INHIBITION OF PROTEASES AND ANTI-CANCER ACTIVITIES OF ETHANOLIC TUBER EXTRACT OF GLOBBA BULBIFERA
HTML Full TextINHIBITION OF PROTEASES AND ANTI-CANCER ACTIVITIES OF ETHANOLIC TUBER EXTRACT OF GLOBBA BULBIFERA
V. Narasinga Rao * and DSVGK Kaladhar
Department of Biochemistry, GIS, GITAM University, Visakhapatnam - 530045, Andhra Pradesh, India.
ABSTRACT: Studies on anticancer and protein inhibition process play a vital role in regulating the cellular process of life and diseases. The present studies have been conducted on ethanolic tuber extract from Globba bulbifera for protease inhibition and breast cancer inhibition. The extract has shown good inhibition for Trypsin, Chymotrypsin and protease K. The extract has also shown MCF-7 (Breast Cancer) inhibition, IC50 value at 235 μg/ml. The inhibition is less compared to the standard compound, tamoxifen (IC50: 37 μg/ml). Hence the present studies have shown good protease inhibition and anticancer (breast cancer) activities.
| Keywords: |
MCF-7, Breast Cancer, Ethanol tuber extract of Globba bulbifera, Protease inhibition
INTRODUCTION: There are several proteins accessible in the living systems present in various parts of the cells that perform numerous functions within the cells of living organisms 1. Biological scientists proposed that enzymatic activity is associated with proteins 2. Proteolytic enzymes show many physiological functions varies from protein digestion to specific regulated processes like blood coagulation, activation of zymogens, the release of bio pharmacologically active peptides and hormones, transport of secretory proteins through membranes, etc. 3 The evolution of proteolytic enzymes changes in structure and function and their inhibitors show diverse and complex functions in higher organisms 4.
Many proteolytic enzymes are synthesized as inactive precursors (Zymogens) with subsequently converted active enzymes by the selective cleavage by peptide bonds and prevent unwanted protein degradation, to enable spatial and temporal regulation of proteolytic activity 5. These enzymes include proteinases like trypsin, proteinase K, Chymotrypsin, etc. 6 The trypsin catalyzes the hydrolysis of dietary protein of the internal peptide bonds that are formed by the basic amino acids due to lysine, arginine and their corresponding amino acid derivatives 7. Human trypsin is strongly inhibited by inhibitory activity of compounds like mercuric chloride, calcium salts, etc. 8
The chymotrypsin catalyzes the hydrolysis of specific ester substrates 9 that are readily soluble and stable to Tris buffer at pH 8 10 The biocatalytic activity of chymotrypsin (CT) ethanol/water solution containing small amounts of metal salts. Calcium acetate accelerates transesterification of amino acid to six fold at 100 μM.
Metal salts can change the secondary and tertiary structures of CT 11. The main proteolytic enzyme was proteinase consists of a single peptide chain containing 277 amino acids concerning its keratin hydrolyzing activity. The specificity of the enzyme for peptide bonds adjacent to the carboxylic group of aliphatic and aromatic amino acids was observed 12.
Cancer is a disease occurring in a series of steps, arising as a consequence of activating mutations (oncogenes) or deactivating mutations (tumor suppressor genes) in proliferating cells 13. Cyclooxygenase-2 (COX-2) recognized as a molecular target of many chemopreventive as anti-inflammatory agents. Previous studies on COX-2 had shown the regulated mechanism by the transcription factor NF-κB. Curcumin, derived from the rhizome of Curcuma longa L. having anti-inflammatory properties, and inhibits chemically induced carcinogenesis in the skin and colon 14. The Tumour-associated macrophages (TAM) are a major inflammatory component of the stroma of tumors and can affect different aspects of the neoplastic tissue 15. The Plant-derived compounds an important source of several clinically useful anti-cancer agents such as vincristine, camptothecin, the vinblastine derivatives, irinotecan, topotecan, and etoposide, derived from paclitaxel an epipodophyllotoxin 16. Some promising new agents are in clinical development and based on selective activity against cancer-related molecular targets, including flavopiridol while some agents who failed in earlier clinical studies are stimulating interest 17.
MATERIALS AND METHODS:
Plant Materials: The fully matured Globba bulbifera plant tubers were collected from Kerala. Plant materials for the study were washed thoroughly in distilled water and air-dried. A tuber from Globba bulbifera is taken and thoroughly washed with double distilled water and cut into pieces and air dried.
Preparation of Ethanolic Extract: The powder from Globba bulbifera tuber is obtained by using a grinder. The powder is placed in a refrigerator. Nearly 30 gm of air-dried powder is taken in a 100ml of ethanol in a 500 ml conical flask by thoroughly mixing by incubating the contents at room temperature in a rotary shaker for 48hrs at 120 rpm. The slurry was then filtered through cheesecloth and Whatman no. 1 filter paper with three times, and then the filtrate was centrifuged at 10,000 rpm for 15 min at 4 °C to remove any cell debris that remains in the preparation. Then the solvent is evaporated through rotavapor and make the final volume one-fourth of the original volume and stored at the 4 ºC in airtight containers. Protease inhibitor activity and anticancer activity is tested for Globba bulbifera plant tubers.
Protein Inhibition Activity: Different metals have been tested for protein inhibition activity. Various metals (5mM) like CaCl2, MgSO4, ZnSo4, CuSo4, FeSO4, MnSO4, HgCl2, BaCl2, CdCl2, Na2MO, Al2O3, Na2EDTA, (CH3COO)2Pb, Na2CrO4, H3BO3 and KI has been used in the present experimentation. By using these metals, inhibition of protease has been observed with tuber extract of Globba bulbifera Linn. Approximately 10 µl of protease inhibitor (plant extract) was mixed with 10µl of protease (0.5 mg/ml) and spotted on to a strip of X-ray film. 10µl of the protease was mixed with 10µl phosphate buffer 0.l M (pH 7.0) as the control and spotted on to the X-ray film. Incubation of X-ray film at 37 °C for 10 min has to be done. Wash the film under tap water for the zone of gelatin hydrolysis to protease activity visualization.
Anti-cancer Activity: Human cancer cell lines used in this study were produced from National Centre for Cell Science, Pune. All cells were grown in Minimal Essential Medium (MEM, GIBCO) and supplemented with 4.5 g/L glucose, 2 mM L-glutamine and 5% fetal bovine serum (FBS) (growth medium) at 37 ºC in 5% CO2 incubator.
The trypsinized cells from T-25 flask were seeded in each well of 96-well flat-bottomed tissue culture plate at a density of 5 × 103 cells/well in growth medium and cultured at 37 ºC in 5% CO2 to adhere. After 48 h incubation, the supernatant was discarded, and the cells were pretreated with growth medium and were subsequently mixed with different concentrations of test compounds (12.5, 25, 50, 100, 200 and 250 µg/ml) in triplicates to achieve a final volume of 100 μl and cultured for 48 hours. The compound separately is prepared as 1.0 mg/ml concentrations of stock solutions using DMSO.
The culture medium and solvent are used as controls. Each well then received 5 microliters of fresh MTT (0.5 mg/ml in PBS) followed by incubation for 2hr at 370c. The supernatant growth medium was removed from the wells and replaced with 100 micro liters of DMSO to solubilize the colored formazan product. After 30 min incubation, the absorbance (OD) of the culture plate is measured at a wavelength of 492 nm on ELISA reader, Anthos 2020 spectrophotometer.
RESULTS: Protease inhibition activity of Globba bulbifera Linn. has been presented. Comparative studies on different proteases and different metals upon plant extract shown small variation. The control (10µl of Chymotrypsin+10µl of phosphate buffer) has been used with enzyme Chymotrypsin and phosphate buffer. There is no inhibition observed in control. Different metals have been tested with enzyme Chymotrypsin. Strong inhibition is not observed in metals. The test sample has been experimented (10µl of Chymotrypsin+10µl of each metal + 10µl of tuber extract) for chymotrypsin inhibition. FeSO4 is acting as a strong activator to Globba bulbifera tuber extract. Remaining tested metals was not shown any inhibition with Globba bulbifera tuber extract.
The control (10µl of protease K+10µl of phosphate buffer) has been used with enzyme protease K and phosphate buffer. There is no inhibition observed in control. Different metals have been tested with enzyme protease K. Strong inhibition is shown by FeSO4 and (CH3COO)2Pb only but remaining metals not shown inhibition. The test sample has experimented (10µl of protease K + 10µl of each metal + 10µl of tuber extract) for protease K inhibition. The CaCl2 shown slight inhibition, FeSO4 and HgCl2 shown strong inhibition means they are acting as strong activators to Globba bulbifera tuber extract, but (CH3COO)2Pb not shown any inhibition with Globba bulbifera tuber extract and remaining metals not shown any inhibition with enzyme protease k.
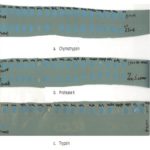
The control (10µl of trypsin+10µl of phosphate buffer) has been used with enzyme trypsin and phosphate buffer. There is no inhibition observed in control. Different metals have been tested with enzyme trypsin. Strong inhibition is shown by FeSO4 and (CH3COO)2Pb only, but remaining metals has not shown inhibition. The test sample has been experimented (10µl of trypsin+10µl of each metal + 10µl of tuber extract) for trypsin inhibition. Except for FeSO4 and (CH3COO)2Pb remaining all metals acting as activators to the Globba bulbifera tuber extract and shown strong inhibition with trypsin Fig. 1.
FIG. 1: PROTEIN INHIBITION ASSAY
TABLE 1: DOSE-RESPONSE OF ETHANOLIC EXTRACT OF GLOBBA BULBIFERA ON MCF-7 (BREAST CANCER) CELL LINE
| Conc Microgm/ml) | OD of Tamoxifen at 450 nm | % of
Cell survival for Tamoxifen |
% of
Cell inhibition for Tamoxifen |
OD of extract at 450nm | % of cell survival for extract | % of
cell inhibition for extract |
| 12.5 | 0.468 | 89.3 | 10.7 | 0.452 | 94.3 | 5.7 |
| 50 | 0.201 | 33.6 | 66.4 | 0.380 | 77.8 | 22.2 |
| 100 | 0.126 | 17.9 | 82.1 | 0.323 | 64.8 | 35.2 |
| 200 | 0.110 | 14.6 | 85.4 | 0.280 | 54.9 | 45.1 |
| 250 | 0.105 | 12.7 | 87.3 | 0.245 | 47.8 | 52.2 |
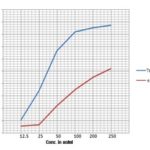
The OD of the blank is 0.040 and control is 0.447. Table 1 shows the %cell survival and %cell inhibition for the standard (tamoxifen) and the plant extract. There is a gradual decrease in the OD values that shown good inhibition results. Fig. 2 shows the IC50 Standard at 37 μg/ml and the IC50 Plant extract at 235 μg/ml.
FIG. 2: ANTICANCER ACTIVITY OF ETHANOLIC EXTRACT OF GLOBBA BULBIFERA
DISCUSSION: Globba bulbifera belongs to the Zingiberaceae family. Most of the medicinal plants are widely used for the treatment of several diseases in India and China 18. The usage of plants like turmeric may reduce the risk of several kinds of cancers and provide other protective biological effects in humans. These biological effects are due to constituent curcumin that widely studied for its wound healing, anti-oxidant, anti-inflammatory and anti-cancer effects 19.
The no steroidal anti-inflammatory drugs (NSAIDs), including aspirin, have clinically significant in anti-carcinogenic effects in the gastrointestinal tract. The epidemiological data indicate that use of these drugs is associated with the risk of sporadic colorectal cancer, and clinical trials of patients with familial polyposis coli show that NSAIDs can lead to the regression of large bowel adenomas 20.
The response of the body to cancer is not a unique process but has many parallels with inflammation and wound healing. The inflammatory cells and cytokines found in tumors are more contribute to tumor growth, progression, and immune suppression than they are to mount an effective host antitumor response. The cancer susceptibility to severity may be associated with functional polymorphisms of inflammatory cytokine genes, deletion or inhibition of inflammatory cytokines inhibits the development of cancer 21.
Pigeon-pea seed extracts have already been analyzed for the protease inhibitors using x-ray film protease inhibitor method 22. Seed extracts of Cajanus cajan were analyzed by Pichare & Kachole, 1996, for protease inhibitor activities using caseinolytic assay and are determined by PAGE. The relative amounts of different trypsin inhibitors and the total trypsin inhibitor activity varied with different mining media. The trypsin inhibitors were not detectable in pigeon pea leaves. The profiles of trypsin and chymotrypsin inhibitors in almost all the cultivars of pigeon pea analyzed were similar those in wild relatives were quite variable 23. The evaluate has been conducted in various plants and animals like Helicoverpa armigera 24 Periplaneta americana 25 Moringa oleifera 26.
The Globba bulbifera is a one of the medicinal plants which is used for the various disorders. The various inhibitors inhibit the proteolytic enzymes, but the present studies indicate that proteolytic enzymes are inhibited by the ethanolic extract of Globba bulbifera along with various metals. The enzyme trypsin showed strong inhibition with various metals along with Globba bulbifera ethanolic extract. The protease K and chymotrypsin showed slight inhibition with ethanolic extract of Globba bulbifera. This study also explains anti-cancer activity by ethanolic extract of Globba bulbifera but shown less activity with standard tamoxifen.
CONCLUSION: Globba bulbifera ethanolic tuber extract has shown good activity for protease inhibition and anti-cancer activity. Hence the tuber extract shows protease inhibition and anti-cancer components.
ACKNOWLEDGEMENT: Author would like to thank management and staff of GITAM University Visakhapatnam, India for their kind support in bringing out the above literature and providing lab facilities.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Bromley EH, Channon K and Moutevelis E: Peptide and protein building blocks for synthetic biology: from programming biomolecules to self-organized biomolecular systems. ACS chemical biology 2008; 3(1): 38-50.
- Whittaker VP: Structure and function of animal cell membranes. British Medical Bulletin 1968; 24(2): 101-106.
- Munro S: Localization of proteins to the Golgi apparatus. Trends in cell biology 1998; 8(1): 11-15.
- Neurath H: Evolution of proteolytic enzymes. Science 1984; 224(4647): 350-357.
- Beynon RJ and Bond JS: Proteolytic enzymes: a practical approach. IRL Press at Oxford University Press 1989
- Neurath H and Walsh KA: Role of proteolytic enzymes in biological regulation (a review). Proceedings of the National Academy of Sciences 1976; 73(11): 3825-3832.
- Dierick NA: Biotechnology aids to improve feed and feed digestion: enzymes and fermentation. Archives of Animal Nutrition 1989; 39(3): 241-261.
- Schwert GW, Neurath H and Kaufman S: The specific esterase activity of trypsin. Journal of Biological Chemistry 1948; 172: 221-239
- Markwardt F, Landmann H and Walsmann P: Comparative studies on the inhibition of trypsin, plasmin, and thrombin by derivatives of benzylamine and benzamidine. European Journal of Biochemistry 1968; 6(4): 502-506.
- Green NM: Competition among trypsin inhibitors. Journal of Biological Chemistry 1953; 205(2): 535-551.
- Hess GP, McConn J and Ku E: Studies of the activity of chymotrypsin. Philosophical Transactions of the Royal Society of London. B, Biological Sciences 1970; 257(813): 89-10.
- DelMar EG, Largman C and Brodrick JW: A sensitive new substrate for chymotrypsin. Analytical biochemistry.1979; 99(2): 316-320.
- Sasaki T and Kise H: Effects of metal salts on the structure and activity of. α-chymotrypsin in Ethanol/Water. Bulletin of the Chemical Society of Japan 1999; 72(6): 1321-1325.
- Jany KD, Lederer G and Mayer B: Amino acid sequence of proteinase K from the mold Tritirachium album Limber: Proteinase K-a subtilisin-related enzyme with disulfide bonds. FEBS letters 1986; 199(2): 139-144.
- Coussens LM and Werb Z: Inflammatory Cells and Cancer Think Different! The Journal of experimental medicine 2001; 193(6): F23-F26.
- Surh YJ: Anti-tumor is promoting the potential of selected spice ingredients with antioxidative and anti-inflammatory activities: a short review. Food and Chemical Toxicology 2002; 40(8): 1091-1097.
- Kawamori T, Lubet R and Steele VE: Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer research 1999; 59(3).
- Sica A, Schioppa T and Mantovani A: Tumor-associated macrophages are a distinct M2 polarized population promoting tumor progression: potential targets of anti-cancer therapy. European journal of cancer 2006; 42(6): 717-727.
- Cragg GM and Newman DJ: Plants as a source of anti-cancer agents. Journal of Ethnopharmacology 2005; 100(1): 72-79.
- Kaladhar DSVGK, Harasreeramulu S and Duddukuri GR: In-vitro regeneration of the medicinal herb, Evolvulus nummularius from shoot tip and flower explants. Indian Journal of Fundamental and Applied Life Sciences 2011; 1(2): 81-89.
- Maheshwari RK, Singh AK and Gaddipati J: Multiple biological activities of curcumin: a short review. Life sciences 2006; 78(18): 2081-2087.
- Baron JA and Sandler RS: No steroidal anti-inflammatory drugs and cancer prevention. Annual review of medicine 2000; 51(1): 511-523.
- Balkwill F and Mantovani A: Inflammation and cancer. The Lancet 2001; 357(9255): 539-545.
- Pichare MM and Kachole MS: Detection of electro-phoretically separated protease inhibitors using X-ray film. Journal of biochemical and biophysical methods 1994; 28(3): 215-224.
- Pichare MM and Kachole MS: Protease inhibitors of pigeon pea (Cajanus cajan) and its wild relatives. Physiologia Plantarum 1996; 98(4): 845-851.
- Parde VD, Sharma HC and Kachole MS: In-vivo inhibition of Helicoverpa armigera gut pro-proteinase activation by non-host plant protease inhibitors. Journal of Insect Physiology 2010; 56(9): 1315-1324.
How to cite this article:
Rao VN and Kaladhar DSVGK: Inhibition of proteases and anti-cancer activities of ethanolic tuber extract of Globba bulbifera. Int J Pharmcognosy 2014; 1(1): 82-86. doi: 10.13040/IJPSR.0975-8232.1(1).82-86.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
11
82-86
507
1978
English
IJP
V. N. Rao and D. S. V. G. K. Kaladhar
Department of Biochemistry, GIS, GITAM University , Visakhapatnam, Andhra Pradesh, India.
narsing.vurukuti@gmail.com
19 November 2013
21 December 2013
26 December 2013
http://dx.doi.org/10.13040/IJPSR.0975-8232.1 (1).82-86
01 January 2014