INVESTIGATING THE ANTIOXIDANT PROPERTIES AND CHEMICAL COMPOSITION OF LINUM USITATISSIMUM L. (FLAXSEED) EXTRACT USING HR-LCMS ANALYSIS
HTML Full TextINVESTIGATING THE ANTIOXIDANT PROPERTIES AND CHEMICAL COMPOSITION OF LINUM USITATISSIMUM L. (FLAXSEED) EXTRACT USING HR-LCMS ANALYSIS
Sanghadeep Ukey * and Dayanand Gogle
Department of Botany, Lokmanya Tilak Mahavidyalaya, Wani, Yavatmal, Sant Gadge Baba Amravati University, Amravati, Maharashtra, India.
ABSTRACT: This study investigates the antioxidant properties and chemical composition of Linum usitatissimum L. (flaxseed) extract using High Resolution-Liquid Chromatography Mass Spectrometry (HR-LCMS) analysis. The primary objective is to identify the bioactive compounds responsible for the antioxidant activity and evaluate the efficacy of flaxseed extracts prepared with different solvents, including methanol, ethanol, acetone, chloroform, petroleum ether, and ethyl acetate. Methodologically, the study employs various antioxidant assays such as DPPH, ABTS, FRAP, and total Antioxidant Activity (TAA), in conjunction with HR-LCMS analysis for comprehensive chemical profiling. Key findings reveal that methanolic and ethanolic extracts exhibit the highest antioxidant activities, with significant amounts of phenolic and flavonoid compounds identified. The HR-LCMS analysis further elucidates the presence of metabolites such as adenosine, abscisic acid glucose ester, and kaempferol 7-O-glucoside, which contribute to the observed bioactivity. The study concludes that flaxseed extracts, particularly those obtained with methanol and ethanol, possess substantial antioxidant potential, underscoring their potential application in nutraceuticals and functional foods.
Keywords: Linum usitatissimum L., Flaxseed, Antioxidant, HR-LCMS analysis, Phenolic compounds, Flavonoids, etc.
INTRODUCTION: Linum usitatissimum L., commonly known as flaxseed or linseed, is a crop cultivated for its seeds and fibres, with a history that dates back thousands of years. Flaxseed is primarily grown for its oil-rich seeds, which are a notable source of alpha-linolenic acid (ALA), an omega-3 fatty acid crucial for human health 1. The cultivation of flaxseed has spread globally, adapting to various climates and soil conditions, making it a versatile agricultural product 2.
Flaxseed is rich in a variety of bioactive compounds, including lignans, which are phytoestrogens with antioxidant properties, as well as mucilage, which contributes to its high dietary fibre content 3. The high lignan content in flaxseed is linked to numerous health benefits, such as reducing the risk of cardiovascular diseases, hormone-related cancers, and improving digestive health 4.
Furthermore, the oil extracted from flaxseed is widely recognized for its high ALA content, which plays a significant role in anti-inflammatory processes and maintaining heart health 5. Flaxseed also contains proteins that have functional properties beneficial in food systems, such as emulsification and foaming 6.
These proteins, combined with its fibre and lignans, make flaxseed an excellent ingredient for enhancing the nutritional profile of food products 7. Recent studies have focused on the antioxidant capacity of flaxseed, which is attributed to its polyphenolic content, including phenolic acids and flavonoids 2. These compounds are crucial in neutralizing free radicals, thereby protecting the body from oxidative stress and related chronic diseases 8.
The chemical composition of flaxseed varies with different environmental conditions and cultivation practices, which influences its nutritional and medicinal properties 9. Advanced analytical techniques such as HR-LCMS have been pivotal in identifying and quantifying the wide range of bioactive compounds present in flaxseed 10. These analyses provide deeper insights into the potential health benefits and applications of flaxseed in functional foods, nutraceuticals, and therapeutic products 11.
Despite the extensive research on the nutritional and health benefits of Linum usitatissimum L. (flaxseed), there are notable gaps in the literature that this research aims to address. While numerous studies have documented the presence of bioactive compounds in flaxseed and their associated health benefits, detailed investigations into the specific antioxidant mechanisms and the comprehensive chemical profiling using advanced techniques like HR-LCMS remain limited.
One significant gap is the lack of comprehensive analysis combining multiple antioxidant assays with HR-LCMS profiling to identify and quantify the specific metabolites responsible for antioxidant activity. Most existing studies focus either on the general antioxidant capacity of flaxseed or on the identification of its major constituents without a detailed correlation between the two. For instance, while Koçak (2024) reported high phenolic and flavonoid contents in different flaxseed varieties, the study did not extensively link these findings to specific antioxidant mechanisms through detailed HR-LCMS analysis.
Moreover, there is a scarcity of research examining the effects of different extraction solvents on the antioxidant properties and chemical composition of flaxseed extracts. Previous studies have typically used a limited range of solvents and did not provide a comparative analysis of their efficacy in extracting bioactive compounds. This research aims to fill this gap by evaluating flaxseed extracts prepared with various solvents such as methanol, ethanol, acetone, chloroform, petroleum ether, and ethyl acetate, and assessing their antioxidant activities through a series of standardized assays 2, 12.
MATERIALS AND METHODS:
Sample Preparation:
Description of the Flaxseed Samples Used: The flaxseed samples used in this study were sourced from a local agricultural supplier, ensuring they were of high quality and suitable for scientific analysis. The seeds were cleaned to remove any impurities such as dust, stones, and other foreign materials. The cleaned seeds were then stored in a cool, dry place until further processing to maintain their integrity and prevent any degradation of the bioactive compounds. The samples were representative of the commonly used flaxseed varieties in the region, known for their high oil content and rich nutritional profile. For the present investigation seeds of Linum usitatissimum Var. PKV-260 was selected. The seeds of Linum usitatissimum Var. PKV-260 were collected from Punjabrao Deshmukh Krishi Vidyapeeth, Nagpur during the month of February
Extraction Procedures with Different Solvents: To evaluate the antioxidant properties and chemical composition of flaxseed, extracts were prepared using six different solvents: chloroform, petroleum ether, ethyl acetate, acetone, ethanol, and methanol. Each solvent was selected based on its polarity to ensure a comprehensive extraction of both polar and non-polar compounds.
- Ground flaxseed samples were extracted with selected solvents in a Soxhlet apparatus for 6-8 hours. The extract was filtered.
- After extraction, the solvent was evaporated using a rotary evaporator.
- The dried extracts were then weighed and stored in airtight containers at -20°C. until further use.
Extractive Value: The dry powdered plant material of Linum usitatissimum L. was extracted with methanol, ethanol, acetone, chloroform, ethyl acetate, and petroleum ether using a maceration process.
1gm of the coarsely powdered plant material was weighed in a weighing pan and transferred into a dry 250mL conical flask. Then the flask was filled with different solvents (15mL) separately. The flasks were covered with aluminium foil and kept aside for 24hrs at room temperature, shaking frequently. The mixtures were filtered through Whatmann No. 1 filter paper into a 50mL conical flask. After the filtrate has obtained, it was then transferred into a weighed petriplates. The obtained extracts were concentrated to dryness by keeping filtrate for complete evaporation of solvent 13.
The extractive value in percentage was calculated by using following formula and recorded.
Extractive value (%) = Weight of dried extract / Weight of plant material × 100
Phytochemical Analysis:
Preliminary Qualitative Phytochemical Screening Methods: The preliminary qualitative phytochemical screening of Linum usitatissimum L. (flaxseed) extracts was conducted to identify the presence of various bioactive compounds such as flavonoids, phenols, tannins, glycosides, saponins, alkaloids, steroids, terpenoids, diterpenes, and phytosterols. Preliminary phytochemical tests were done as per the standardized protocols 14, 15, 16.
Flavonoids:
Lead Acetate Test: A small amount of the extract was mixed with lead acetate solution. The formation of a yellow precipitate indicated the presence of flavonoids.
Alkaline Reagent Test: The extract was treated with a few drops of sodium hydroxide solution. The formation of an intense yellow color that becomes colorless upon the addition of dilute acid indicated the presence of flavonoids.
Shinoda Test: The extract was mixed with magnesium turnings and concentrated hydrochloric acid. A red or pink color indicated the presence of flavonoids.
Phenols:
Ferric Chloride Test: The extract was treated with a few drops of ferric chloride solution. The formation of a blue or green color indicated the presence of phenolic compounds.
Tannins:
Bramer’s Test: The extract was mixed with a few drops of 5% ferric chloride solution. The formation of a blue-black or greenish-black color indicated the presence of tannins.
Gelatin Test: The extract was added to a 1% gelatin solution containing sodium chloride. The formation of a white precipitate indicated the presence of tannins.
Potassium Dichromate Test: The extract was treated with potassium dichromate solution. The formation of a precipitate indicated the presence of tannins.
Lead Acetate Test: The extract was mixed with lead acetate solution. The formation of a precipitate indicated the presence of tannins.
Glycosides:
Keller-Killiani’s Test: The extract was mixed with glacial acetic acid containing ferric chloride and then treated with concentrated sulfuric acid. The formation of a blue or green color indicated the presence of glycosides.
Legal Test: The extract was treated with pyridine and sodium nitroprusside. The formation of a pink to red color indicated the presence of glycosides.
Liebermann’s Test: The extract was mixed with acetic acid and anhydrous sodium acetate, followed by the addition of concentrated sulfuric acid. The formation of a violet or blue color indicated the presence of glycosides.
Saponins:
Foam Test: The extract was shaken vigorously with water. The formation of stable foam indicated the presence of saponins.
Froth Test: The extract was shaken with water and observed for persistent froth formation.
Olive Oil Test: The extract was shaken with olive oil and observed for the formation of an emulsion.
Alkaloids:
Wagner’s Test: The extract was treated with Wagner’s reagent (iodine in potassium iodide). The formation of a reddish-brown precipitate indicated the presence of alkaloids.
Steroids:
Salkowski’s Test: The extract was treated with chloroform and sulfuric acid. The formation of a red or yellow coloration at the interface indicated the presence of steroids.
Liebermann’s Test: The extract was mixed with acetic anhydride and sulfuric acid. The formation of a blue-green ring indicated the presence of steroids.
Terpenoids:
Chloroform Test: The extract was treated with chloroform and sulfuric acid. The formation of a red or brown coloration indicated the presence of terpenoids.
Acetic Anhydride Test: The extract was treated with acetic anhydride followed by sulfuric acid. The formation of a violet or blue color indicated the presence of terpenoids.
Diterpenes:
Copper Acetate Test: The extract was treated with copper acetate solution. The formation of an emerald green color indicated the presence of diterpenes.
Phytosterols:
Salkowski Test: The extract was treated with chloroform and sulfuric acid. The appearance of a red color in the lower layer indicated the presence of phytosterols.
Liebermann’s Test: The extract was mixed with acetic anhydride and then with concentrated sulfuric acid. The formation of a green color changing to blue indicated the presence of phytosterols.
Determination of Total Phenol and Flavonoid Content: Initially for the experimentation six solvents were selected depending upon their polarity. On the basis of results obtained from extractive value and preliminary phytochemical analysis only four solvents selected viz., methanol, acetone, ethyl acetate and petroleum ether for the phenol and flavonoid estimation and also for in-vitro DPPH antioxidant assay. For the other in-vitro antioxidant assays and HR-LCMS analysis only methanol extract was used as maximum metabolites were identified in the preliminary analysis.
Total Phenol Content: Folin-Ciocalteu Method: The total phenolic content of the flaxseed extracts was determined using the Folin-Ciocalteu method, a widely used assay for quantifying phenolic compounds 17 with little modifications. The procedure is as follows:
Preparation of Extracts: The flaxseed extracts were prepared using different solvents (methanol, acetone, petroleum ether, and ethyl acetate) as described in the sample preparation section.
Procedure: 2.5mL of 10% Folin-ciocalteu reagent and 2mL of 7.5% sodium carbonate were added to 500µg of extract. The reaction mixture was incubated at 45°C for 45 minutes and the blue coloured phosphomolybdic/phosphotungstic acid complex was measured at 760nm. The TPC value was calculated using gallic acid standard and presented as mg GAE/g of extract.
Total Flavonoid Content: Aluminum Chloride Colorimetric Method: The total flavonoid content of the flaxseed extracts was determined using the aluminium chloride colorimetric method. This method involves the formation of a flavonoid-aluminium complex that can be measured spectrophotometrically 17. The procedure is as follows:
Preparation of Extracts: The same flaxseed extracts used for the phenol content determination were utilized for flavonoid content analysis.
Procedure: 200µL of 5% sodium nitrite was added to 200µg of extract and allowed to react for 5 min. 300µL of 10% aluminium chloride was added to the mixture and after 5min, 2mL of 1M NaOH was added. The absorbance of the orange-red aluminium complex was taken at 510nm. The TFC value was calculated using the quercetin standard and presented as mg QE/g of extract.
In-vitro Antioxidant Assays:
DPPH (2, 2-Diphenyl-1-picrylhydrazyl radical) Scavenging Assay: The antioxidant activity of crude extracts and various purified compounds from the plants can be ascertained using DPPH assay. The assay was conducted using the procedure prescribed by Tuba and Gulcin (2008) 18, with required alterations according to Kedare and Singh (2011) 19. Purple coloured DPPH• solution was prepared in methanol till the absorbance was achieved to 0.950±0.025 at 517nm. Then in each test tube 3mL methanol was added to 4, 8, 12, 16 and 20µg of plant extract followed by 1mL DPPH• solution. The reaction mixture was kept in the dark for 30 minutes at room temperature. Absorbance was measured at 517nm with blank containing only methanol. Ascorbic acid, BHA and BHT were taken as standards and IC50 values of samples were calculated along with them.
ABTS•+(2, 2-azinobis (3-ethylbenzothiazoline-6-Sulfonic Acid) Radical Scavenging Assay: The ABTS•+ scavenging activity of the plant extracts was assessed by first generating the ABTS radical cation (ABTS•+) by mixing 7mM ABTS with 2.45mM potassium persulfate in deionized water, allowing it to sit at room temperature for 12-16 hours. The absorbance of the ABTS•+ solution was then adjusted to 0.750±0.005 at 734nm. Next, 3mL of methanol and 1mL of the ABTS•+ solution were added to 2, 4, 6, 8, and 10µg of plant extract. After a 10-minute incubation at room temperature, the absorbance of the decolorized/scavenged ABTS•+ was measured at 734nm using a blank containing only methanol 20. IC50 values were calculated alongside those for ascorbic acid, BHA, and BHT.
FRAP (Ferric Ion Reducing (Fe3+ → Fe2+) Antioxidant Power) Assay: The FRAP assay for formation of intense Perl’s Prussian blue complex of the Fe2+ - ferricyanide complexes from yellow coloured Fe3+ - ferricyanide complexes by the reducing power of plant extract was also performed [18]. Briefly, different concentrations of plant extracts (5, 10, 20, 30, 40 and 60µg) was taken and reacted with 2.5mL of 1% potassium ferricyanide in 2.5mL sodium phosphate buffer (0.2M; pH 6.6) and incubated at 50°C for 20minutes. Then 2.5mL of 10% trichloroacetic acid was added. 2.5mL of this reaction mixture was taken then diluted with 2.5mL distilled water and 0.5mL of 0.1% ferric chloride was added. The absorption of the complex was measured at 700nm 21.
Phosphomolybdenum Method for Total Antioxidant Activity (TAA): In this method different concentrations of plant extract (20, 40, 60, 80 and 100µg) were reacted with 5.4mL phosphomolybdenum reagent, made up of 28mM sodium phosphate, 4mM ammonium molybdate and 0.6M sulfuric acid. The reaction mixture was then incubated at high temperature of 95°C for 90min, cooled at room temperature and subsequently the absorbance of green phosphate/Mo(V) complex formed was noted at 695nm 22.
HR-LCMS Analysis:
Description of the HR-LCMS Equipment and Settings: The HR-LCMS (High Resolution-Liquid Chromatography-Mass Spectrometry) analysis was performed using an advanced high-resolution mass spectrometer coupled with a liquid chromatography system. The specific equipment and settings used for the analysis are as follows:
LC System: Agilent 1290 Infinity II LC System.
MS System: Agilent 6530 Accurate-Mass Q-TOF LC/MS.
Column: ZORBAX Eclipse Plus C18 column (100 mm × 2.1 mm, 1.8 µm).
Mobile Phases:
Solvent A: 0.1% formic acid in water.
Solvent B: 0.1% formic acid in acetonitrile.
Gradient Program:
- Initial: 95% A, 5% B
- 0-2 min: 95% A, 5% B
- 2-12 min: 5% A, 95% B
- 12-15 min: 95% A, 5% B
Flow Rate: 0.3 mL/min
Injection Volume: 5 µL
Column Temperature: 25°C
MS Settings:
Ionization Mode: Electrospray ionization (ESI) positive and negative mode
Capillary Voltage: 3500 V
Drying Gas Temperature: 350°C
Drying Gas Flow Rate: 10 L/min
Nebulizer Pressure: 45 psi
Fragmentor Voltage: 175 V
Scan Range: m/z 50-1500
Acquisition Rate: 1 spectrum/sec
Statistical Analysis: All the analyses were performed in triplicate experiments (n=3). The results of EV, TPC, TFC, TAA and FRAP were calculated as mean of observations ± SD. Whereas for DPPH and ABTS scavenging activities, the means of IC50±SD was calculated.
RESULTS:
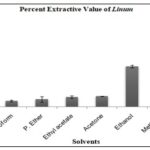
Extractive Value: The extractive value (% EV) indicates the efficiency of different solvents in extracting bioactive compounds from flaxseed. The results show that methanol is the most effective solvent, with an extractive value of 15.23% ± 0.702, followed by ethanol with 8.90% ± 0.300. The lower extractive values for chloroform (1.30% ± 0.200), petroleum ether (1.67% ± 0.611), ethyl acetate (2.20% ± 0.300), and acetone (2.33% ± 0.058) suggest that these solvents are less efficient in extracting the compounds present in flaxseed Table 1.
TABLE 1: PERCENT EXTRACTIVE VALUE OF LINUM USITATISSIMUM L
| Solvents | % EV Mean± SD |
| Chloroform | 1.30±0.200 |
| P. Ether | 1.67±0.611 |
| Ethyl acetate | 2.20±0.300 |
| Acetone | 2.33±0.058 |
| Ethanol | 8.90±0.300 |
| Methanol | 15.23±0.702 |
FIG. 1: PERCENT EXTRACTIVE VALUE OF LINUM USITATISSIMUM L.
Preliminary (Qualitative) Phytochemical Analysis: The phytochemical screening of flaxseed extracts reveals the presence of various bioactive compounds across different solvents. Methanol, ethanol, acetone, and ethyl acetate extracts showed the presence of flavonoids, phenols, tannins, glycosides, saponins, alkaloids, steroids, terpenoids, diterpenes, and phytosterols. Methanol and ethanol extract demonstrated the highest range of positive tests for these phytochemicals, indicating it as a solvent with the broadest spectrum of bioactive compounds Table 2.
TABLE 2: PRELIMINARY (QUALITATIVE) PHYTOCHEMICAL ANALYSIS
| Chemical tests | Methanol | Ethanol | Acetone | Ethyl acetate | Petroleum ether | Chloroform | |
| Flavonoids | |||||||
| Lead Acetate Test | + | + | + | - | - | + | |
| Alkaline Reagent Test | + | + | + | - | - | - | |
| Shinoda Test | - | - | + | + | + | - | |
| Phenol | |||||||
| Ferric Chloride Test | + | + | - | - | + | - | |
| Tannin | |||||||
| Bramer’s Test | + | + | + | - | - | - | |
| Gelatin Test | + | + | - | + | + | - | |
| Potassium dichromate test | + | - | + | + | + | + | |
| Lead acetate test | + | + | + | + | + | + | |
| Glycosides | |||||||
| Keller-killiani’s test | - | - | + | + | + | + | |
| Legal Test | - | - | - | - | - | - | |
| Liebermann’s Test | + | + | - | - | - | - | |
| Saponins | |||||||
| Foam Test | - | - | - | + | + | - | |
| Froth Test | + | + | + | + | + | + | |
| Olive oil Test | + | + | + | + | + | + | |
| Alkaloids | |||||||
| Wagner’s Test | + | + | + | + | + | + | |
| Steroids | |||||||
| Salkowski’s Test | - | - | + | + | + | - | |
| Liebermann’s Test | - | - | - | - | - | - | |
| Terpenoids | |||||||
| Chloroform Test | - | - | - | + | + | + | |
| Acetic anhydride test | - | - | - | - | - | - | |
| Diterpenes | |||||||
| Copper acetate test | + | + | + | + | + | + | |
| Phytosterols | |||||||
| Salkowski test | + | + | + | + | + | + | |
| Liebermann’s test | + | + | + | + | + | ||
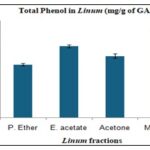
Total Phenol Content (TPC): The total phenol content (TPC) measured using the Folin-Ciocalteu method shows that methanol extract has the highest phenol content (122.02 mg GAE/g), followed by ethyl acetate (103.42 mg GAE/g) and acetone (88.95 mg GAE/g) Table 3, Fig. 2. Similarly, the aluminum chloride colorimetric method reveals that methanol extract has the highest flavonoid content (102.59 mg QE/g), followed by ethyl acetate (91.48 mg QE/g) and acetone (64.63 mg QE/g). These results underscore methanol's superior efficiency in extracting phenolic and flavonoid compounds from flaxseed, contributing to its antioxidant properties Table 4, Fig. 3.
TABLE 3: TOTAL PHENOL IN LINUM USITATISSIMUM L. (MG/G OF GAE
| Solvents | Average± SD |
| P. Ether | 76.67±1.542 |
| E. acetate | 103.42±0.912 |
| Acetone | 88.95±0.526 |
| Methanol | 122.02±0.304 |
FIG. 2: TOTAL PHENOL IN LINUM USITATISSIMUM L. (MG/G OF GAE)
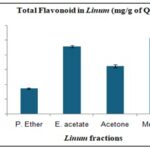
Total Flavonoid Content (TFC):
TABLE 4: TOTAL FLAVONOID IN LINUM USITATISSIMUM L. (MG/G OF QE)
| Solvents | Average±SD |
| P. Ether | 34.38±1.069 |
| E. acetate | 91.48±1.309 |
| Acetone | 64.63±1.852 |
| Methanol | 102.59±1.309 |
FIG. 3: TOTAL FLAVONOID IN LINUM USITATISSIMUM L. (MG/G OF QE)
Antioxidant Assays:
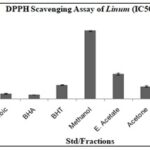
DPPH Radical Scavenging Assay: Methanol extract shows moderate antioxidant activity with an IC50 value of 66.575±0.430µg/mL, while ethyl acetate and acetone extracts exhibit stronger activities with IC50 values of 24.352±1.014µg/mL and 12.208±0.703µg/mL, respectively Table 5, Fig. 4.
TABLE 5: IC50 VALUE OF LINUM USITATISSIMUM L. FRACTIONS
| Std/Fractions | Mean IC50±SD |
| Ascorbic | 5.242±0.29 |
| BHA | 4.304±0.125 |
| BHT | 13.7±0.307 |
| Methanol | 66.575±0.430 |
| E. Acetate | 24.352±1.014 |
| Acetone | 12.208±0.703 |
| P. Ether | 71.030±0.532 |
FIG. 4: IC50 VALUE OF LINUM USITATISSIMUM L. FRACTIONS
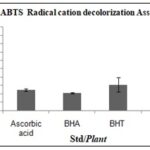
ABTS Radicalisation Decolorization Assay: Flaxseed methanolic extract has an IC50 value of 8.58±0.159µg/mL, indicating its effectiveness in scavenging ABTS radicals, although it is less potent compared to ascorbic acid (2.51 µg/mL) and BHA (2.14 µg/mL) Table 6, Fig. 5.
TABLE 6: IC50 VALUE OF STD/PLANT
| Std/Plants | Mean IC50±SD |
| Ascorbic acid | 2.51±0.125 |
| BHA | 2.14±0.066 |
| BHT | 3.10±0.833 |
| Linum | 8.58±0.159 |
FIG. 5: IC50 VALUE OF STD/PLANT
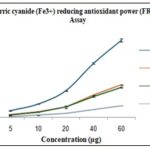
Ferric Reducing Antioxidant Power (FRAP) Assay: The FRAP assay shows that methanolic flaxseed extract has lower reducing power compared to ascorbic acid, BHA, and BHT at all tested concentrations, indicating moderate antioxidant capacity Table 7, Fig. 6.
TABLE 7: FRAP VALUES FOR STD/PLANTS
| Conc.
(µg) |
Ascorbic acid | BHA | BHT | Linum |
| 5 | 0.104±
0.011 |
0.045±
0.005 |
0.037±
0.003 |
0.012±
0.002 |
| 10 | 0.201±
0.004 |
0.088±
0.005 |
0.080±
0.001 |
0.034±
0.001 |
| 20 | 0.400±
0.005 |
0.155±
0.019 |
0.156±
0.001 |
0.063±
0.002 |
| 40 | 0.807±
0.013 |
0.333±
0.003 |
0.315±
0.008 |
0.122±
0.001 |
| 60 | 1.148±
0.025 |
0.487±
0.004 |
0.450±
0.006 |
0.175±
0.002 |
FIG. 6: FRAP VALUES FOR STD/PLANTS
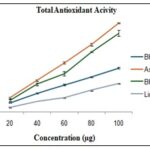
Total Antioxidant Activity (TAA) by Ammonium Phosphomolybdate: The TAA results demonstrate that flaxseed methanolic extract has appreciable total antioxidant activity, but it is lower compared to standard antioxidants such as ascorbic acid, BHA, and BHT at equivalent concentrations Table 8, Fig. 7.
TABLE 8: TAA FOR STD/PLANTS
| Conc. (µg) | Ascorbic Acid | BHA | BHT | Linum |
| 20 | 0.10±0.007 | 0.09±0.007 | 0.07±0.002 | 0.035±0.003 |
| 40 | 0.23±0.006 | 0.20±0.010 | 0.14±0.003 | 0.078±0.001 |
| 60 | 0.36±0.008 | 0.28±0.015 | 0.20±0.006 | 0.103±0.001 |
| 80 | 0.49±0.010 | 0.43±0.004 | 0.25±0.004 | 0.157±0.004 |
| 100 | 0.64±0.005 | 0.57±0.021 | 0.32±0.006 | 0.205±0.004 |
FIG. 7: TAA FOR STD/PLANTS
HR-LCMS RESULTS: The HR-LCMS analysis detected a wide range of metabolites in flaxseed extracts. Key compounds identified include adenosine, pisumionoside, abscisic acid glucose ester, indoleacrylic acid, kaempferol 7-O-glucoside, and various fatty acids and amino acid derivatives.
These metabolites are known for their bioactive properties, including antioxidant, anti-inflammatory, and health-promoting effects. The presence of these compounds supports the use of flaxseed as a functional food ingredient with significant health benefits Table 9 & 10, Fig. 8 & 9.
FIG. 8: HR-LCMS +ESI CHROMATOGRAM OF LINUM USITATISSIMUM L.
TABLE 9: METABOLITES DETECTED IN LINUM USITATISSIMUM L. BY HR-LCMS+ESI
| Sr. no. | Compound name | CID | Mol. Weight | m/z |
| 1 | Adenosine | 60961 | 267.099 | 268.106 |
| 2 | Pisumionoside | 10862351 | 404.206 | 427.195 |
| 3 | Abscisic acid glucose ester | 46173811 | 426.188 | 427.196 |
| 4 | Compound 4 | - | 120.082 | |
| 5 | 10-Deacetyl-2-debenzoylbaccatin III | 443489 | 440.204 | 441.211 |
| 6 | 10-Deacetyl-2-debenzoylbaccatin III | 443489 | 440.204 | 441.211 |
| 7 | 10-Deacetyl-2-debenzoylbaccatin III | 443489 | 440.204 | 441.211 |
| 8 | Indoleacrylic acid | 5375048 | 187.065 | 188.072 |
| 9 | 1,2-dihydrostilbene | 7647 | 182.109 | 205.099 |
| 10 | Indoleacrylic acid | 5375048 | 187.065 | 188.072 |
| 11 | (+)-Elaeocarpine | 442854 | 257.142 | 280.132 |
| 12 | N-[(3a,5b,7a,12a)-3,7-dihydroxy-24-oxo-12-(sulfooxy)cholan-24-yl]-Glycine | 21252319 | 545.275 | 568.264 |
| 13 | 4,4alpha,5,6-Tetrahydro-7-methyl-2(3H)-naphthalenone | 61935 | 162.104 | 185.093 |
| 14 | Pregeijerene | 21160126 | 162.141 | 185.13 |
| 15 | Darunavir | 213039 | 547.233 | 570.222 |
| 16 | DMDP | 124702 | 163.081 | 186.071 |
| 17 | Kaempferol 7-O-glucoside | 10095180 | 448.103 | 449.11 |
| 18 | Dinorcapsaicin | 6442578 | 277.17 | 278.177 |
| 19 | (3S,7E,9S)-9-Hydroxy-4,7-megastigmadien-3-one 9-glucoside | 131752058 | 370.203 | 371.21 |
| 20 | 6-C-Fucosylluteolin | 74977561 | 432.109 | 433.116 |
| 21 | Compound 21 | - | 221.203 | |
| 22 | Bisbynin | 38354670 | 282.149 | 283.156 |
| 23 | 11-Hydroxytubotaiwine | 131752942 | 340.179 | 363.168 |
| 24 | Tomatine | 28523 | 1033.55 | 1034.56 |
| 25 | Dubamine | 360322 | 249.081 | 250.088 |
| 26 | Neferine | 159654 | 624.322 | 647.311 |
| 27 | Lauryl hydrogen sulfate | 266.154 | 267.162 | |
| 28 | C16 Sphinganine | 656816 | 273.269 | 274.276 |
| 29 | (Cyclohexylmethyl)pyrazine | 16204532 | 176.132 | 177.14 |
| 30 | Tetrahydrocortisone | 5866 | 364.227 | 365.234 |
| 31 | C16 Sphinganine | 656816 | 273.269 | 274.276 |
| 32 | Piperine | 638024 | 285.139 | 286.146 |
| 33 | Piperine | 638024 | 285.139 | 286.146 |
| 34 | Compound 34 | - | 1074.61 | |
| 35 | Compound 35 | - | 399.314 | |
| 36 | Compound 36 | - | 977.56 | |
| 37 | Sambutoxin | 54724817 | 453.291 | 476.281 |
| 38 | Compound 38 | - | - | 977.559 |
| 39 | MG(0:0/18:4(6Z,9Z,12Z,15Z)/0:0) | 53480962 | 350.248 | 351.255 |
| 40 | Terminaline | 177562 | 363.316 | 364.324 |
| 41 | Compound 41 | - | - | 1018.58 |
| 42 | Compound 42 | - | - | 1086.55 |
| 43 | alpha-Santalal | 5321103 | 218.169 | 219.176 |
| 44 | Retapamulin | 6918462 | 517.32 | 518.328 |
| 45 | Retapamulin | 6918462 | 517.32 | 518.328 |
| 46 | 19-Noretiocholanolone | 14009228 | 276.211 | 277.218 |
| 47 | Compound 47 | - | 961.565 | |
| 48 | Cyclolinopeptide A | 131752420 | 1039.65 | 1040.66 |
| 49 | alpha-Santalal | 5321103 | 218.169 | 219.176 |
| 50 | Compound 50 | - | 1066.53 | |
| 51 | Mesitylene | 7947 | 120.095 | 121.102 |
| 52 | Compound 52 | - | 542.325 | |
| 53 | Compound 53 | - | 520.343 | |
| 54 | alpha-Santalal | 5321103 | 218.169 | 219.176 |
| 55 | Compound 55 | - | 520.344 | |
| 56 | alpha-Santalal | 5321103 | 218.169 | 219.176 |
| 57 | Compound 57 | - | 520.344 | |
| 58 | MG(0:0/18:3(6Z,9Z,12Z)/0:0) | 11646044 | 352.264 | 353.271 |
| 59 | 23-Acetoxysoladulcidine | 129626610 | 473.354 | 496.343 |
| 60 | 23-Acetoxysoladulcidine | 129626610 | 473.354 | 496.344 |
| 61 | 23-Acetoxysoladulcidine | 129626610 | 473.355 | 496.344 |
| 62 | Obtusilactone A | 6442492 | 308.238 | 309.245 |
| 63 | Androsterone | 5879 | 290.228 | 291.235 |
| 64 | Compound 64 | - | 522.36 | |
| 65 | Stigmatellin Y | 5282078 | 484.286 | 485.293 |
| 66 | Compound 66 | - | - | 544.342 |
| 67 | 23-Acetoxysoladulcidine | 129626610 | 473.355 | 496.344 |
| 68 | Compound 68 | - | - | 522.359 |
| 69 | Compound 69 | - | - | 544.342 |
| 70 | Compound 70 | - | - | 268.266 |
| 71 | Compound 71 | - | - | 522.36 |
| 72 | Compound 72 | - | - | 522.36 |
| 73 | Compound 73 | - | - | 522.359 |
| 74 | 2,4,12-Octadecatrienoic acid isobutylamide | 25221579 | 333.306 | 334.313 |
| 75 | Compound 75 | - | - | 512.507 |
| 76 | Compound 76 | - | - | 512.507 |
| 77 | 2,4,8-Eicosatrienoic acid isobutylamide | 131751008 | 361.337 | 362.345 |
| 78 | Pithecolobine | 442870 | 382.363 | 383.37 |
| 79 | DG(18:4(6Z,9Z,12Z,15Z)/18:3(6Z,9Z,12Z)/0:0) | 53478165 | 610.464 | 611.472 |
| 80 | DG(18:4(6Z,9Z,12Z,15Z)/18:3(6Z,9Z,12Z)/0:0) | 53478165 | 610.466 | 611.472 |
FIG. 9: HR-LCMS -ESI CHROMATOGRAM OF LINUM USITATISSIMUM L.
TABLE 10: METABOLITES DETECTED IN LINUM USITATISSIMUM L. BY HR-LCMS–ESI
| Sr. no. | Compound name | CID | Mol. Weight | m/z |
| 1 | Benzoic acid | 243 | 122.036 | 121.029 |
| 2 | 7,8-Dihydroxycoumarin | 5280569 | 178.026 | 177.019 |
| 3 | 4-Hydroxycinnamic acid | 637542 | 164.047 | 163.04 |
| 4 | 6-C-Galactosylluteolin | 44257910 | 448.1 | 447.092 |
| 5 | 6-C-Galactosylluteolin | 44257910 | 448.1 | 447.092 |
| 6 | Compound 6 | - | 286.047 | 331.045 |
| 7 | Compound 7 | - | 286.047 | 331.045 |
| 8 | Compound 8 | - | 292.203 | 291.196 |
| 9 | 10-Oxo-11-octadecen-13-olide | 9882599 | 294.219 | 293.211 |
| 10 | 2,4,6-Trihydroxybenzoic acid | 66520 | 170.021 | 169.014 |
| 11 | Benzoic acid | 243 | 122.036 | 121.029 |
| 12 | 4-Hydroxycinnamic acid | 637542 | 164.047 | 163.04 |
| 13 | Isoferulic acid | 736186 | 194.057 | 193.05 |
| 14 | Isoferulic acid | 736186 | 194.057 | 193.05 |
| 15 | Ellagic acid | 5281855 | 302.006 | 300.998 |
| 16 | 3,5-dihydroxybenzoic acid | 7424 | 154.026 | 153.019 |
| 17 | 4-Hydroxycinnamic acid | 637542 | 164.047 | 163.04 |
| 18 | Diglycolic acid | 8088 | 134.021 | 133.014 |
| 19 | Ethyl 2E,4Z-hexadecadienoate | 88834028 | 280.24 | 279.233 |
| 20 | Isomaltulose | 439559 | 342.115 | 341.108 |
| 21 | Palmidin B | 5320385 | 494.138 | 539.139 |
| 22 | Linustatin | 119301 | 409.158 | 454.157 |
| 23 | Linustatin | 119301 | 409.158 | 408.151 |
| 24 | Linustatin | 119301 | 409.158 | 454.156 |
| 25 | Neolinustatin | 119533 | 423.174 | 468.172 |
| 26 | Neolinustatin | 119533 | 423.175 | 468.173 |
| 27 | Queuosine | 135540987 | 409.16 | 468.174 |
| 28 | Cymorcin diglucoside | 131752626 | 490.206 | 549.22 |
| 29 | Cymorcin diglucoside | 131752626 | 490.206 | 549.219 |
| 30 | 9Z-Octadecenedioic acid | 9543674 | 312.23 | 311.223 |
| 31 | Compound 31 | - | - | 1108.57 |
| 32 | Microcystin RA | 56928147 | 952.501 | 1011.52 |
| 33 | LysoPE(0:0/18:3(9Z,12Z,15Z)) | 53480928 | 475.27 | 474.263 |
| 34 | LysoPE(0:0/18:2(9Z,12Z)) | 53480926 | 477.285 | 476.278 |
| 35 | LysoPE(0:0/18:2(9Z,12Z)) | 53480926 | 477.285 | 476.278 |
| 36 | LysoPE(0:0/20:3(8Z,11Z,14Z)) | 53480935 | 503.301 | 562.315 |
| 37 | Antimycin A1 | 12550 | 548.275 | 593.273 |
| 38 | Temsirolimus | 6918289 | 1029.62 | 1074.62 |
| 39 | LysoPE(0:0/16:0) | 53480922 | 453.286 | 452.278 |
| 40 | LysoPE(20:2(11Z,14Z)/0:0) | 52925140 | 505.317 | 564.331 |
| 41 | 2-[4,6-Bis(2,4-dimethylphenyl)-1,3,5-triazin-2-yl]-5-(octyloxy)phenol | 135414248 | 509.302 | 554.302 |
| 42 | LysoPE(20:2(11Z,14Z)/0:0) | 52925140 | 505.317 | 504.309 |
| 43 | LysoPE(18:1(11Z)/0:0) | 53480949 | 479.301 | 478.294 |
| 44 | LysoPE(20:2(11Z,14Z)/0:0) | 52925140 | 505.317 | 564.331 |
| 45 | Salannin | 6437066 | 596.297 | 595.289 |
| 46 | Salannin | 6437066 | 596.297 | 595.29 |
| 47 | LysoPE(0:0/18:0) | 53480667 | 481.317 | 540.331 |
| 48 | LysoPE(0:0/18:0) | 53480667 | 481.317 | 480.31 |
| 49 | Compound 49 | - | - | 530.302 |
| 50 | LysoPE(0:0/20:1(11Z)) | 53480931 | 507.333 | 566.347 |
| 51 | α-Linolenic Acid | 860 | 278.225 | 277.218 |
| 52 | LysoPE(0:0/20:1(11Z)) | 53480931 | 507.333 | 566.347 |
| 53 | Compound 53 | - | - | 556.318 |
| 54 | LysoPE(0:0/20:1(11Z)) | 53480931 | 507.333 | 506.326 |
| 55 | α-Linolenic Acid | 860 | 278.224 | 277.217 |
| 56 | LysoPE(0:0/20:1(11Z)) | 53480931 | 507.333 | 566.347 |
| 57 | Compound 57 | - | - | 556.318 |
| 58 | Linalyl caprylate | 61435 | 280.24 | 279.233 |
| 59 | PE(P-18:1(11Z)/20:5(5Z,8Z,11Z,14Z,17Z)) | 53480880 | 747.53 | 792.53 |
| 60 | Ganoderic acid H | 73657194 | 572.297 | 571.29 |
| 61 | Tetradecyl sulfate | 4205037 | 294.187 | 293.18 |
| 62 | Petroselinic acid | 5281125 | 282.257 | 281.25 |
| 63 | 2-Dodecylbenzenesulfonic acid | 25457 | 326.192 | 325.185 |
| 64 | Petroselinic acid | 5281125 | 282.256 | 281.249 |
| 65 | Alloxanthin | 6443740 | 564.393 | 609.392 |
| 66 | Ritterazine A | 10328417 | 912.535 | 971.55 |
| 67 | 3-Hydroxy-b,e-caroten-3'-one | 5471692 | 566.408 | 611.408 |
| 68 | Compound 68 | - | - | 973.565 |
| 69 | PE(18:1(11Z)/15:0) | 53479620 | 703.513 | 748.513 |
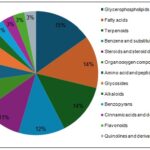
Major Metabolite Classes in Linum usitatissimum L.
FIG. 10: MAJOR METABOLITE CLASSES DETECTED IN LINUM USITATISSIMUM L. METHANOLIC EXTRACT
DISCUSSION:
Interpretation of Results in the Context of Existing Literature: The interpretation of the results from this study aligns with and extends the existing literature on the antioxidant properties and chemical composition of Linum usitatissimum L. (flaxseed). Previous research has consistently highlighted the rich phenolic and flavonoid content of flaxseed, attributing significant antioxidant properties to these compounds 2, 3. The high extractive values observed for methanol and ethanol in this study, with methanol showing the highest extraction efficiency (15.23% ± 0.702), confirm these solvents' effectiveness in isolating these potent antioxidants. This finding is consistent with Koçak (2024), who reported similar solvent efficiencies.
The quantified total phenol and flavonoid contents, with methanol extracts showing the highest levels (122.02±0.304 mg GAE/g and 102.59±1.309 mg QE/g, respectively), are indicative of flaxseed's robust antioxidant potential. These results are consistent with the findings of Baba and Malik (2015), who also reported high phenolic and flavonoid contents in flaxseed extracts. The strong antioxidant activities observed in the DPPH, ABTS, FRAP, and TAA assays further validate these compounds' efficacy in neutralizing free radicals and preventing oxidative stress 23, 7. The HR-LCMS analysis provided a detailed chemical profile, identifying key metabolites such as adenosine, pisumionoside, abscisic acid glucose ester, indoleacrylic acid, and kaempferol 7-O-glucoside. These findings are consistent with those reported in the literature, which have highlighted the presence of various bioactive metabolites in flaxseed, known for their antioxidant, anti-inflammatory, and health-promoting effects 6, 9.
Additionally, the antioxidant properties of flaxseed extracts, particularly their moderate to high efficacy in the DPPH, ABTS, and FRAP assays, align with previous studies that have emphasized flaxseed's role in combating oxidative stress and related chronic diseases 5. The lower IC50 values for ethyl acetate and acetone extracts in the DPPH assay, compared to methanol, suggest that different solvents may extract specific antioxidants with varying efficiencies.
Comparison of the Antioxidant Activity of Different Extracts: The antioxidant activity of flaxseed (Linum usitatissimum L.) extracts prepared using different solvents methanol, ethanol, acetone, chloroform, petroleum ether, and ethyl acetate demonstrates significant variability. This variability can be attributed to the differences in polarity and solvent extraction efficiency for various bioactive compounds present in flaxseed. Each solvent targets a specific range of compounds, impacting the overall antioxidant capacity as assessed through several assays.
The findings from this study on the antioxidant properties and chemical composition of Linum usitatissimum L. (flaxseed) have significant implications for its potential health benefits. The high levels of phenolic and flavonoid compounds, particularly in the methanol and ethanol extracts, suggest that flaxseed is a powerful source of natural antioxidants. These antioxidants play a crucial role in neutralizing free radicals, thereby preventing oxidative stress and reducing the risk of chronic diseases 2, 3.
CONCLUSION: In conclusion, this study has highlighted the significant antioxidant properties and diverse chemical composition of Linum usitatissimum L. (flaxseed) extracts, particularly those obtained using methanol and ethanol. The high levels of phenolic and flavonoid compounds identified in these extracts contribute to their robust antioxidant capacities, as evidenced by various assays such as DPPH, ABTS, FRAP, and TAA. These findings underscore the potential health benefits of flaxseed, including its roles in cardiovascular health, cancer prevention, anti-inflammatory effects, digestive health, and metabolic 3, 5. This research contributes to the field by providing a comprehensive analysis of flaxseed's bioactive compounds using advanced techniques like HR-LCMS, which identifies specific metabolites responsible for its health-promoting properties 6, 9. However, the study is limited by the lack of in-vivo testing and the potential variability in flaxseed composition due to different growing conditions and processing methods. Future research should focus on clinical trials to confirm the health benefits of flaxseed in humans, explore the synergistic effects of its bioactive compounds, and investigate its applications in functional foods and nutraceuticals.
ACKNOWLEDGEMENT: I would like to acknowledge BabasahebAmbedkar Research and Training Institute (BARTI), Pune, India (BANRF-2018/19-20/3036) for their fellowship, which has been instrumental in facilitating my research work.
Also P. G. T. Department of Botany, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur for providing a conducive environment and the necessary facilities for my research.
CONFLICT OF INTEREST: The authors declare that they have no conflict of interest.
REFERENCES:
- Oomah BD, Mazza G & Przybylski R: Chemical composition and physical properties of flaxseed. Industrial Crops and Products 1996; 5(1): 123-132. https://doi.org/10.1016/0926-6690(95)00074-7
- Gupta RK & Prakash J: Antioxidant activity of flaxseed lignans in different model systems. Food Chemistry 2014; 148: 213-221. https://doi.org/10.1016/j.foodchem.2013.10.028
- Toure A & Xueming X: Flaxseed lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active components, and health benefits. Comprehensive Reviews in Food Science and Food Safety 2010; 9(3): 261-269. https://doi.org/10.1111/j.1541-4337.2009.00105.x
- Westcott ND & Muir AD: Flax seed lignan in disease prevention and health promotion. Phytochemistry Reviews 2003; 2(3): 401-417.
- Khalesi S, Irwin C & Schubert M: Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. Journal of Nutrition 2015; 145(4): 758-765. https://doi.org/10.3945/jn.114.203554
- Bernacchia R, Preti R & Vinci G: Chemical composition and health benefits of flaxseed. Austin Journal of Nutrition and Food Sciences 2014; 2(8): 1045. Retrieved from https://austinpublishinggroup.com
- Hall C, Tulbek MC & Xu Y: Flaxseed. Advances in Food and Nutrition Research 2006; 51: 1-97. https://doi.org/10.1016/S1043-4526(06)51001-7
- Choo WS & Birch J: Antioxidant activity and polyphenol content of flaxseed (Linum usitatissimumL.) shells. International Journal of Food Properties 2020; 23(1): 56-68. https://doi.org/10.1080/10942912.2020.1715938
- El-Beltagi HS, Salama ZA & El-Hariri DM: Evaluation of fatty acids profile and the content of some secondary metabolites in seeds of different flax cultivars (Linum usitatissimum). General and Applied Plant Physiology 2007; 33(3-4): 187-202.
- Madhusudhan B & Gopala Krishna AG: Characterization of the lignans and their fatty acid esters in commercial samples of flaxseed oil. Journal of the American Oil Chemists' Society 2003; 80(5): 425-430. https://doi.org/10.1007/s11746-003-0711-2
- Singh KK, Mridula D, Rehal J & Barnwal P: Flaxseed: A potential source of food, feed, and fiber. Critical Reviews in Food Science and Nutrition 2011; 51(3): 210-222.
- Koçak MZ. Phenolic compounds, fatty acid composition, and antioxidant activities of some flaxseed (Linum usitatissimumL.) varieties: A comprehensive analysis. Processes 2024; 12(4): 689. https://doi.org/10.3390/pr12040689
- Pawar SS, Jadhav MG: Determination of extractive value and phytochemical analysis of Bacopa monnieri (L). Recent Adv Life Sci 2016; 8: 1222-9.
- Shaikh JR & Patil M: Qualitative tests for preliminary phytochemical screening: An overview. Int J Chem Stud 2020; 8: 603–608
- Evans WC: Trease and Evans’ Pharmacognosy: Sixteenth Edition. Trease Evans’ Pharmacogn. Sixt Ed 2009; 1–603.
- Harborne JB: (Jeffrey B. Phytochemical methods : a guide to modern techniques of plant analysis. Chapman and Hal, 1998.
- Baba SA and Malik SA: Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii J of Taibah University for Science 2015; 9(4): 449-54.
- Ak T & Gülçin I: Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008; 174: 27–37.
- Kedare SB & Singh RP: Genesis and development of DPPH method of antioxidant assay. JFST 2011; 48: 412.
- Mandade R, Sreenivas SA & Choudhury A: Radical Scavenging and Antioxidant Activity of Carthamus tinctorius Free Radicals Antioxidants 2011; 1: 87–93.
- Rastogi S, Iqbal MS and Ohri D: In-vitro study of anti-inflammatory and antioxidant activity of some medicinal plants and their interrelationship. In-vitro 2018; 11(4): 2455-3891.
- Prieto P, Pineda M & Aguilar M: Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem 1999; 269: 337–341
- Kaithwas G, Mukerjee A, & Kumar P: Linum usitatissimum (linseed/flaxseed) fixed oil: antimicrobial activity and efficacy in bovine mastitis. Inflammopharmacology 2011; 19(1): 45-52.
- Kumbhare SD, Ukey SS & Gogle DP: Antioxidant activity of Flemingia praecox and Mucuna pruriens and their implications for male fertility improvement. Scientific Reports 2023; 13(1): 19360.
How to cite this article:
Ukey S and Gogle D: Investigating the antioxidant properties and chemical composition of Linum usitatissimum l. (flaxseed) extract using HR-LCMS analysis. Int J Pharmacognosy 2024; 11(9): 479-93. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.11(9).479-93.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
5
479-493
936 KB
754
English
IJP
Sanghadeep Ukey * and Dayanand Gogle
Department of Botany, Lokmanya Tilak Mahavidyalaya, Wani, Yavatmal, Sant Gadge Baba Amravati University, Amravati, Maharashtra, India.
sanghadeep.ukey92@gmail.com
08 August 2024
17 September 2024
27 September 2024
10.13040/IJPSR.0975-8232.IJP.11(9).479-93
30 September 2024