose (CMC-Na) (1ml/kg body weight, p.o.) for 7 days, and olive oil (1ml/kg body weight, s.c.) on days 2nd and 3rd.
Group II (CCl4) received 0.5% CMC-Na (1ml/kg body weight, p.o.) for 7 days, and a 1:1 mixture of CCl4 and olive oil (2mL/kg body weight, s.c.) on days 2nd and 3rd.
Group III was treated with the standard drug silymarin (50mg/kg body weight, p.o.) daily for 7 days, and also received the CCl4–olive oil mixture (1:1, 2ml/kg body weight, s.c.) on days 2nd and 3rd, 30 min after administration of silymarin.
Groups IV-VII (test group animals) was administered orally hydroalcoholic extract of SX and JC at the dose of (200 and 400 mg/kg body weight, p.o.) for 7 days respectively. Additionally, 30 min after administration of test drug, they received a dose of CCl4-olive oil mixture (1:1, 2 ml/kg, s.c.) on 2nd and 3rd day.
On the 7th day, animals were anesthetized by thiopentone sodium (45-50 mg/kg, i.p.), blood was collected, allowed to clot, and serum was separated for assessment of enzyme activity. The rats were sacrificed by bleeding; the livers were carefully dissected then removed and rinsed with ice-cold isotonic saline then kept on ice. The liver was separated and weighed. A 10% (w/v) tissue homogenates were prepared in 0.1 M phosphate buffer (pH 7.4). The homogenates were centrifuged at 10,000 × g for 15 min, and aliquots of the supernatants were separated and used for biochemical tissue estimation. Some parts of the liver tissue were immediately transferred into 10% formalin for histopathological investigation 28, 29.
Estimation of Serum Biochemical Parameters: Biochemical parameters were assayed according to standard methods. Estimation of the serum biochemical parameters like Serum glutamate oxaloacetate transaminase (SGOT), Serum glutamate pyruvate transaminase (SGPT), Alkaline phosphatase (ALP) 30, and Total bilirubin (TB) 31 was measured using commercial enzymatic biochemical diagnostic kits.
Estimation of Tissue Biochemical Parameters:
Measurement of Lipid Peroxidation: The extent of lipid peroxidation in the liver was determined quantitatively by performing the method as developed by Ohkawa et al., 1979. The amount of malondialdehyde (MDA) was measured by reaction with thiobarbituric acid at 532 nm using Shimadzu spectrophotometer (Japan). The values were calculated using the molar extinction coefficient of the chromophore (1.56 × 105 M-1 cm-1) and expressed as a percentage of control 32.
Measurement of Nitrite: The accumulation of nitrite in the supernatant, an indicator of the production of nitric oxide was determined by a colorimetric assay with Greiss reagent (0.1% N-(1-Napththyl) ethylenediamine dihydrochloride, 1% sulphanilamide and 5% phosphoric acid). Equal volumes of the supernatant and Greiss reagent were mixed, and the mixture was incubated for 10 min at room temperature in the dark. The absorbance was measured at 540 nm using Shimadzu spectrophotometer (Japan). The nitrite concentration in the supernatant was determined from a sodium nitrite standard curve and expressed as a percentage of control 33.
Measurement of Reduced Glutathione: Reduced glutathione was estimated according to the method by Ellman 1959. 1ml supernatant was precipitated with 1 ml of 4% sulphosalicylic acid and cold digested for 1 h at 48 °C. The samples were then centrifuged at 1200 × g for 15 min at 4 °C. To 1 ml of the supernatant obtained, 2.7ml of phosphate buffer (0.1 mmol/l, pH 8) and 0.2 ml of 5, 5’dithio-bis (2-nitrobenzoic acid) (DTNB) was added. The yellow color developed was measured at 412 nm using Shimadzu spectrophotometer (Japan). Results were calculated using molar extinction coefficient of the chromophore (1.36 × 104 (mol/l)-1cm-1) and expressed as a percentage of control 34.
Measurement of Catalase: Briefly, the assay mixture consisted of 12.5mM H2O2 in phosphate buffer (50mM of pH7.0) and 0.05 ml of supernatant from the tissue homogenate (10%) and the change in absorbance was recorded at 240 nm. Results were expressed as mM of H2O2 decomposed per milligram of protein/min 35.
Measurement of Protein Content: The protein content was estimated by the Biuret method using bovine serum albumin as a standard 36.
Histopathological Studies: Liver tissues were fixed in 10% formalin for at least 24 h, embedded in paraffin, and cut into 5µm-thick sections using a rotary microtome. The sections were stained with eosin methylene blue dye and observed under a microscope (Olympus, Japan) to observe histopathological changes in the liver.
Statistical Analysis: All experiments were done in triplicate and results were reported as mean ± S.E.M. (n = 6). The data analyzed was done by one-way ANOVA, and statistically significant effects were further analyzed by means comparison using Tukey’s multiple comparison analysis. The p<0.05 was considered to be statistically significant.
RESULTS:
Phytochemical Screening: Preliminary phytochemical screening of hydroalcoholic extract of SX and JC are shown in Table 1.
TABLE 1: PRELIMINARY PHYTOCHEMICAL SCREENING OF HYDROALCOHOLIC EXTRACTS OF SOLANUM XANTHOCARPUM AND JUNIPERUS COMMUNIS
| Class of compound | Hydroalcoholic extract of plants | |
| Solanum xanthocarpum | Juniperus communis | |
| Carbohydrates | + | - |
| Glycosides | + | + |
| Amino acids | + | - |
| Proteins | + | - |
| Steroids and triterpenoids | ++ | ++ |
| Alkaloids | + | + |
| Phenolic compound and Tannins | ++ | ++ |
| Flavonoids | + | + |
| Saponins | + | - |
(+) = Positive, (-) = Negative
In-vitro Free Radical Scavenging Activity:
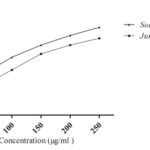
DPPH Radical Scavenging Activity: The antioxidant activity of Solanum xanthocarpum and Juniperus communis were determined by its capacity to scavenge DPPH radical. The hydroalcoholic extract of SX and JC showed DPPH radical scavenging activity with an IC50 of 69.41 ± 0.76 µg/ml, 117 ± 0.34 µg/ml respectively. Ascorbic acid (IC50 24.14 ± 0.16 µg/ml) showed an excellent activity. The activity of hydroalcoholic extract of SX has significantly higher free radical quenching capacity when compared to the hydroalcoholic extract of JC are shown in Fig. 1.
FIG. 1: DPPH RADICAL SCAVENGING ACTIVITY OF DIFFERENT CONCENTRATION OF SOLANUM XANTHOCARPUM AND JUNIPERUS COMMUNIS
Values expressed as means ± SEM
In-vivo CCl4 Induced Hepatotoxicity in Rats:
Effect of Hydroalcoholic Extract of Solanum xanthocarpum and Juniperus communis on Hepatic Markers: The hepatoprotective effect of hydroalcoholic extract of Solanum xanthocarpum and Juniperus communis were assessed by measuring liver enzymes biochemically. The biochemical parameter like SGOT, SGPT, ALP, and serum bilirubin were significantly (P<0.05) elevated and as compared to control group indicating liver damage. However, rats treated with hydroalcoholic extract of SX and JC at the dose of 200, 400 mg/kg shown significant (p<0.05) decrease in the levels of liver enzymes SGOT, SGPT and ALP in the CCl4-treated rats suggesting their hepatoprotective potential. A hydroalcoholic extract of SX showed a more significant effect to reduce the SGOT, SGPT, ALP and bilirubin levels Table 2.
TABLE 2: EFFECT OF SOLANUM XANTHOCARPUM AND JUNIPERUS CUMMINUS ON BIOCHEMICAL PARAMETERS OF CCl4 DAMAGED LIVERS IN RATS
| Groups | SGOT
(U/L) |
SGPT
(U/L) |
ALP
(U/L) |
TB
(mg/dl) |
Protein
(gm/dl) |
Albumin (gm/dl) |
| Normal-control | 30.5±1.5 | 32±1.2 | 145.25±1.49 | 0.7±0.09 | 6.425±0.08 | 3.975±0.1 |
| CCl4-control | 106.75±6.9a | 195±7.5a | 428.5±60.0a | 1.0475±0.007a | 3.95±0.3a | 3.725±0.2a |
| Silymarin (50mg/kg) | 43.75±3.7 | 57.25±3.7 | 147.75±2.2 | 0.615±0.06 | 6.725±0.2 | 3.95±0.2 |
| SXE(200mg/kg) | 61.25±2.3b | 142.25±10.3b | 155.5±3.6b | 0.7125±0.01b | 5.675±0.1b | 3.775±0.3b |
| SXE (400mg/kg) | 58.5±4.2b,c | 70.75±4.8b,c | 146.25±1.6b,c | 0.4±0.04b,c | 6.6±0.2b,c | 4.375±0.08b,c |
| JCE (20mg/kg) | 89.5±7.7b | 181.25±9.03b | 214±29.7b | 0.7625±0.02b | 6.15±0.3b | 3.45±0.1b |
| JCE (400mg/kg) | 72.9±1.4b,c | 127.75±11.3b,c | 149.5±3.4b,c | 0.4975±0.03b,c | 5.675±0.2b,c | 4.12±0.1b,c |
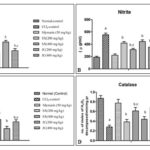
FIG. 2: EFFECT OF HYDROALCOHOLIC EXTRACTS OF SOLANUM XANTHOCARPUM AND JUNIPERUS COMMUNIS ON BIOCHEMICAL ALTERATION IN CCl4 TREATED RATS. A. MDA LEVEL B. NITRITE CONCENTRATION C. REDUCED GLUTATHIONE (GSH) D. CATALASE. Results were expressed as mean ± S.D; ap < 0.05 vs. Normal control. bp < 0.05 vs. CCl4 control group, cp < 0.05 vs. Silymarin (50 mg/kg).
Effect of Hydroalcoholic Extract of Solanum xanthocarpum and Juniperus communis on Oxidative Stress Parameters (Lipid Peroxidation, Nitrite, Catalase and Reduced Glutathione) in CCl4 Induced Hepatotoxicity in Rats: Chronic administration of CCl4 significantly caused oxidative stress (increased MDA level, nitrite concentration, depleted catalase (CAT) and reduced glutathione (GSH) enzyme activity) as compared to normal control group. The hydroalcoholic extract of SX and JC (200, 400mg/kg b.w.) treated rats significantly (p<0.05) decrease oxidative stress (MDA levels, nitrite concentration and restored the level of endogenous antioxidant enzyme viz. catalase CAT and reduced GSH) dose-dependently as compared to CCl4 treated rats indicating antioxidant effect. Moreover, the administration of standard silymarin (50mg/kg) significantly (p < 0.05) attenuated the oxidative damage in CCl4 induced liver injury as shown in Fig. 2A-D.
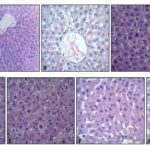
Histopathological Studies: The different groups of rats were studied for the cellular architecture of the liver tissue by a histopathological analysis which is presented in Fig. 3A-G.
FIG. 3: HISTOPATHOLOGY OF LIVER TISSUES. PHOTOMICROGRAPHS WERE TAKEN AT 100X
A: Liver section of normal control rats showing central vein surrounded by a hepatic cord of cells (normal architecture)
B: Liver section of CCl4 treated rats showing massive fatty changes along with congestion in a central vein, necrosis, ballooning degeneration and the loss of cellular boundaries.
C: Liver section of rats treated CCl4 and 50 mg/kg b.w. of Silymarin showing normal liver architecture.
D: Liver section of rats treated CCl4 and 200 mg/kg b.w. of hydroalcoholic extract of SX showing inflammatory collections and focal necrosis with sinusoidal dilatation.
E: Liver section of rats treated CCl4 and 400 mg/kg b.w. of hydroalcoholic extract of SX showing regeneration of hepatocytes toward near normal liver architecture.
F: Liver section of rats treated CCl4 and 200 mg/kg b.w. of hydroalcoholic extract of JC showing inflammatory collections around the central vein and focal necrosis.
G: Liver section of rats treated CCl4and 400 mg/kg b.w. of hydroalcoholic extract of JC showing less inflammatory cells and, absence of necrosis.
DISCUSSION: Oxidative stress is a process where the physiological balance between pro-oxidants and antioxidants is disrupted, resulting in potential damage to the body organs 37. Oxidative stress is responsible for the liver diseases resulting in one of the serious health issues worldwide 38. Antioxidants derived from natural source help in counteracting the oxidative stress induced by the number of hepatotoxins 39. Therefore, in the present study the comparative liver protective activity of ethanolic extract of Solanum xanthocarpum Schradt. and Wendl. and Juniperus communis Linn. (whole plant) was demonstrated against CCl4 induced liver toxicity. Preliminary phytochemical screening of SX and JC showed the presence of steroids, triterpenoids, glycosides, flavonoids and phenolic compounds. These phytoconstituents have been previously reported to have antioxidant as well as hepatoprotective potential 40, 41. Hydroalcoholic extract of SX and JC showed remarkable antioxidant activity in DPPH radical scavenging assay. The radical scavenging activity of SX was more significant as compared to JC. Antioxidant activity of both plants extracts on DPPH radicals may be attributed to a direct role in trapping free radicals by donating hydrogen atom or electron. The antioxidant activity of both plants may be due to the high flavonoids and phenolic contents as phenolic compounds received attention for their potential antioxidant activity 42.
CCl4 is conventionally used to induce liver toxicity in rats. CCl4 is actively metabolized in the liver tissues to its highly reactive trichloromethyl free radical CCl3. Trichloromethyl free radical reacts with cellular macromolecular protein and polyunsaturated fatty acids in the presence of molecular oxygen to form more toxic trichloromethyl peroxyl radicals along with H2O2, O2, OH that leads to liver damage 37. The liver toxicity induced by CCl4 elevates the liver marker enzymes as seen in the blood 43. The rise in serum levels of SGOT and SGPT indicate the damaged structural integrity of the liver 44. The level of enzyme SGOT and SGPT were increased with the toxicant CCl4 treatment but after treatment with hydroalcoholic extract of SX and JC the elevated level were changed which indicates the protective action of plant extract.
The enzyme alkaline phosphate (ALP) is a membrane-bound glycoprotein enzyme with a high concentration in sinusoid and endothelium. It is excreted into the bile, but on treatment with toxicant CCl4, there is the elevation of serum ALP level due to hepatobiliary disorder 45. In the present study, the treatment with hydroalcoholic extract of plants reduced the level of ALP in treated animals. CCl4 induced elevation of ALP is in line with high levels of serum bilirubin. The decrease in the raised ALP enzyme activity along with the fall of higher bilirubin level indicated some benefits in biliary functions in rats with hepatic injury. The significant control of ALP and bilirubin levels in treated groups points toward an early improvement in the secretary mechanism of hepatocytes 28.
Treatment with SX and JC hydroalcoholic extract reduces the biochemical enzyme level, which indicates the preservation of structural and functional integrity of the hepatocellular membrane in rats. The decrease in the total protein (TP) is attributed to the initial damage produced and localizes in the endoplasmic reticulum which results in the loss of cytochrome - 450 enzymes indicating the functional failure of protein synthesis and accumulation of triglycerides leading to fatty liver 46. Treatment with both plant extract enhances the total protein level accelerate the regeneration and protection of liver cells indicating the hepatoprotective activity of plants.
GSH is a non-enzymatic antioxidant bio-molecules present in the tissue. It removes the free oxygen species, such as H2O2, superoxide anions and alkoxy radicals, maintains the membrane protein thiols, and act as a substrate for GPx and glutathione S-transferase (GST) 47. Thus GSH maintains the body’s endogenous antioxidant defense mechanism and conjugates with free radicals directly to protect the integrity of cell membranes 48. Increase in MDA levels; intoxicant CCl4 treated rats; indicate an increase in lipid peroxidation leading to tissue damage and failure of antioxidant defense mechanisms 49. The level of GSH decrease and the LPO increase in treatment with toxicant CCl4 treatment. Animals treated with plant extract significantly restore the hepatic GSH and LPO content toward normal level shown in Fig. 2A, C.
Catalase plays an essential role in protection against the harmful effects of hydrogen peroxide and lipid peroxidation in diseases related to oxidative stress 50. The suppression of CAT level is an indication of liver damage in CCl4 treated animal group. On the administration of both plant extracts there is the significant restoration of the reduced catalase (CAT) level as shown in Fig. 2D. Nitrite is a stable metabolite produced from the metabolism of NO. The increased NOS activity has been observed in liver homogenate of rats exposed to CCl4, that led to increased nitrite levels indicates the oxidative and nitrosative stress in animals. The exposure to reactive and nitrogen species RNOS may cause the lipid peroxidation in cell membranes, which generates reactive species that damage the cell proteins and promote their degradation 51. On treatment with SX and JC hydroalcoholic extract there was a significant reduction in the elevated nitrite concentration as shown in Fig. 2B. The hepatoprotective potential of hydroalcoholic extract SX and JC is dose-dependent. As the results have shown the hydroalcoholic extract, SX (400 mg/kg) showed a maximum reduction in MDA level, nitrite concentration and resorted the catalase (CAT), reduced GSH level.
Histopathological examinations of treated CCl4 animal liver showed hepatic toxicity which was evidenced by cellular necrosis, nodal formation, profound steatosis and fibrosis as compared to normal hepatic architecture of normal animal liver section, which is clearly shown in Fig. 3A, B. On treatment with SX and JC hydroalcoholic extract the animal showed recovery of damaged parenchyma, which was comparable to that of the standard drug Silymarin treated animal liver section shown in Fig. 3C-G.
The in-vitro and in-vivo antioxidant activities of SX and JC may be associated with the flavonoids, phenolic, and terpenoid compounds present in the extract which has been known for their antioxidant and hepatoprotective activities 52.
CONCLUSION: In a nutshell it is concluded that both the plants Solanum xanthocarpum and Juniperus communis extracts may have promising hepatoprotective properties due to their antioxidant potential. The results suggested that the plants exhibited hepatoprotective effect that may be due to the presence of phenolic compounds which acts as antioxidants. It is also observed that Solanum xanthocarpum plant has more significant hepatoprotective activity compared to Juniperus communis plant.
ACKNOWLEDGEMENT: We express our sincere thanks to the Management and Shri. S. P. Singh, Honorable Chairman, Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences & Research, Balawala India for providing necessary facilities.
CONFLICTS OF INTEREST: None to declare.
REFERENCES:
- Zhang A, Sun H and Wang X: Recent advances in natural products from plants for the treatment of liver diseases. European Journal of Medicinal Chemistry 2013; 63: 570-77.
- Rao GMM, Rao CV, Pushpangadan P and Shirwaikar A: Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Journal of Ethnopharmacology 2006; 103: 484-490.
- Amat N, Upur H and Blazekovi B: In vivo hepatoprotective activity of the aqueous extract of Artemisia absinthium against chemically and immunologically induced liver injuries in mice. Journal of Ethnopharmacology 2010; 131: 478-484.
- Sheetal V and Singh SP: Current and future status of herbal medicine. Veterinary World 2008; 1(11): 347-50.
- Deng JS, Chang YC, Wen CL, Liao JC, Hou WC, Amagaya S, Huang SS and Huang GJ: Hepatoprotective effect of the ethanol extract of Vitis thunbergii on carbon tetrachloride-induced acute hepatotoxicity in rats through anti-oxidative activities. Journal of Ethnopharmacology 2012; 142: 795803.
- Muriel P: Nitric oxide protection of rat liver from lipid peroxidation, collagen accumulation, and liver damage induced by carbon tetrachloride. Biochemical Pharmacology 1998; 56: 773-79.
- Maling HM, Eichelbaum FM, Saul W, Sipes IG, Brown EA and Gillette JR: Nature of the protection against carbon tetrachloride-induced hepatotoxicity produced by pretreatment with dibenamine N-(2-chloroethyl) dibenzylamine. Biochemical Pharmacology 1974; 23: 1479-91.
- Maes M, Vinken M and Jaeschke H: Experimental models of hepatotoxicity related to acute liver failure. Toxicology and Applied Pharmacology 2016; 290: 86-97.
- Kiritikar KR and Basu BD: Indian Medicinal Plants. 2nd International Book Distributors: Dehradun 1994.
- Singh OM and Singh TP: Phytochemistry of Solanum xanthocarpum: An amazing traditional healer. Journal of Scientific and Industrial Research 2010; 69: 732-40.
- Anwikar S and Bhitre M: Study of the synergistic anti-inflammatory activity of Solanum xanthocarpum Schrad and Wendl and Cassia fistula International Journal of Ayurveda Research 2010; 1: 167.
- Rahman M, Ahmed M, Alimuzzaman M and Shilpi J: Antinociceptive activity of the aerial parts of Solanum xanthocarpum. Fitoterapia 2003; 74: 119-121.
- Parmar S, Gangwal A and Sheth N: Evaluation of the antiasthmatic activity of a polyherbal formulation containing four plant extracts. International Journal of Current Pharmaceutical Research 2010; 2: 40–44.
- Yang RY, Tsou S, Lee TC, Wu WJ, Hanson PM, Kuo G, Engle LM and Lai PY: Distribution of 127 edible plant species for antioxidant activities by two assays. Journal Science of Food and Agriculture 2006; 86: 2395–2403.
- Gupta AK, Ganguly P, Majumder UK and Ghosal S: Hepatoprotective and antioxidant effects of total extracts and steroidal saponins of Solanum xanthocarpum and Solanum nigrumin paracetamol induced hepatotoxicity in rats. Pharmacologyonline 2009; 1: 757-768.
- Shahiladevi S, Jayanthi G and Jagadeesan M: Comparative diuretic activity of seeds and fruit wall extracts of Solanum surattense F. and Solanum melongenavar. Insanum (L.) Prain. Indian Drugs 2006; 43: 856-859.
- Allen D and Hatfield G: Medicinal Plants in Folk Tradition, an Ethnobotany of Britain and Ireland. Timber Press: Cambridge 2004.
- Khare CP: Indian Medicinal Plants: An Illustrated Dictionary: Springer 2007.
- Handa SS, Sharma A and Chakraborthi KK: Natural products and plants as liver protecting agents. Fitoterapia 1986; 5: 307-51.
- McCutcheon AR, Stokes RW, Thorson LM, Ellis SM, Hancock REW and Towers GHN: Anti-mycobacterial screening of British Columbian medicinal plants. International Journal of Pharmacognosy 1997; 35: 77-83.
- Chatterjee TK, Ghosh C and Raychaudhuri P: Anti-inflammatory and antipyretic action of Juniperus communis (Linn.) leaf extract in rats. Indian Drugs 1991; 28(9): 430.
- Banerjee S, Mukherjee A and Chatterjee TK: Evaluation of Analgesic activities of methanolic extract of medicinal plant Juniperus communis Inter Journal of Pharmacy and Pharmaceutical Sciences 2012; 4(5): 547-50.
- Farnsworth NR: Biological and phytochemical screening of plants. Journal of Pharmaceutical Sciences 1966; 55: 225-76.
- Harborne JB: Phytochemical methods. 3rd Chapman and Hall; London 1998.
- Trease GE and Evans WC: Text Book of Pharmacognosy. 12th, Tindall; London 1983.
- Shimada K, Fujikawa K, Yahara K and Nakamura T: Antioxidative properties of xanthin on autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry 1992; 40: 945-48.
- Joshi BC, Mukhija M and Semwal S: Antioxidant potential and total phenolic content of Urtica dioica (whole plant). Journal of Applied Pharmacy 2015; 7(2): 120-28.
- Joshi BC, Prakash A and Kalia AN: Hepatoprotective potential of antioxidant potent fraction from Urtica dioica(whole plant) in CCl4 challenged rats. Toxicology Reports 2015; 2: 1101-10.
- Shuangchan W, Yuan Y, Hui T, Zhike L, Xiaofei L, Wei H and Hong D: Carthamus red from Carthamus tinctorius exerts antioxidant and hepatoprotective effect against CCl4-induced liver damage in rats via the Nrf2 pathway, Journal of Ethnopharmacology 2013; 148: 570-78.
- King J: The hydrolases-acid and alkaline phosphatases, in: Practical Clinical Enzymology. Nostrand Company Ltd.; London 1965.
- Malloy HT and Evelyn KA: The determination of bilirubin with the photometry colorimeter. The Journal of Biological Chemistry 1937; 119: 481-90.
- Ohkawa H, Ohishi N and Yagi K: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 1979; 95: 351–358.
- Green LC, Wagner DA and Glagowski J: Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. The Journal of Biological Chemistry 1982; 193: 265-75.
- Ellman GL: Tissue sulfhydryl groups. Arch. Biochem Biophys 1959; 82: 70–77.
- Luck H: Catalase, in: H.U. Bergmeyer (Ed.), Methods of Enzymatic Analysis, 3rd, Academic Press; New York 1971; 618(3).
- Gornall AG, Bardawill CJ and David MM: Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry 1949; 177: 751-66.
- Maheshwari D, Kumar Y, Verma SK, Singh VK and Singh SN: Antioxidant and hepatoprotective activities of phenolic-rich fraction of Seabuckthorn Hippophae rhamnoides leaves. Food and Chemical Toxicology 2011; 49: 2422-28.
- Huang B, Ban XQ, He JS, Zeng H, Zhang P and Wang YW: Hepatoprotective and antioxidant effects of the methanolic extract from Halenia elliptica. Journal of Ethnopharmacology 2010; 131: 276-81.
- Sundararajan R, Haja NA, Venkatesan K, Mukherjee K, Saha BP, Bandyopadhyay A and Mukherjee PK: Cystisus scoparius- a natural antioxidant. BMC Complementary and Alternative Medicine 2006; 16: 6-8.
- Krishnaiah D, Sarbatly R and Nithyanandam R: A review of the antioxidant potential of medicinal plant species. Food and Bioproducts Processing 2011; 89: 217-33.
- Sandhar HK, Kumar B, Prasher S, Tiwari P, Salhan M and Sharma P: A review of phytochemistry and pharmacology of flavonoids. International Journal of Pharmaceutical Sciences and Research 2011; 1: 25-41.
- Rice-Evans CA, Miller NJ and Paganga G: Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine 1996; 20: 933-56.
- Drotman RB and Lawhorn GT: Serum enzymes are indicators of chemical-induced liver damage. Drug and Chemical Toxicology 1978; 1: 163-71.
- Sallie R, Tredger JM and William R: Drugs and the liver. Part I testing liver function. Biopharmaceutics and Drug Disposition 1991; 12: 251-59.
- Burtes CA and Ashwood ER: Textbook of Clinical Chemistry. WB Sunders Co; Philadelphia 1986.
- Suresh KSV, Sujatha C, Syamala J, Nagasudha B and Mishra SH: Hepatoprotective activity of extracts from Pergularia daemai Forsk against carbon tetra chloride induced toxicity in rats. Pharmacognosy Magazine 2007; 3: 11-15.
- Townsend DM, Tew KD and Tapiero H: The importance of glutathione in human disease. Biomedicine and Pharmacotherapy 2003; 57; 145-55.
- He J, Huang B, Ban X, Tian J, Zhu L and Wang Y: In-vitro and in-vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. Journal of Ethnopharmacology 2012; 141: 104-10.
- Goel A, Dani V and Dhawan DK: Protective effects of zinc on lipid peroxidation, antioxidant enzymes and hepatic histoarchitecture in chlorpyrifos induced toxicity. Chemico-Biological Interactions 2005; 156: 131-40.
- Zhu R, Wang Y, Zhang L and Guo Q: Oxidative stress and liver disease. Hepatology Research 2012; 42: 741–749.
- Das SK and Vasudevan D: Modulation of lecithin activity by Vitamin-B complex to treat long term consumption of ethanol-induced oxidative stress in liver. Indian Journal of Experimental Biology 2006; 44: 791.
- Hsiao G, Shen MY and Lin KH: Antioxidative and hepatoprotective effects of Antrodia camphorate Journal of Agricultural and Food Chemistry 2003; 51: 3302-08.
How to cite this article:
Joshi BC, Uniyal S and Sukanya: Investigation on novel role of Solanum xanthocarpum and Juniperus communis extract against CCl4 induced liver injury. Int J Pharmacognosy 2017; 4(12): 399-07. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.4(12).399-07.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
399-407
748
1431
English
IJP
B. C. Joshi *, S. Uniyal and Sukanya
Department of Pharmacognosy, Sardar Bhagwan Singh Post Graduate Institute of Biomedical Sciences and Research, Balawala, Dehradun, Uttarakhand, India.
bhuwan.joshi000@gmail.com
24 June 2017
07 July 2017
17 September 2017
10.13040/IJPSR.0975-8232.IJP.4(12).399-07
01 December 2017