INHIBITORY EFFECT OF SOLVENT EXTRACTS AND THEIR FRACTIONS OF MEMECYLON SPECIES ON RNASE AND PROTEASE ACTIVITY
HTML Full TextINHIBITORY EFFECT OF SOLVENT EXTRACTS AND THEIR FRACTIONS OF MEMECYLON SPECIES ON RNASE AND PROTEASE ACTIVITY
T. R. Bharathi * and H. S. Prakash
Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore - 570006, Karnataka, India.
ABSTRACT: Genus Memecylon belonging to the family Melastomataceae is a large shrub or a small tree native to Western Ghats of India. Memecylon species are reported to show various biological activities and are also used as medication in herpes virus infection. RNases and proteases are involved in the replication of herpes simplex virus (HSV). The present study was taken up to screen RNase and protease (trypsin and thrombin) inhibitors in different solvent extracts of Memecylon species viz., M. umbellatum, M. malabaricum, M. wightii, M. edule and M. talbotianium. The methanol extract of the leaves of Memecylon species was observed to strongly inhibit RNase, trypsin, and thrombin functions in a dose-dependent manner. The percent inhibition of RNase activity ranged from 53-84% in 50 ug/mL of plant extracts. The highest inhibition was observed in M. malabaricum and M. wightii for RNase. Similarly, for trypsin the percent inhibition ranged from 55-68% in 1 mg/mL of plant extracts. Highest inhibition was observed in M. malabaricum, M. wightii, and M. talbotianium. For thrombin the percent inhibition was between 44-52% in 1 mg/mL of plant extracts. The extracts from M. umbellatum, M. edule showed moderate inhibition. M. malbaricum extracts that had shown promising results were further fractionated using silica gel column chromatography, where 20:80 ethyl acetate: methanol fraction showed good protease as well as ribonuclease inhibitory properties. This study provides an important clue for scaling up further work on managing the herpes virus infection using Memecylon extracts, and this is the first report on RNase and protease inhibition in Memecylon species.
| Keywords: |
Memecylon species, RNase, Trypsin, Thrombin, Plant extracts
INTRODUCTION: Plant sources as targets to isolate antiviral compound was limited, mostly due to the highly infectious nature of viruses and difficulty in identifying plant metabolites. Antiviral potential of plant extracts against viral strains resistant to conventional antiviral agents have challenged the modern drug discovery practices and explored the natural antiviral components of medicinal plants 1, 2.
Several studies pertaining to traditional ayurvedic plants and other potential medicinal plants have confirmed the presence of diverse compounds with anti-viral/HSV inhibiting properties examples include curcumin, podophyllotoxin, and ursolic acid exhibits potent antiviral potential against HSV-1 and HSV-2 3. Two compounds called geraniin and 1,3,4,6-tetra-O-galloyl-betad-glucose (1346TOGBG), isolated from the acetone extract of another plant Phyllanthus urinaria, also suppressed HSV-2 and HSV-1 4. Anti protease activity (trypsin) are reported from several plant examples include Acer plantanoides, Rhus typhina, and Tamarix gallica 5.
In the field of pharmaceutical research, enzyme inhibition is an important area that has resulted in the discovery of a number of useful drugs. Specific inhibitors interact with enzymes and block their activity towards their corresponding physiological substrates. The importance of enzyme inhibitors as drugs is enormous, since these molecules have been used for treating a large number of physiological conditions. Plant-derived protease inhibitors serve in the defense mechanisms of plants against pests and plant pathogens. These inhibitors can be classified into a number of families on the basis of their specificities to inhibit the cleavage of specific peptide sequences within proteins 6.
Ribonucleases are a ubiquitous and functionally diverse group of enzymes that have a common ability to cleave RNA. Bovine pancreatic ribonuclease A (RNase A) provides an illuminating example of enzymatically mediated general acid-base catalysis. This digestive enzyme functions to hydrolyze RNA to its component nucleotides 7.
Memecylon is used in local medicine practices to treat mild bacterial infections to inflammations and skin ailments such as herpes and chickenpox 8. Various biochemical activities such as anti-oxidant, anti-inflammatory, anti-cancer, Hepato-protective, neuro-protective, nephroprotective, and cyto-protective properties of this plant have already been studied 9. It is evident from ethnic medicine practices that the plant shows the inhibitory effect on the herpes virus, which could be studied further, and also, these enzymes are involved in the replication of herpes simplex virus (HSV) 7. The present aim of the study is to explore viral replication inhibitory activities (anti-protease and anti-RNase) of plant extracts derived from five species of Memecylon using in-vitro biochemical assays.
MATERIALS AND METHODS:
Plant Sample Collection: Different Memecylon species viz., M. umbellatum, M. malabaricum, M. wightii and M. edule, were collected from Sringeri region in Western Ghats of Karnataka State, India. Leaves of the collected plant samples were authenticated by plant taxonomists. The leaves were separated, washed to remove adhering dust particles under running tap water, followed by a slow drying at a regulated temperature of 30 °C. The dried leaves were ground to a coarse powder using the mechanical grinder and stored at 4 °C.
Preparation of Extracts: Leaf powder (250 g) was extracted sequentially using 500 mL of non-polar, moderately polar, and polar solvents (E. Merck, Bangalore, India) in increasing polarity (hexane < ethyl acetate < methanol < water) using Soxhlet apparatus by continuous hot percolation (boiling point, 52 to 62 °C) until the solvent became colorless. The resultant solvent extracts were concentrated in a rotary evaporator (Speed Vac, Savant SPD 2010, Thermo Scientific, Germany) under controlled pressure. The required amount of extracts were weighed and solubilized in minute quantities of dimethyl sulphoxide (DMSO) and water.
RNase Enzyme Activity Assay: RNase assay was carried out spectrophotometrically Galiana et al., 1997 10 with slight modification using 0.1 M sodium acetate buffer (pH 5.2) for extraction instead of 50 mM Tris–HCl buffer (pH 7.0). The plant extracts (50 µl) were incubated for 2 h at 37 °C in 250 µl of 50 mM Tris–HCl (pH 7.0) buffer containing BSA (0.01%) and yeast RNA (400 µg mL-1). After incubation, the remaining RNA in the reaction mixture was precipitated with ethanol in the presence of 2.5 M ammonium acetate (30:1) and resuspended in 100 µl of distilled water. Optic density was measured at 260 and 280 nm (the ratio ranged from 1.7 to 2.0). One enzyme unit is defined as the change in one OD at 260 nm mg-1 of protein per hour. Diethylpyrocarbonate (DEPC) was used as a positive (standard) inhibitor.
Protease Inhibition Assay (Trypsin and Thrombin): All the extracts were subjected to protease inhibition assay according to the method of Jedinak et al., 2010 5, with some modification. Tris buffer (100 mM) of pH 7.5 was prepared by dissolving 12.1 g of Tris (hydroxymethyl)-amino-methane in distilled water and adjusted pH 7.5 with HCl (5 M). The stock solution of trypsin was prepared by dissolving 2 mg of trypsin in 10 mL of 1.0 mM HCl. N𝛼-benzoyl-DL-arginine-paranitro-anilide hydrochloride (BApNA) was dissolved in DMSO (20 mg/mL). An enzyme (0.3 mL) and inhibitor (100 𝜇L) were incubated at 37 °C for 15 minutes, and then 0.6 mM substrate was added, and the final volume was made 2.5 mL with Tris buffer. The reaction mixture was incubated at 37 °C for 30 min. The reaction was quenched by adding 100 𝜇L of acetic acid (30%) and read the absorbance at 410 nm using UV/Vis spectrophotometer. Phenyl-methanesulfonylfluoride (PMSF) was used as a positive inhibitor. A similar procedure is carried out for thrombin.
% Inhibition = Absorbance (blank) − Absorbance (test) / Absorbance (blank) × 100
Partial-purification of Methanol Crude Extract of M. malabaricum: Fifty grams of powdered leaves of Memecylon species were extracted in a Soxhlet apparatus with hexane, ethyl acetate, methanol and water to obtain extracts with different polarities. The extracts obtained from hexane, ethyl acetate, methanol and water were 0.86, 1.21, and 4.32 g, respectively. Only the methanol extract was selected for further purification as it exhibited highest RNase and antiprotease activity. Soxhlet methanol extract (2 g) (5 mg/mL) was subjected to separation by silica gel column chromatography. A glass column (55 cm - 2.2 cm dia) was equilibrated bypassing ethyl acetate repeatedly. The methanol extract was loaded on to the packed glass column. The flow rate was set to 10 mL/min. The column was eluted with a methanol-ethylacetate mobile phase with the following increasing polarity: 100:0, 80:20, 60:40, 40:60, 20:80, and 0:100. Six fractions were collected from the methanol extract to obtain active fractions. The effluents were collected in a flask and allowed to dry. The yields of six fractions collected were 0.136, 0.246, 0.312, 0.263, 0.353, and 0.472 g. In-vitro bioassays (RNase & Protease) were carried out on all collected fractions to find the active fraction.
Statistical Analysis: All experiments / measurements were made in triplicate, and all the values are expressed as the Mean ± SE of three replicates.
RESULTS:
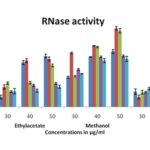
In-vitro RNase Activity: RNase assay is widely used to evaluate and control pathogen infection. Different extracts of Memecylon species at various concentrations, i.e., 30, 40, and 50 μg/mL, showed RNase activity in a dose-dependent manner with respect to standard Diethylpyrocarbonate (DEPC) Fig. 1. The methanol extract of both M. malabaricum and M. wightii showed the highest percent inhibition at 50 μg/mL with 81 and 84% inhibition of RNase activity compared to all other extracts, whereas M. umbellatum, M. edule, and M. talbotianum showed moderate activity.
FIG. 1: PERCENT INHIBITION OF RNASE ACTIVITY BY VARIOUS SOLVENT EXTRACTS OF MEMECYLON SPECIES
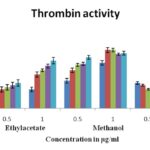
In-vitro Antiprotease Activity: Memecylon plant extracts obtained from the leaves were screened for antiprotease (Trypsin and thrombin) activity using chromogenic bioassay. In this study methanol extracts of M. malabaricum, M. wightii and M. talbotianum showed strong inhibition of protease trypsin with percent inhibiton of 62-68% in 1 mg/mL of plant extract with respect to positive control phenyl methane sulfonyl fluoride (PMSF). Similarly, for thrombin, the percent inhibition of 52-55% was observed in M. malabaricum, M. wightii, and M. talbotianum. All other extracts showed moderate activity Fig. 2 & 3. However, M. malabaricum and M. wightii showed the broadest spectrum of inhibition activity against in tested proteases and seemed to be a prospective new source of natural products as inhibitors of proteases.
FIG. 2: PERCENT INHIBITION OF TRYPSIN ACTIVITY BY VARIOUS SOLVENT EXTRACTS OF MEMECYLON SPECIES
FIG. 3: PERCENT INHIBITION OF THROMBIN ACTIVITY BY VARIOUS SOLVENT EXTRACTS OF MEMECYLON SPECIES
Effect of Methanol Solvent Fraction of M. malabaricum: The methanol extract of M. malabaricum was subjected to silica gel column chromatography. Among the eluted fractions, the fraction with an ethyl acetate–methanol ratio of 20:80 showed the highest activity for both RNase and Protease assays Table 1. Hence, this fraction was selected as an active fraction.
TABLE 1: RNASE AND PROTEASE ACTIVITY FOR THE FRACTIONATED METHANOL CRUDE EXTRACT OF M. MALABARICUM (AT 50 μg/ml)
| S. no. | Fractions | Percent inhibition | ||
| RNase activity | Protease activity | |||
| 50 μg/mL | Trypsin | Thrombin | ||
| 1 | 100% Methanol | 53.20 | 55 | 53 |
| 2 | 80:20 | 63.40 | 62 | 58 |
| 3 | 60:40 | 71.98 | 68 | 64 |
| 4 | 40:60 | 66.25 | 63 | 66 |
| 5 | 20:80 | 85.40 | 83 | 80 |
| 6 | 100% EA | 81.43 | 82 | 81 |
DISCUSSION: Scientific validation of natural bioactive metabolites helps in drug development. Memecylon species are reported to show various biological activities. It is also used as a medication in herpes virus infection 11, 12. RNase and protease are involved in the replication of HSV and HIV 13, 14. In the present study, the solvent extracts of Memecylon species viz., M. umbellatum, M. malabaricum, M. wightii, M. edule, and M. talbotianum were evaluated for their RNase and Protease inhibitor activities. The methanol extract of the leaves of different Memecylon species viz., M. umbellatum, M. malabaricum, M. wightii, M. edule, and M. talbotianum were observed to strongly inhibit RNase, trypsin, and thrombin functions in a dose-dependent manner. The percent inhibition of RNase activity ranged from 53-84% in 50 μg/mL of plant extracts with respect to standard DEPC equivalents. However, the highest inhibition was observed in M. malabaricum and M. wightii for RNase.
Similarly, for trypsin the percent inhibition ranged from 55-68% in 1 mg/mL of plant extracts with respect to standard PMSF. The highest inhibition was observed in M. malabaricum, M. wightii and M. talbotianum. For thrombin the percent inhibition was between 44-52% in 1 mg/mL of plant extracts Fig. 1, 2, and 3. The extracts from M. umbellatum, M. edule showed moderate inhibition. Jedinak 5 reported protease inhibition by Rhus typhina. Although the metabolites responsible for this activity were not investigated, tannins and triterpenes have been isolated from the leaves of Memecylon 12. Elsewhere, both tannins and triterpenes have shown to be strong inhibitors of HSV and HIV-1 Reverse transcriptase in-vitro 13, 15, with potential specificity of action 16. Similar results were observed in the study of antiviral potential by checking protease inhibition of medicinal plants such as Vernonia cinerea, Hemigraphis reptans, Hedyotis auricularia, Laurentia longiflora, Tridax procumbers and Senna angustifolia against dengue virus infection 17.
In previous studies, extracts obtained from Memecylon species have been shown to have strong antioxidant, anti-inflammatory, antidiabetic and antimicrobial effects against Staphylococcus aureus and Candida albicans 11-12. S. aureus is implicated in skin infections, while infection with C. albicans manifests as oral thrush and vulvovaginitis. The prevalence of S. aureus, and C. albicans as opportunistic infections in viral diseases such as Herpes, HIV/AIDS are well documented 18-20. It is also possible that the beneficial effects of decoctions/paste made from M. malabaricum leaves on Herpes patients may be linked to its inhibition of common opportunistic infections of bacterial or fungal etiology. We are employing a bioassay-guided fractionation protocol to isolate and chemically characterize the molecule responsible for its activity.
Our observations, fraction 20:80 might contain several phenolic and flavonoids compounds, and significant enzyme inhibition activities in-vitro this fraction should be considered as a new source of natural product for pharmaceutical studies similar conclusion drawn by Jalali et al., 2014 21 while fractionating Solanum xanthocarpum leaf extracts.
Since RNases and Proteases are effective in removing damaged and infected tissues from wounds and thus play an important role in the wound healing process 6, 22. These enzymes may be used to debride both adherent slough and eschar. Since Memecylon species have been used traditionally in the treatment of skin infections, this study provides an important clue for scaling up further work on managing the herpes virus infection using Memecylon extracts or metabolites.
CONCLUSION: In conclusion, the study had shown that M. malbaricum extracts have promising RNase and protease inhibition, the characterization of bioactive compounds from this plant will have the highest bioactive applications in the treatment of several viral infections.
ACKNOWLEDGEMENT: The authors acknow-ledge the UGC fellowship scheme (Or. No. DV9/192/NON-NETFS/2013-14 dated: 11-11-2013) and recognition of the University of Mysore as an Institution of Excellence and financial support from the Ministry of Human Resource Development, Government of India through UGC under UOM/IOE/RESEARCH/1/2010-11, dt 22-04-2010 project.
CONFLICTS OF INTEREST: None
REFERENCES:
- Serkedjieva J: Influenza virus variants with reduced susceptibility to inhibition by a polyphenol extract from Geranium sanguineum Die Pharmazie-An International Journal of Pharmaceutical Sciences 2003; 58(1): 53-57.
- Tolo FM, Rukunga GM, Muli FW, Njagi EN, Njue W, Kumon K, Mungai GM, Muthaura CN, Muli JM, Keter LK and Oishi E: Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. Journal of Ethnopharmacology 2006; 104(1-2): 92-99.
- Bedows E and Hatfield GM: An investigation of the antiviral activity of Podophyllum peltatum. Journal of Natural Products 1982; 45(6): 725-29.
- Yang CM, Cheng HY, Lin TC, Chiang LC and Lin CC: The in-vitro activity of geraniin and 1, 3, 4, 6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. Journal of ethnopharmacology 2007; 110(3): 555-58.
- Jedinak A, Valachova M, Maliar T and Šturdík E: Antiprotease activity of selected Slovak medicinal plants. Die Pharmazie-An International Journal of Pharmaceutical Sciences 2010; 65(2): 137-40.
- Vidyalakshmi A and Selvi SE: Protease activity of floral extracts of Jasminum grandiflorum L., a wound healing herb. Journal of Medicinal Plants 2013; 1(4): 11-15.
- Bystrická M and Russ G: Immunity in latent Herpes simplex virus infection. Acta Virol 2005; 49(3): 159-67.
- Prakasha HM, Krishnappa M, Krishnamurthy YL and Poornima SV: Folk medicine of NR Pura taluk in Chikmagalur district of Karnataka 2010; 9(1): 55-60.
- Bharathi TR, Sampathkumara KK and Prakash HS. Memecylon species: A review of traditional information and taxonomic description. Int J Pha Pha Sci 2016; 8: 1-9.
- Galiana E, Bonnet P, Conrod S, Keller H, Panabières F, Ponchet M, Poupet A and Ricci P: RNase activity prevents the growth of a fungal pathogen in tobacco leaves and increases upon induction of systemic acquired resistance with elicitin. Plant Physiology 1997; 115(4): 1557-67.
- Bharathi TR, Madhusudhan MC, Kumar PP, Nayaka CS and Prakash HS: Antimicrobial potential of Memecylon species from Western Ghats against clinical isolates of pathogenic bacteria. Research Journal of Pharmaceutical Biological and Chemical Sciences 2015; 6(4): 1280-87.
- Bharathi TR, Shailasree S, Kumara SKK, Madhusudan MC and Prakash HS: Metabolite profiling by UPLC-PDA-ESI/HDMS and antibacterial activity of Memecylon talbotianum Phcog Commn 2016; 6: 225-31.
- Tan GT, Pezzuto JM, Kinghorn AD and Hughes SH: Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J of Natural products 1991; 54(1): 143-154.
- Smiley JR: Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? Journal of Virology 2004; 78(3): 1063-68.
- Notka F, Meier GR and Wagner R: Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antiviral Research 2003; 58(2): 175-86.
- Zhu M, Phillipson JD, Greengrass PM, Bowery NE and Cai Y: Plant polyphenols: biologically active compounds or non-selective binders to protein? Phytochemistry 1997; 44(3): 441-47.
- Rothan HA, Zulqarnain M, Ammar YA, Tan EC, Rahman NA and Yusof R: Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay. Tropical Biomedicine 2014; 31(2): 286-96.
- Miller M, Cespedes C, Vavagiakis P, Klein RS and Lowy FD: Staphylococcus aureus colonization in a community sample of HIV infected and HIV-uninfected drug users. Eur J Clin Microbiol Infect Dis 2003; 22(8): 463-69.
- Bertagnolio S, Gaetano DK, Tacconelli E, Scoppettuolo G, Posteraro B and Fadda G: Hospital acquired candidemia in HIV-infected patients. Incidence, risk factors and predictors of outcome. J Chemotheraphy 2004; 16(2): 172-178.
- Lattif AA, Banerjee U, Prasad R, Biswas A, Wig N, Sharma N, Haque A, Gupta N, Baquer NZ and Mukhopadhyay G: Susceptibility pattern and molecular type of species-specific Candida in oropharyngeal lesions of Indian human immunodeficiency virus positive plants patients. J Clin Microbiol 2004; 42(3): 1260-62.
- Ghassam JB, Ghaffari H, Prakash HS and Kini KR: Antioxidant and hepatoprotective effects of Solanum xanthocarpum leaf extracts against CCl4-induced liver injury in rats. Pharmaceutical Biology 2014; 52(8): 1060-68.
- Shivakumar PD, Vasanthi NS, Shetty HS and Smedegaard-Petersen V: Ribonucleases in the seedlings of pearl millet and their involvement in resistance against downy mildew disease. European Journal of Plant Pathology 2000; 106(9): 825-36.
How to cite this article:
Bharathi TR and Prakash HS: Inhibitory effect of solvent extracts and their fractions of memecylon species on rnase and protease activity. Int J Pharmacognosy 2020; 7(12): 376-81. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.7(12).376-81.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
376-381
878
821
English
IJP
T. R. Bharathi * and H. S. Prakash
Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore, Karnataka, India.
bharathi99tr@gmail.com
21 July 2020
27 September 2020
22 November 2020
10.13040/IJPSR.0975-8232.IJP.7(12).376-81
31 December 2020