INVESTIGATION OF ANALGESIC ACTIVITY OF AMARANTHUS SPINOSUS LINN. (LEAVES) FROM BANGLADESH IN SWISS ALBINO MICE
HTML Full TextINVESTIGATION OF ANALGESIC ACTIVITY OF AMARANTHUS SPINOSUS LINN. (LEAVES) FROM BANGLADESH IN SWISS ALBINO MICE
Pritesh Ranjan Dash * 1, Fowzia Afsana Chowdhury 2, Zakia Sultana 3 and Nishat Jahan 3
Department of Pharmacy 1, Jahangirnagar University, Savar, Dhaka - 1342, Bangladesh.
Department of Pharmacy 2, BRAC University, Mohakhali, Dhaka, Bangladesh.
Department of Pharmacy 3, University of Asia Pacific, Dhaka, Bangladesh.
ABSTRACT: The current study was aimed to investigate the analgesic activity of the methanolic extract of Amaranthus spinosus leaf (Family: Amaranthaceae). The analgesic activity was assessed with this extract for their peripheral and central anti-nociceptive potentials using three standard test methods, for instance, acetic acid induced writhing, hot plate and tail immersion methods in Swiss albino mice, at the doses of 200 and 400 mg/kg body weight. In acetic acid induced writhing method, methanolic extract of Amaranthus spinosus leaf (200 and 400 mg/kg) showed significant peripheral analgesic activity with writhing inhibition of 58.02% and 63.2% respectively whereas the standard drug diclofenac-Na (25 mg/kg) showed 73.88% writhing inhibition. In the hot plate test, methanolic extract of the leaf (200 and 400 mg/kg body weight) showed 55.09%, and 66.22% central nociception inhibition of thermal stimulus respectively, where the standard drug morphine (5 mg/kg) displayed 50.35% nociception inhibition. In the tail immersion test, maximum 68.19% and 73.91% nociception inhibition of thermal stimulus was exhibited with methanol extract of the leaf (200 and 400 mg/kg, b.w. respectively). The methanolic extract of Amaranthus spinosus at higher dose showed more prominent central and peripheral analgesic activity. However, further investigation can also assist in inventing unexplored pharmacological activities of this plant as well as to familiarize the plant as a new source of medicine.
| Keywords: |
Amaranthus spinosus Linn., Writhing method, Tail immersion, Hot plate method, Amaranthaceae
INTRODUCTION: Amaranthus spinosus Linn. (Family Amaranthaceae), is an annual or perennial herb grows annually as a branched erect, glabrous, monoecious weed varying in color from green to purple. It is found throughout the United States of America, and all tropical and subtropical regions of Africa, Southeast Asia and India 1.
It is a very common Bangladeshi plant commonly known as ‘Kantanotey” in ‘Bengali” 2. Traditionally the natural crude extracts from the plant have been used to treat bronchitis, biliousness, appetizer, digestible, nausea, haematinic, galactagogue, stomachic, flatulence, blood diseases, anorexia, piles, leucorrhoea, and leprosy.
The plant is also used as antipyretic, diuretic, laxative and febrifuge. The leaves of this plant are considered a good emollient and applied externally to treat burning sensation, abscess, eczema, arthritis and hemorrhoids and rheumatism 3. The leaves are also effective against gall bladder inflammation, abdominal pain, fever, hysteria, malaria, mania, dysurea, dysentery, vomiting, and tonsillitis. The plant is a rich source of alkaloids, quercetin, flavonoids, glycosides, amino acids, betalains, betaxanthin, betacyanins, isomaranthine, steroids, phenolic acids, terpenoids, lipids, saponin, β-sitosterol, stigmasterol, linoleic acid, rutin, polyuronides, hydroxycinnamates, anthriquinone derivatives, organic acids, volatile oils, catechuic tannins and carotenoids.
Various studies have been executed on Amaranthus spinosus by the researchers and these studies showed that the plant is devoid of a wide range of pharmacological actions like anti-diabetic, analgesic, antimicrobial, antiviral, antibacterial, bronchodilator, antitumor, anti-inflammatory, spasmolytic, anti-malarial, anti-hyperglycemic, anti-hyperlipidemic, hepato-protective, immuno-stimulating, spermatogenic, antifertility, anti-oxidant properties 4. Currently, the analgesic drugs that are available, for instance, NSAIDs and opiates are not useful in all cases because of their adverse effects. Therefore, it is important to explore new compounds with improved pain management capacity and minimal side effects. As a part of our ongoing investigations on local medicinal plants of Bangladesh 5, 6, 7. In this paper, the analgesic activity of the leaves of Amaranthus spinosus Linn has been reported.
MATERIALS AND METHODS:
Chemicals: Acetic acid was a product of Merck, Germany. Diclofenac sodium and aspirin were purchased from Square Pharmaceuticals Ltd., Bangladesh; morphine was purchased from Gonoshasthaya Pharmaceuticals Ltd., Bangladesh; 0.9% sodium chloride solution (Normal saline) was purchased from Orion Infusion Ltd., Bangladesh, and other reagents were of analytical grade.
Plant Material: At first, the plant of Amaranthus spinosus Linn. were collected from the local area of Savar, Dhaka, Bangladesh in November 2018. For authentication, the plant was given to National Herbarium of Bangladesh (NHB), Mirpur, Dhaka. After one week, the collected plant was taxonomically identified as (Accession Number: (DACB: 46,794). After that, the plant was authenticated by the taxonomist of Bangladesh National Herbarium, Mirpur, Dhaka.
Extraction: At first, the dried leaves were coarsely powdered and extracted using a mixture of methanol: water (8:2, v/v) at room temperature with a cold extraction method. After that, the solvent was completely removed with a rotary evaporator, and the dried crude extract was obtained, which was used for investigation.
Animal: Male Swiss albino mice, 3-4 weeks old with weighing between 20-25 gm, were collected for the experiment from Animal Production Unit of Animal House at the Department of Pharmacy, Jahangirnagar University. Animal was acclimatized to the standard environmental conditions at a temperature of (24.0 ± 1.0) °C, relative humidity: 55-65% under 12h light and 12h dark cycle for one week before each experiment. They had free access to feed and water ad libitum 8. The study was conducted following the approval by the Institutional Animal Ethical Committee (IAEC) of Jahangirnagar University, Savar, Dhaka, Bangladesh.
Acute Toxicity Study: To determine toxicity, mice were divided into control and test groups (n=6). As a single dose, the test groups were administered the methanolic extract of the leaf of Amaranthus spinosus orally at the doses of 500, 1000, 1500 and 2000 mg/kg. Then in separate cases, the animals were kept and were allowed to food and ad libitum. On the other hand, the control group received the water. The animals were observed periodically for possible behavioral changes, allergic reactions, and mortality for the next 72 h 9.
Tests for Analgesic Activity:
Acetic Acid-Induced Writhing Test: The peripheral analgesic activity of methanolic extract of Amaranthus spinosus leaf was evaluated using the acetic acid-induced writhing model in mice. Animals were divided into four groups, each group having six members. The control group received only water (5 ml/kg, p.o.). Reference standard group was administered diclofenac-Na 15 min before injected of 0.7% acetic acid intraperitoneally but third and fourth groups were administered methanolic extract orally at the doses of 200 and 400 mg/kg body weight respectively 30 min before intraperitoneal administration of 0.7% acetic acid. After 5 min, the number of writhes produced was counted for the next 10 min 10.
Contraction of the abdominal muscles with stretching of hind limbs was considered as a writhing movement. Percentage of writhing inhibition was calculated using the following formula:
Writhing inhibition (%) = Mean no. of writhings (control) - Mean no. of writhings (test) / Mean no. of writhings (control) × 100
Hot Plate Test: Eddy and Leimbach’s hot plate test was used to study centrally acting antinociceptive activity of methanolic extract of Amaranthus spinosus leaf 11. The control group received only water (5 ml/kg, p.o.) while the standard group was given morphine sulfate (5 mg/kg, i.p.). The test groups were treated with methanolic extract of Amaranthus spinosus orally at doses of 200 and 400 mg/kg body weight, respectively. Then the animals were allowed to place on Eddy’s hot plate having a temperature of 52± 0.5 °C. A cut off period of 28s was set for all mice to avoid damage to the tissue. Latency time was recorded when animals licked their fore or hind paws or tried to jump out before 0, 30, 60 and 90 min after the administration of the standard and test crude extract Amaranthus spinosus leaf. Percentage of elongation was calculated using the following formula:
Elongation (%) = Latency (test) – Latency (control) / Latency (test) × 100
Tail Immersion Test: Tail immersion model was also used to evaluate centrally acting anti-nociceptive activity of methanolic extract of Amaranthus spinosus leaf. The procedure is based on the observation that centrally acting analgesics selectively prolongs the reaction time (the time taken by the mice to deflect their tails) 12. The animals were treated as discussed above. 1 to 2 cm of the tail of mice was immersed in warm water (at a constant 55°C temperature).
The first reading was discarded, and the reaction time was recorded as a mean of the next three readings. A latency period of 20 s was considered as complete analgesia, and the measurement was then stopped to avoid injury to mice. The latent period of the tail-flick response was recorded before 0, 30, 60, and 90 min after the administration of drugs. Percentage of elongation was calculated using the following formula:
Elongation (%) = Latency (test) – Latency (control) / Latency (test) × 100
RESULTS:
Acute Toxicity: Oral administration of Amaranthus spinosus Linn at the doses of 500-2000 mg/kg did not produce any mortality or noticeable behavioral changes in mice within 72 h observation period. Therefore, it can be suggested that Amaranthus spinosus have low toxicity profile with LD50 higher than 2000 mg/kg.
Analgesic Activity:
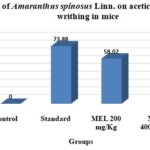
Acetic Acid-Induced Writhing Test: Acetic acid induced writhing test displayed that the methanolic extract of Amaranthus spinosus leaf produced potent inhibition of writhing reaction in a dose-dependent manner. In this test, MEL (400 mg/kg) inhibited maximum 63.2% writhing, whereas the standard drug (25 mg/kg) diclofenac-Na (25 mg/kg) showed 73.88% writhing inhibition.
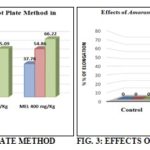
The results of the hot plate test showed that analgesic potentials increase in latency time at a dose-dependent manner. The results were found to be statistically significant (p<0.05-0.001). In the hot plate test, MEL (200 and 400 mg/kg) showed maximum 55.09 and 66.22% central nociception inhibition of thermal stimulus respectively, whereas the standard drug morphine (5 mg/kg) displayed maximum 44.52% nociception inhibition. Results of the hot plate test are presented in Table 2.
TABLE 1: EFFECT OF THE METHANOL EXTRACT OF AMARANTHUS SPINOSUS LINN. ON ACETIC ACID-INDUCED WRITHING IN MICE
| Groups | Dose (mg/kg) | No. of writhing | % of writhing | % of writhing inhibition |
| Control | 5 ml/kg | 45±0.53 | 100 | - |
| Standard | 25 | 11.75±0.34 | 26.11 | 73.88 |
| MEL | 200 | 18.89±0.53 | 22.22 | 58.02 |
| 400 | 16.56±0.56 | 28.44 | 63.2 |
MEL = Methanolic extract of leaves, the control group, received water 5 ml/kg body weight (p.o.), standard received diclofenac-Na 25 mg/kg body weight, MEL were treated with 200 and 400 mg/kg body weight (p.o.) of the crude extract of Amaranthus spinosus. Values are mean ± SEM, (n = 6).
FIG. 1: EFFECT OF MEL ON HOT PLATE TEST. MEL = Methanolic extract of leaves, the control group, received water 5 ml/kg body weight (p.o.), standard received diclofenac-Na 25 mg/kg body weight, MEL were treated with 200 and 400 mg/kg body weight (p.o.) of the crude extract of Amaranthus spinosus. Values are mean ± SEM, (n = 6).
Tail Immersion Test: The results of the tail immersion test showed that crude extract of Amaranthus spinosus increases the tail withdrawal reflex time with increasing of doses. In this test, maximum effect was observed after 60 and 90 min of drug administration.
TABLE 2: EFFECTS OF THE METHANOL EXTRACT OF A. SPINOSUS LINN. ON HOTPLATE METHOD
| Groups | Dose
(mg/kg) |
Mean reaction time (s) before and after
drug administration |
%
inhibition |
|||||
| 0 min | 30 min | 60 min | 90 min | 30 min | 60 min | 90 min | ||
| Control | 5 ml/kg | 9.53±0.63 | 8.89±0.69 | 9.93±0.75 | 9.43±0.36 | - | - | - |
| Standard | 5 | 10.95±1.19 | 16±3.02** | 20±0.43** | 20±2.19** | 44.43 | 50.35 | 44.52 |
| MEL | 200 | 10.29±2.19 | 14±1.85* | 16±2.33* | 21±2.33* | 36.5 | 37.93 | 55.09 |
| 400 | 8.28±0.67 | 14.29±1.83* | 22±2.36* | 24.67±1.67* | 37.78 | 54.86 | 66.22 | |
MEL = Methanolic extract of leaves, the control group, received water 5 ml/kg body weight (p.o.), standard groups received morphine 5 mg/kg body weight (i.p.), MEL were treated with 200 and 400 mg/kg body weight (p.o.) of the crude extract of Amaranthus spinosus. Values are mean ± SEM, (n = 6).
TABLE 3: EFFECTS OF THE METHANOL EXTRACT OF AMARANTHUS SPINOSUS LINN. ON TAIL WITHDRAWAL REFLEX OF MICE INDUCED BY TAIL IMMERSION METHOD
| Groups | Dose
(mg/kg) |
Mean reaction time (s) before and after
drug administration |
%
inhibition |
|||||
| 0 min | 30 min | 60 min | 90 min | 30 min | 60 min | 90 min | ||
| Control | 5 ml/kg | 1.79±0.19 | 1.88±0.29 | 1.91±0.36 | 1.87±0.17 | - | - | - |
| Standard | 5 | 2.63±0.49 | 4.95±0.95** | 5.56±0.43** | 5.95±0.93** | 62.02 | 65.64 | 68.57 |
| MEL | 200 | 2.39±0.16 | 4.39±0.93* | 5.29±0.96* | 5.88±0.87* | 57.17 | 63.89 | 68.19 |
| 400 | 2.44±0.19 | 4.71±0.17* | 6.63±0.36* | 7.17±0.30* | 60.08 | 71.19 | 73.91 | |
MEL = Methanolic extract of leaves, the control group, received water 5 ml/kg body weight (p.o.), standard groups received morphine 5 mg/kg body weight (i.p.), MEL were treated with 200 and 400 mg/kg body weight (p.o.) of the crude extract of Amaranthus spinosus. Values are mean ± SEM, (n = 6).
MEL = Methanolic extract of leaves, the control group, received water 5 ml/kg body weight (p.o.), standard groups received morphine 5 mg/kg body weight (i.p.), MEL were treated with 200 and 400 mg/kg body weight (p.o.) of the crude extract of Amaranthus spinosus. Values are mean ± SEM, (n = 6).
The results were statistically significant (p<0.05-0.001). In tail immersion test, maximum 73.91% and 68.19% nociception inhibition of thermal stimulus was exhibited with the MEL at the doses of 200 and 400 mg/kg, body weight respectively, whereas the standard drug morphine produced 68.57% nociception inhibition.
DISCUSSION: No acute toxicity was observed after oral administration of Amaranthus spinosus even at the dose of 2000 mg/kg in mice. The present study demonstrates that methanolic leaf extract of Amaranthus spinosus possess potent antinociceptive activity in chemical and heat-induced models. No acute toxicity was observed after oral administration of Amaranthus spinosus even at the dose of 2000 mg/kg in mice. Acetic acid induced writhing test is frequently used to evaluate the peripherally acting analgesics 13. Acetic acid stimulates endogenous mediators of pain like prostaglandin E2, prostaglandin E2α, Kinins 14, 15 synthesis within peritoneum and pain is induced by the activation of local peritoneal pain receptors due to which abdominal constrictions (writhing) get started with stretching of hind limbs 16. Nociceptive neurons stimulation is NSAIDs and narcotics 17. The findings of the present study indicate that methanolic extract of Amaranthus spinosus had significant antinociceptive effect in the acetic acid-induced writhing test, which may be achieved through inhibition of cyclooxygenase COX-synthesized prostaglandins.
The hot plate test and tail immersion model are used to evaluate the centrally acting analgesics 18. In the hot plate test, the paw-licking or jumping responses in the hot plate are considered as a complex supraspinal organized behavior of mice 19. So, the centrally acting analgesic property of the treatment is indicated by a decrease in licking or increase in latency. The results of the hot plate test showed that the methanolic extract of Amaranthus spinosus has a central anti-nociceptive effect against heat-induced pain. The effect was evident from the elongation of the latency time until the 4th observation (90 min).
Tail immersion model is considered as an acute pain model 20. In the tail immersion experiment, Amaranthus spinosus extract displayed a significant increase in tail-withdrawal time. The results of the tail immersion test showed that the methanolic extract of Amaranthus spinosus has a central anti-nociceptive effect against thermal stimulated pain may be inhibition of prostaglandin and bradykinins synthesis.
The activity of adenylyl cyclase may be decreased by Influx of calcium ions at the terminal of the axon of the afferent nerve due to the response of methanolic extract. This may cause the subsequent decreased cAMP level, potassium ion efflux. Consequent hyperpolarization of the nerves gives the anti-nociceptive effect 21. The anti-nociceptive effect of the extracts in these three models implies that the extracts contain pharmacologically active phytoconstituents that may act both centrally and peripherally.
CONCLUSION: Based on the results of the above three experiments, it can be concluded that the plant extract possesses remarkable analgesic potential. However, further studies are needed to be conducted to understand the exact mechanisms of analgesic action and to isolate the compound (s) responsible for such acts as well as to invent unexplored pharmacological activities of this plant and to familiarize the plant as a new source of medicine.
ACKNOWLEDGEMENT: Authors are grateful to Jahangirnagar University, Department of Pharmacy, Savar, and Dhaka, Bangladesh, for providing the necessary facility to carry out the study.
CONFLICT OF INTEREST: Authors declare no conflict of interest.
REFERENCES:
- Asha S, Rekha R and Sadiq AM: Amaranthus spinosus- A review. Bulletin of Environment, Pharmacology and Life Sciences 2016; 5(9): 102-07.
- Tanmoy G, Arijit M, Tanushree S, Jagadish S and Kumar MT: Pharmacological actions and phytoconstituents of Amaranthus spinosus A review. International Journal of Pharmacognosy and Phytochemical Research 2014; 6(2): 405-13.
- Hema ES, Sivadasan M and Anil KN: Studies on edible species of Amaranthaceae and Araceae used by Kuruma and Paniya tribes in Wayanad district, Kerala, India. Ethnobotany 2006; 18: 122-26.
- Tripathi KD: Essentials of Medical Pharmacology. Jaypee Brothers Medical Publishers (P) Ltd. New Delhi, India, Edition 4th, 1999: 432.
- Dash PR, Nasrin M, Raihan SZ and Ali MS: Study of anti-diarrhoeal activity of two medicinal plants of Bangladesh in castor-oil induced diarrhoea. Int J Pharm Sci Res 2015; 5(9): 3864-8.
- Dash PR, Rana MS and Sohrab MH: Biological activities of Polygonum flaccidum. Int J Pharmacognosy 2016; 3(5): 100-04.
- Mou KW and Dash PR: A comprehensive review on Gynura procumbens Int J Pharmacognosy 2016; 3(4): 167-74.
- Chatterjee TK: Handbook of Laboratory Mice and Rats. Department of Pharmaceutical Technology, Jadavpur University, India, Edition 1st, 1993: 157.
- Walker CI, Trevisan G, Rossato MF, Franciscato C, Pereira ME and Ferreira J: Anti-nociceptive activity of Mirabilis jalapain mice. J Ethnopharmacol 2008; 120: 169-75.
- Ahmed F, Selim MST, Das AK and Choudhuri MSK: Anti-inflammatory and anti-nociceptive activities of Lippia nodiflora Pharmazie 2004; 59: 329-33.
- Ishtiaq S, Ali T, Ahmad B, Anwar F, Afridi MSK and Shaheen H: Phytochemical and biological evaluations of methanolic extract of Amaranthus graecizans silvestris (Vill.) Brenan. British Journal of Pharmaceutical Research 2017; 15(3): 1-11.
- Toma W, Graciosa JS, Hiruma-Lima CA, Andrade FDP, Vilegas W and Souza Brita ARM: Evaluation of the analgesic and anti-edematogenic activities of Quassia amara bark extract. J Ethnopharmacol 2003; 85: 19-23.
- Sánchez-Mateo CC, Bonkanka CX, Hernández-Pérez M and Rabanal RM: Evaluation of the analgesic and topical anti-inflammatory effects of Hypericum reflexum fil. J Ethnopharmacol 2006; 107: 1-6.
- Deraedt R, Jouquey S, Delevallée F and Flahaut M: Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol 1980; 61: 17-24.
- Bentley GA, Newton SH and Starr J: Studies on the antinociceptive action of alpha-agonist drugs and their interactions with opioid mechanisms. Br J Pharmacol 1983; 79: 125-34.
- Muhammad N, Saeed M and Khan H: Antipyretic, analgesic and anti-inflammatory activity of Viola betonicifolia whole plant. BMC Complementary and Alternative Medicine 2012; 12(1): 1.
- Adzu B, Amos S, Kapu SD and Gamaniel KS: Anti-inflammatory and anti-nociceptive effects of Sphaeranthus senegalensis. J Ethnopharmacol 2003; 84: 169-73.
- Wong CH, Dey P, Yarmush J, Wu WH and Zbuzek VK: Nifedipine induced analgesia after epidural injection in rats. Anesth Analg 1994; 79: 303-06.
- Chapman, CR, Casey KL, Dubner R, Foley KM and Gracely RH: Pain measurement. An overview. Pain 1985; 22: 1-31.
- Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V and Kumar D: Antinociceptive and antipyretic activities of Pongamia pinnata Phytother Res 2003; 17: 259-64.
- Yaksh TL: Spinal systems and pain processing. Development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol Sci 1999; 20: 329-37.
How to cite this article:
Dash PR, Chowdhury FA, Sultana Z and Jahan N: Investigation of analgesic activity of Amaranthus spinosus Linn. (leaves) from Bangladesh in Swiss albino mice. Int J Pharmacognosy 2019; 6(5): 187-92. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP. 6(5).187-92.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
187-192
623
1092
English
IJP
P. R. Dash *, F. A. Chowdhury, Z. Sultana and N. Jahan
Department of Pharmacy, Jahangirnagar University, Savar, Dhaka, Bangladesh.
pritesh.ju@gmail.com
15 April 2019
20 May 2019
26 May 2019
10.13040/IJPSR.0975-8232.IJP.6(5).187-92
31 May 2019