INHIBITORY ACTIVITY OF LEAF AND STEM EXTRACTS OF COSCINIUM FENESTRATUM (GAERTN.) COLEBR. AGAINST CLINICAL PATHOGENS
HTML Full TextINHIBITORY ACTIVITY OF LEAF AND STEM EXTRACTS OF COSCINIUM FENESTRATUM (GAERTN.) COLEBR. AGAINST CLINICAL PATHOGENS
Mala, A. Hannah Hepsibah and G. Jeya Jothi *
Department of Plant Biology and Biotechnology, Loyola College, Chennai, Tamil Nadu, India.
ABSTRACT: Medicinal plants have been estimated as the richest holdings of traditional medicines and from these plants, many types of modern medicines were developed. Coscinium fenestratum (Gaertn.) Colebr. belongs to the family Menispermaceae and is considered a critically endangered medicinal plant. Traditionally it is used for antimicrobial agents, diabetes mellitus, cancer, and arthritis. In this study in-vitro pharmacological activities of leaf and stem extracts of C. fenestratum were evaluated. The solvent extracts were screened for antimicrobial activity against some clinically isolated bacterial and fungal cultures using the disc diffusion method. The antioxidant activity of extracts were tested by 2,2-diphenyl-1-picrylhydrazyl (DPPH) method. The acetone extract of stem showed potential antimicrobial activity than other extracts. All the pathogens were inhibited at very low concentration, very specific Klepsiella pneumoniae was inhibited at 0.156mg/mL and Phialophora verrucosa was inhibited at 0.078mg/mL of the stem extract. The methanol extracts of stem showed the highest antioxidant activity of 99.59%. followed by stem extracts of ethyl acetate (99.02%), acetone (98.79%), and petroleum ether(90.55%) were showed. Hence, the present study clearly showed that the stem of C. fenestratum has higher antimicrobial and antioxidant properties capacity than the leaves.
Keywords: Yellow vine, Clinical pathogens, Pharmacological evaluation, Therapeutics, Folk medicine
INTRODUCTION: Coscinium fenestratum, commonly known as yellow vine, a woody climber with yellow wood and sap, is widely used as a medicinal plant in many Southeast Asian countries 1, 2, 3. The entire plant is reported to possess medicinal properties. The high demand for and the slow growth of this plant makes it vulnerable to poaching and the destruction of its habitat. Traditionally it has been used for fever, muscle pain, abdominal pain, inflammation 4 and malaria 5.
In addition, C. fenestratum has been reported to possess antioxidant 6 hypotensive 7 antidiabetic 8 lipid-lowering 9 antiplasmodial 5 and antibacterial 10 activities. It is used as a tea extract with other herbals as an immunity booster. The chief constituent of Cosciniumis the yellow crystalline alkaloid berberine; it also contains saponins 11.
The roots of C. fenestratum contain alkaloids berlambine, dihydroberlambine, 12, 13 – dihydro – 8-oxo berberine, tetra - hydroberberine, oxy-berberine and noroxy hydrastinine 12. Stems of Coscinium fenestratum from Thailand furnished the new protoberberine alkaloids oxypalmatine, (-)-8-oxotetrahydrothalifendine, (-)-8-oxoisocorypalmine and either (-)-8-oxothaicanine or (-)-8-oxo- 3-hydroxy-2, 4, 9, 10-tetramethoxyberbine in addition to berberine, the major alkaloid and (-)-8-oxocanadine (Pinhoet al., 1992). Berberine is present in both vegetative and reproductive parts, indicating the secretion of berberine in all parts of the plant. The plant extracts act as an antimicrobial agent 13 and are used as an antioxidant source in the Ayurveda and Siddha medicine 14. Plants are an alternate source for existing antibiotics; there is a desperate requirement to find new inhibitory sources against the pathogens. Many of the synthetic antioxidants available in the market have proved some of the side effects to humans 15 there is a need for highly dynamic and less harmful antioxidants. It has been found that plants having polyphenolic compounds such as flavonoids possess antioxidant activity 16. Polyphenols have antioxidative properties which is due to their high reactivity as hydrogen donor or electron donor, which stabilize and delocalize the unpaired electron 17. The present study was to assess the comparative inhibitory activity against clinical pathogens and free radicals scavengers of leaf and stem.
MATERIALS AND METHODS:
Plant Collection and Extraction: Fresh leaves and stems of C. fenestratum (Gaertn.) Colebr. were collected from Kannikatty, Agasthiyamalai hills, Tamil Nadu, India. And a sample was deposited at the herbarium in Loyola College (LCH 438). The collected plant materials were washed in running tap water to remove dirt and shade-dried at room temperature to remove moisture. The shade-dried plants were ground into a fine powder and stored in airtight containers. The ground powders were serially extracted in an incubator shaker with the selected solvents based on their polarity (petroleum ether-ethyl acetate- acetone- methanol) and mixed in the ratio1:4 for 72 h each. Each solvent was used 3 to 4 times until the extract turns colorless. A Rotary evaporator concentrated the obtained crude extracts at reduced pressure. The solvent-free extracts were used for further experimental studies.
Antimicrobial Activity against Human Pathogens by Disc Diffusion Method: Antimicrobial activity was screened by the disc diffusion method. The sterile Agar plates were inoculated with the test organisms. 200 mg of each extract was dissolved in 1000µl of Di Methyl Sulfoxide (DMSO), and 25µl of the extract was loaded on sterile discs. The discs were placed on the petriplates inoculated with the test organism and plates were kept for incubation at 37 ℃ for 24 h for bacteria and 48 h for fungi. The antibiotics Ciprofloxacin and Fluconazole were used as positive controls for bacteria and fungi, respectively. The inhibition zones formed were measured in millimeters. The experiment was done in triplicates, and the mean value of the zone of inhibition was calculated. DMSO was taken as a negative control.
Minimal Inhibitory Concentration: MIC was done for the extracts of selected plants that showed good antimicrobial activity. The serial microtiter plate dilution method developed by Eloff in 1998 was followed with little modification. 200mg of the extract was dissolved in 1ml of DMSO, from which 50µl was loaded on the first well and serially diluted with sterile water. 25µl of test organism was added to each well. The final well was loaded with 50µl of the test organism. The microtiter plates were incubated at 37℃ for 24h. After incubation, 50μl of 2 mg/ml P-Iodonitrotetrazolium Violet (INT) was added to all the wells and incubated for half an hour to one hour to note the colour change. INT is converted to formazanincase of microbial growth, giving restored colour. The lowest concentration in which red colour is not seen was taken as the MIC of the extract. The MIC values were expressed as mg/ml.
Antioxidant Activity: The antioxidant activity of the extracts was assayed by the DPPH scavenging activity assay. 1 mL of DPPH solution (0.3mM) was added to 2Ml of the extracts of different concentrations. The concentrations of the extracts were 50µg, 100µg, 150µg, 200µg, and 250µg. The reaction mixture was kept in the dark for 30 min, and the absorbance was measured at 517nm with a spectrophotometer. The percentage inhibitions of free radicals were calculated using the formula:
% inhibition = Absorbance of blank –Absorbance of sample / Absorbance of blank × 100
Ascorbic acid was taken as standard, with concentrations ranging from 20µg to 100µg.
Phytochemical Analysis: Preliminary phytochemical analysis was performed following standard procedures described by Sofowora (1993), Trease and Evans (1989), and Harborne (1973).
Test for Alkaloids: Fifty milligrams of the extract were dissolved in 5ml of dilute hydrochloric acid and filtered and the filtrate was used for analysis. To 2ml of the filtrate, a few drops Dragen droff’s reagent was added. A yellow precipitate indicated the test as a positive.
Test for Flavonoids: To 1ml of the aqueous filtrate of the extract, 0.5ml of dilute ammonia was added and shaken well. Yellow color a Reaction of the ammonia layer indicates the presence of flavonoids.
Test for Phenols: To 2mL of the aqueous extract, Potassium hydroxide solution was added. The appearance of dirty white precipitate indicates the presence of phenols.
Test for Tannins: To 2ml of the extract dissolved in water, 1% ferric chloride was added in drops. Appearance of dark green or deep blue colour indicated the presence of tannins.
Test for Saponins: Five milli gram of extract was dissolved in 3ml water and shaken well for 5min. Persistent foam formation indicated the presence of Saponins.
Test for Terpenoids and Sterols: To 2ml of extract inchloro form, a few drops of concentrated Sulfuric Acid were added. The development of the red dish brown interface indicates the presence of terpenoids, and the development of green fluorescence indicates the presence of sterols.
Statistical Analysis: All the studies were done in triplicates, and the mean± SD values were calculated and tabulated.
RESULTS:
Antimicrobial Activity: Coscinium fenestratum stem extracts showed very good antibacterial activity against 17 bacterial strains. All the four extracts showed antibacterial activity Table 1. The acetone extract showed the highest antibacterial activity against 16 bacterial species. The highest zone of inhibition of 18 mm was observed against Streptococcus agalactiae by acetone and methanol extracts, followed by a zone of 15mm against Enterococcus faecalis and a zone of 14mm against Salmonella paratyphi and Methicillin-resistant Staphylococcus aureus by acetone extract.
The petroleum ether and ethyl acetate extracts showed activity against only 4 bacteria. The Coscinium fenestratum leaf extracts showed very poor activity. Among the four extracts, acetone extract only showed antibacterial activity against Salmonella paratyphi (10mm), Staphylococcus aureus (7mm) and Methicillin resistant Staphylococcus aureus (7mm).
The results from the antibacterial studies carried out on the stem extracts of C. fenestratum showed good activity gainst 11 bacterial strains. Hence, MIC was performed on all the four extracts of the stem against the bacterial strains against which they showed activity as in Table 1.
TABLE 1: ANTIBACTERIAL ACTIVITY OF COSCINIUM FENESTRATUM LEAF AND STEM
| S. no. | Name of the Strains | Activity Of the Plant Extracts (mm) | CIP
5µg |
|||||||
| Leaf | Stem | |||||||||
| PE | EA | AC | ME | PE | EA | AC | ME | |||
| 1 | Micrococcus luteus | - | - | - | - | - | - | 9±0.2 | - | 14 |
| 2 | Staphylococcus epidermis | - | - | - | - | - | - | 11±0.0 | 7±0.1 | - |
| 3 | Yersiniaent erocolitica | - | - | - | - | - | - | 8±0.3 | - | 13 |
| 4 | Enterobactera erogenes | - | - | - | - | - | - | 10±0.5 | 9±0.3 | 17 |
| 5 | Salmonella typhimurium | - | - | - | - | - | - | - | - | - |
| 6 | Proteus vulgaris | - | - | - | - | - | - | 9±0.1 | 8±0.2 | 08 |
| 7 | Klebsiella pneumonia | - | - | - | - | - | - | 10±0.3 | 8±0.2 | 17 |
| 8 | Streptococci pneumonia | - | - | - | - | - | - | 8±0.2 | - | - |
| 9 | Streptococcus pyogenes | - | - | - | - | 9±0.1 | 11±0.1 | - | - | 10 |
| 10 | Streptococcus agalactiae | - | - | - | - | - | - | 18±0.2 | 18±0.6 | 08 |
| 11 | Escherichia coli | - | - | - | - | 8±0.1 | - | 8±0.1 | - | - |
| 12 | Pseudomonas aeruginosa | - | - | - | - | - | - | 8±0.0 | - | 16 |
| 13 | Staphylococcus aureus | - | - | 7±0.2 | - | - | - | 11±0.2 | 9±0.4 | - |
| 14 | Shigella dysentriae | - | - | - | - | - | - | 11±0.5 | - | 09 |
| 15 | Mucoid pneumonia | - | - | - | - | - | - | - | - | - |
| 16 | Salmonella paratyphi | - | - | 10±0.4 | - | - | 8±0.2 | 14±0.3 | 9±0.3 | 11 |
| 17 | Enterococcus faecalis | - | - | - | - | 7±0.0 | 7±0.2 | 15±0.0 | 12±0.4 | 12 |
| 18 | Serratia marcescens | - | - | - | - | - | - | - | 13 | |
| 19 | MRSA | - | - | 7±0.4 | - | - | - | 14±0.3 | 9±0.2 | - |
| 20 | Proteus mirabilis | - | - | - | - | - | - | - | - | - |
| 21 | Acinetobacter | - | - | - | - | - | 9±0.5 | 9±0.0 | - | 10 |
CIP- Ciprofloxacin, PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol; MRSA-Methicillin resistant Staphylococcus aureu.
The acetone extract showed very good activity with MIC less than 0.078mg against 10 of the tested bacterial strains. It also showed a statistically significant MIC of 0.625 mg against Salmonella paratyphi and Enterococcus faecalis.
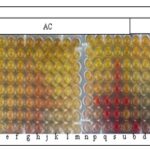
FIG. 1: MINIMAL INHIBITORY CONCENTRATION (MIC) OF COSCINIUM FENESTRATUM (GAERTN.) COLEBR STEM EXTRACTS AGAINST BACTERIAL STRAINS. PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
TABLE 2: MIC OF THE COSCINIUM FENESTRATUM STEM EXTRACTS AGAINST BACTERIAL STRAINS
| S. no. | Name of the strains | Activity of the Plant Extracts (mg) | |||
| PE | EA | AC | ME | ||
| 1 | Staphylococcus epidermis | - | - | ++ | 0.156 |
| 2 | Yersiniaent erocolitica | - | - | ++ | - |
| 3 | Enterobacter aerogenes | - | - | ++ | ++ |
| 4 | Salmonella typhimurium | - | - | ++ | - |
| 5 | Proteus vulgaris | - | - | ++ | ++ |
| 6 | Klebsiella pneumonia | - | - | ++ | 1.25 |
| 7 | Streptococci pneumonia | - | - | 2.5 | - |
| 8 | Streptococcus pyogenes | 0.312 | 1.25 | - | - |
| 9 | Streptococcus agalactiae | - | - | ++ | 0.312 |
| 10 | Escherichia coli | 0.156 | - | 1.25 | - |
| 11 | Pseudomonas aeruginosa | - | - | ++ | - |
| 12 | Staphylococcus aureus | - | - | ++ | 0.625 |
| 13 | Shigella dysentriae | - | - | ++ | - |
| 14 | Salmonella paratyphi | - | 1.25 | 0.625 | 0.312 |
| 15 | Enterococcus faecalis | 1.25 | - | 0.625 | 0.625 |
| 16 | MRSA | - | 5 | 5 | 10 |
| 17 | Acinetobacter | - | - | 0.625 | - |
PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
The methanol extract also showed a MIC of ˂0.078mg against 2 of the tested bacterial strains, followed by an MIC of 0.312 mg against Streptococcus agalactiae and Salmonella paratyphi MIC of 0.625mg against Staphylococcus aureus and Enterococcus faecalis. The petroleum ether extract showed a MIC of 0.312mg against Streptococcus pyogenes. The PE and ME extracts showed MIC of 0.156 mg against Escherichia coli and Staphylococcus epidermis, respectively. The EA extract showed the least activity against the entire tested organism Table 2 and Fig. 1.
Antifungal Activity of Coscinium fenestratum Leaf and Stem Extracts: The Coscinium fenestratum stem and leaf extracts were tested for their antifungal potency against 16 fungal strains. The stem extract showed good activity than the leaf extracts Table 3. The acetone extract of the stem showed very good activity against the fungal strains, Scedosporium sp., Aspergillus terreus, Rhizopus sp., Phialophora verrucosa, Trichophyton rubrum, Fusarium sp., Scytalidium dimidiatum, Paecilomyces sp., Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, Fusarium oxysporum, Candida albicans, Candida krusei and Candida tropicalis. The methanol, ethyl acetate extracts showed moderate activity and Petroleum ether extract showed very little activity. The leaf extracts showed very little activity against 5 fungal strains alone.
TABLE 3: ANTIFUNGAL ACTIVITY OF COSCINIUM FENESTRATUM LEAF AND STEM
| S. no. | Name of the Strains | Activity of the Plant Extracts (mm) | FLC | |||||||
| Leaf | Stem | |||||||||
| PE | EA | AC | ME | PE | EA | AC | ME | |||
| 1 | Scedosporium sp., | - | - | 10±0.5 | - | - | - | 14±0.2 | 12±0.7 | - |
| 2 | Phialo phora | - | - | - | - | 7±0.3 | - | 8±0.0 | - | - |
| 3 | Verruc osa | 7±0.3 | 8±0.4 | - | - | 7±0.0 | 7±0.2 | 10±0.5 | - | - |
| 4 | Aspergillu sterreus | - | - | - | - | - | 7±0.3 | 8±0.4 | - | - |
| 5 | Trichophyton rubrum | - | 7±0.1 | - | - | 8±0.0 | 7±0.3 | 7±0.2 | - | - |
| 6 | Rhizopus sp., | - | - | - | - | - | - | - | - | - |
| 7 | Fusarium sp., | - | - | - | - | - | - | 9±0.6 | - | - |
| 8 | Scytalidium dimidiatum | - | - | - | - | 8±0.5 | - | 9±0.4 | - | - |
| 9 | Paecilo mycessp., | 9±0.1 | - | - | - | - | 12±0.5 | 12±0.5 | 9±0.4 | - |
| 10 | Aspergillusfumigatus | - | - | - | - | - | - | 10±0.3 | 9±0.5 | - |
| 11 | Aspergillus niger | - | - | - | - | - | - | 9±0.2 | 7±0.0 | - |
| 12 | Aspergillus flavus | - | - | - | - | - | - | 9±0.4 | - | - |
| 13 | Fusarium oxysporum | - | - | - | - | - | - | 10±0.6 | 10±0.2 | - |
| 14 | Candida albicans | 7±0.4 | 7±0.2 | - | - | 8±0.4 | 8±0.3 | 11±0.5 | 7±0.5 | - |
| 15 | Candida krusei | - | - | - | - | - | - | 8±0.2 | - | - |
FLU- Flucanazole, PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
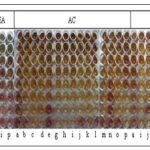
Minimum Inhibitory Concentration: The acetone extract of the stem showed significant activity. The best activity (MIC ˂0.078mg) was recorded against seven of the tested fungal strains, followed by an MIC of 0.078mg against Phialophora verrucosa and a MIC of 0.156 mg against Aspergillus niger, Fusarium oxysporium, and Candida tropicalis; finally with the MIC of 0.625 mg against Scedosporium sp. The PE extract showed the second-best activity, with a MIC of 0.156 mg against Aspergillus terreus and Fusarium sp., Table 4 and Fig. 2.
FIG. 2: MINIMAL INHIBITORY CONCENTRATION (MIC) OF COSCINIUM FENESTRATUM (GAERTN.) COLEBR STEM EXTRACTS AGAINST FUNGAL STRAINS. PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
TABLE 4: MIC OFTHE COSCINIUM FENESTRATUM STEMEXTRACTS AGAINST FUNGAL STRAINS
| S. no. | Name of the Strains | Activity of the Plant (mg) | |||
| Stem | |||||
| PE | EA | AC | ME | ||
| 1 | Scedosporium sp., | - | 5 | 0.625 | 10 |
| 2 | Phialophora verrucosa | 5 | - | 0.078 | - |
| 3 | Aspergillus terreus | 0.156 | - | ++ | - |
| 4 | Trichophyton rubrum | - | - | ++ | - |
| 5 | Rhizopus sp., | 0.156 | - | 2.5 | - |
| 7 | Scytalidium dimidiatum | - | - | ++ | - |
| 8 | Paecilomyces sp., | ++ | - | ++ | - |
| 9 | Aspergillus fumigatus | - | 2.5 | ++ | ++ |
| 10 | Aspergillus niger | - | - | 0.156 | ++ |
| 11 | Aspergillus flavus | - | - | ++ | 2.5 |
| 12 | Fusarium oxysporum | - | - | 0.156 | - |
| 13 | Candida albicans | - | - | ++ | 5 |
| 14 | Candida krusei | ++ | - | 1.25 | 0.156 |
| 15 | Candida tropicalis | - | - | 0.156 | - |
PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
Antioxidant Activity of Stem Extracts was showed in Table 5: The antioxidant activity of the extracts was assayed by DPPH scavenging activity assay. Among the stem extracts of C. fenestratum, the highest antioxidant activity was observed in methanol extract (99.59%), followed by ethyl acetate extract (99.02%), acetone extract (98.79%) and petroleum ether extract (90.55%). Compared with the activity of standard Ascorbic acid (100µg), 100µg of ethyl acetate extract showed better percentage inhibition of 95.99% followed by acetone extract (94.52%), methanol extract (93.71%) and petroleum ether extract (85.16%).
TABLE 5: ANTIOXIDANT ACTIVITY OF EXTRACTS OF COSCINIUM FENESTRATUM STEM
| S. no. | Conc. of the extracts (µg/ml) | % Inhibition of the extracts | Conc. of the standard (µg/ml) | % Inhibition of the standard | |||
| PE | EA | AC | ME | ||||
| 1 | 50 | 83.33±0.34 | 92.56±0.36 | 94.20±0.62 | 90.64±0.72 | 20 | 97.87±0.66 |
| 2 | 100 | 85.16±0.65 | 95.99±0.54 | 94.52±0.22 | 93.71±0.44 | 40 | 97.89±0.38 |
| 3 | 150 | 85.92±0.29 | 96.61±0.64 | 94.96±0.45 | 95.32±0.46 | 60 | 98.63±0.64 |
| 4 | 200 | 86.81±0.42 | 98.48±0.70 | 95.59±0.33 | 96.08±0.28 | 80 | 98.93±0.24 |
| 5 | 250 | 90.55±0.48 | 99.02±0.32 | 98.79±0.14 | 99.59±0.52 | 100 | 99.24±0.32 |
PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
Antioxidant Activity of Leaf Extracts was showed in Table 6: A mongall the extracts of Coscinium fenestratum leaf, the highest activity was observed in methanol extract (99.59%), followed by ethyl acetate extract (99.02%), acetone extract (98.79%) and petroleum ether extract (90.55%). Compared with the activity of standard Ascorbic acid (100 µg), 100 µg of ethyl acetate extract showed the better percentage of inhibition (95.99%) followed by acetone extract (94.52%), methanol extract (93.71%), and petroleum ether extract (85.16%).
TABLE 6: ANTIOXIDANT ACTIVITY OF EXTRACTS OF COSCINIUM FENESTRATUM LEAF
| S. no. | Conc. of the extracts (µg/ml) | % Inhibition of the extracts | Conc. of the standard (µg/ml) | % Inhibition of the standard | |||
| PE | EA | AC | ME | ||||
| 1 | 50 | 72.29±0.36 | 54.78±0.55 | 79.06±0.63 | 86.54±0.20 | 20 | 97.87±0.66 |
| 2 | 100 | 85.61±0.80 | 66.90±0.65 | 85.74±0.12 | 92.24±0.34 | 40 | 97.89±0.38 |
| 3 | 150 | 95.63±0.74 | 73.18±0.22 | 86.54±0.31 | 93.31±0.43 | 60 | 98.63±0.64 |
| 4 | 200 | 98.79±0.52 | 80.80±0.45 | 89.48±0.47 | 97.23±0.25 | 80 | 98.93±0.24 |
| 5 | 250 | 98.93±0.44 | 89.75±0.38 | 97.19±0.52 | 97.81±0.42 | 100 | 99.24±0.32 |
PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol.
Phytochemicals Present in Coscinium fenestratum Stem: The petroleum ether extract showed the presence of phenols, tannins, and sterols. The ethyl acetate extract showed the presence of flavonoids, phenols, tannins, saponins, and sterols. The acetone and methanol extracts were rich in alkaloids, flavonoids, phenols, tannins, saponins, terpenoids, and sterols Table 7 and Fig. 3.
Phytochemicals Present in Coscinium fenestratum Leaf: The petroleum ether and ethyl acetate extracts showed the presence of phenols, tannins and sterols.
The acetone extract showed the presence of alkaloids, phenols, tannins, terpenoids and sterols. The methanol extract was rich in alkaloids, flavonoids, phenols, tannins, saponins and terpenoids Table 7 and Fig. 3.
TABLE 7: PHYTOCHEMICALS SCREENING OF COSCINIUM FENESTRATUM STEM AND LEAF EXTRACTS
| S. no. | Name of the tests | Name of the Extracts | |||||||
| Stem | Leaf | ||||||||
| PE | EA | AC | ME | PE | EA | AC | ME | ||
| 1 | Alkaloids | - | - | + | + | - | - | + | + |
| 2 | Flavonoids | - | + | + | + | - | - | - | + |
| 3 | Phenolics | + | + | + | + | + | + | + | + |
| 4 | Tannins | + | + | + | + | + | + | + | + |
| 5 | Saponins | - | + | + | + | - | - | - | + |
| 6 | Terpenoids and Sterols | + | + | + | + | + | + | + | + |
PE-Petroleum Ether, EA-Ethyl Acetate, AC-Acetone and ME-Methanol + - Presence, - -Absent.
FIG. 3: PHYTOCHEMICALS SCREENING OF COSCINIUM FENESTRATUM STEM AND LEAF EXTRACTS
DISCUSSION: Different parts of the selected plant C. fenestratum is used for fever, muscle pain, stomach pain, malaria, diarrhea, ulcers, and infection of the eyes 11, 18. The present study showed the presence of alkaloids, flavonoids, tannins, saponins and terpenoids, and sterols in serially extracted methanol extracts of leaf and stem. Based on our observation, alkaloids, flavonoids, and saponins have extracted with mid and high polar solvents, and terpenoids, phenols and tannins were extracted even low polar solvents 19 reported the presence of phytochemicals in C. fenestratum were alkaloids, glycosides, tannins and flavonoids extracted with high polar solvent methanol. Some of the phytoconstituents were known to possess potent antioxidant capacity 19.
Hence, the presence of any of these phytoconstituents might act as a free radical scavenger. Stable diamagnetic molecules are formed from paired off with the corresponding hydrazine, when accepting hydrogen or electrons released from the stable nitrogen centered free radicals such as DPPH 20. DPPH radicals reacted with plant extracts lose their color stoichiometrically depending on the number of paired electrons 21. The DPPH inhibition property of stem and leaf extracts is shown in Tables 5 and 6. In the DPPH assay, the methanolic stem extract showed more antioxidant activity than methanolic leaf extract. The presence of flavonoids reported in phytochemical screening might be the reason for a percentage of free radical scavenging activity. Stem showed more potent antioxidant capacity than the leaf extract; the same concept was reported by 25. The reduction in the number of DPPH molecules can be correlated with the available number of hydroxyl groups. Hence the significant scavenging activity may be due to the presence of hydroxyl groups present in the extracts. The antioxidant activity of the extracts might be due to the presence of phenolic compounds (Pinto-Garcia et al., 2010), and flavonoids 23 may be due to chelation of metal ions or scavenging of free radicals.
The flavonoids also contain a broad spectrum of biological activity 24. Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and Bacillus subtilis were studied and reported C. Fenestratum stem has more potent antibacterial activity than the leaf methanolic extract 25. The present study proved that the stem extracts of Coscinium fenestratum have significant antimicrobial activity against a number of pathogenic bacterial and fungal strains that are multiple drug-resistant. In our studies, we have found that both leaf and stem have antioxidant and antibacterial activity comparable.
CONCLUSION: Coscinium fenestratum has been reported to possess various pharmacological actions such as antioxidant, laxative, antiproliferative, antidiabetic, anti-hypotensive, anti-plasmodial, and antibacterial activities. The present study also authenticated the inhibitory properties against clinically isolated pathogens and free radical scavenging activity. Hence, the above results confirmative evidence that the acetone extract of Coscinium fenestratum stem is potent against a wide range of drug-resistant bacteria and fungi that are causing enterococcal infections, UTI nosocomial infections and skin infections. The MIC results were showed remarkable activity at very low concentrations compared to all the solvent extracts; the acetone extract of the stem showed the highest activity against both bacterial and fungal strains, with the MIC value of 0.078mg, 0.156mg, 0.312mg and 0.625mg which are more potent when compared with standard drugs.
ACKNOWLEDGEMENT: The authors would like to thank Dr. R. Ravindhran, Head of the Department of Plant Biology and Biotechnology, and Rev. Fr. A. Thomas, Principal, Affiliated to University of Madras, Chennai, Tamil Nadu, India, for their encouragement and support. We would like to thank ICMR (Indian Council of Medical Research), New Delhi, India, for providing financial support through project No.59/37/2011/BMS/TRM.
CONFLICTS OF INTEREST: The authors declared no potential conflicts of interest concerning research, authorship and /or publication of this article.
REFERENCES:
- Pinho PMM, Pinto MMM, Kijjoa A, Pharadai K, D´ıaz JG and Herz W: Protoberberine alkaloids from Coscinium fenestratum. Phytochemistry 1992; 31(4): 1403–7.
- Singh GB, Singh S, Bani S and Malhotra S: Hypotensive action of a Coscinium fenestratum stem extract. Journal of Ethnopharmacology 1990; 30(2): 151–155.
- Siwon J, Verpoorte R, Van Essen GFA and Baerheim Svendsen A: Studies on Indonesian medicinal plants. III: the alkaloids of Coscinium fenestratum. Planta Medica 1980; 38(1): 24–32.
- Sudharshan S, Prashith Kekuda T and Sujatha M: Antiinflammatory activity of Curcuma aromatic Salisb and Coscinium fenestratum Colebr: a comparative study. Journal of Pharmacy Research 2010; 3(10): 24–25.
- Tran QL, Tezuka Y and Ueda JY: In-vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. Journal of Ethnopharmacology 2003; 86(2): 249–52.
- Venukumar MR and Latha MS: Antioxidant effect of Coscinium fenestratum in carbon tetrachloride treated rats. Indian J of Physiology and Pharma 2002; 46(2): 223–8.
- Wongcome T, Panthong A, Jesadanont S, Kanjanapothi D, Taesotikul T and Lertprasertsuke N: Hypotensive effect and toxicology of the extract from Coscinium fenestratum (Gaertn.) Colebr. J of Ethnopharma 2007; 111(3): 468– 75.
- Shirwaikar A, Rajendran and Punitha ISR: Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. Journal of Ethnopharmacology 2005; 97(2): 369–74.
- Jittaprasatsin W, Banlunara V, Sommitr D, Patanachai M and Yibchok-Anan S: Subacute effects of Coscinium fenestratum ethanol extract on blood glucose level, lipid profiles and blood chemistry in normal and streptozotocin-induced diabetic rats. Thai J of Pharma 2005; 27: 109–120.
- Nair GM, Narasimhan S, Shiburaj S and Abraham TK: Antibacterial effects of Coscinium fenestratum. Fitoterapia 2005; 76(6): 585–587.
- Rojsanga P and Gritsanapan W: Variation of berberine content in Coscinium fenestratum stem in Thailand Market. The Mahidol University Journal of Pharmaceutical Sciences 2005; 32(3): 66–70.
- Shirwaikar A, Rajendran K and Punitha IS: Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J. Ethnopharmacol 2005; 97(2): 369-74.
- Kumar GS, Jayaveera KN, Kumar A, Sanjay CK, Swamy UP and Kumar BMV: Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Tropical Journal of Pharmaceutical Research 2005; 6(2): 717-23.
- Punitha ISR, Rajendran K, Shirwaikar A and Shirwaikar A: Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin-nicotinamide induced diabetic rats. Oxford Journal 2005; 2(3): 375-81.
- Ito N, Fukushima S, Hagiwara A, Shibata M and Ogiso T: Carcinogenicity of butylatedhydroxyanisole in F344 rats. J Natl Cancer Inst 1983; 70: 343-347.
- Cook NC and Samman S: Flavonoids- chemistry, metabolism, cardio protective effects, and dietary sources. Nutritional Biochemistry 1996; 7: 66- 76.
- Rice-Evans C, Miller N and Paganga G: Antioxidant properties of phenolic compounds. Trends Plant Sci 1997; 2: 152-159.
- Malhotra S, Taneja SC and Dhar KL: Minor alkaloid from Coscinium fenestratum. Phytoch 1989; 28(7); 1998–9.
- Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM and Aggarwal BB: Berberine modifies cysteine 179 of I𝜅B𝛼 kinase, suppresses nuclear factor-𝜅B-regulated antiapoptotic gene products and potentiates apoptosis. Cancer Research 2008; 68(13): 5370–9.
- Tang J, Feng Y, Tsao S, Wang, Curtain R and Wang Y: Berberine and coptidisrhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J of Ethnopharma 2009; 126(1): 5–17.
- Sun Y, Xun K, Wang Y and Chen X: A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009; 20(9): 757–69.
- Pinto-Garcia L, Efferth T, Torres A, Hoheisel JD and Youns M: Berberine inhibits cell growth and mediates cas paseinde pendent cell death in human pancreatic cancer cells. Planta Medica 2010; 76(11): 1155–61.
- Benavente-Garcia O, Castillo J, Marin FR, Ortuno A and Del Rio JA: Uses and properties of Citrus flavonoids J Agric Food Chem1997; 45: 4505–15.
- Miliauskas G, Venskutonis PR and van Beek TA: Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem 2004; 85: 231-7.
- Santhosh W. Goveas and Asha Abraham: Evaluation of antimicrobial and antioxidant activity of stem and leaf extracts of Coscinium fenestratum. Asian Journal of Pharmaceutical and Clinical Research 2013; 6(3): 218-21.
How to cite this article:
Mala M, Hepsibah AH and Jothi GJ: Inhibitory activity of leaf and stem extracts of Coscinium fenestratum (Gaertn.) colebr. against clinical pathogens. Int J Pharmacognosy 2022; 9(3): 52-61. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.9(3).52-61.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
52-61
2024 KB
763
English
IJP
M. Mala, A. Hannah Hepsibah and G. Jeya Jothi *
Department of Plant Biology and Biotechnology, Loyola College, Chennai, Tamil Nadu, India.
gjjothiloyola@gmail.com
22 February 2022
26 March 2022
28 March 2022
10.13040/IJPSR.0975-8232.IJP.9(3).52-61
31 March 2022