IN-VITRO EVALUATION OF EUCALYPTUS CITRIODORA LEAF ESSENTIAL OIL AND EXTRACTS ON SELECTED PATHOGENS IMPLICATED IN RESPIRATORY TRACT INFECTIONS
HTML Full TextIN-VITRO EVALUATION OF EUCALYPTUS CITRIODORA LEAF ESSENTIAL OIL AND EXTRACTS ON SELECTED PATHOGENS IMPLICATED IN RESPIRATORY TRACT INFECTIONS
Samuel Ehiabhi Okhale *, Peters O. Oladosu, Mercy I. Aboh, Chinyere Imoisi and Josiah James Gana
Department of Medicinal Plant Research & Traditional Medicine , National Institute for Pharmaceutical Research & Development, P.M.B. 21, Garki, Abuja, Nigeria.
ABSTRACT: Public health challenge due to respiratory tract infections has necessitated vehement research for promising substances against the causative agents. The leaves of Eucalyptus citriodora (E. citriodora, Myrtaceae) are used in Nigeria as tea, infusion or steam inhalation by patients as an alternative medicine to clear the airway and relieve symptoms; however, no scientific evidence exists to support or debunk this claim. This study aimed to evaluate the in-vitro antimicrobial effect of E. citriodora leaf essential oil and crude extracts against selected pathogens implicated in respiratory tract infections. The fresh leaves of E. citriodora were collected, identified and subjected to exhaustive fractionated extraction. The leaf essential oil was obtained by hydrodistillation using Clevenger-type apparatus and characterized using GC-MS. Aqueous extracts were obtained by hot infusion and decoction and characterized using HPLC-UV-DAD. The susceptibility of test pathogens Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Salmonella paratyphi ATCC 9150 Candida albicans ATCC 10231, Klebsiella pneumonia and Streptococcus pneumonia to the extracts were evaluated by agar well diffusion and agar dilution methods. Hydrodistillation gave colourless essential oil in yield of 3.5% (v/w). Monoterpenoids, citronellal (47%), isopulegol (8%), citronellol (8%), tetradecane-3-of (5%), citronellic acid (4%), p-methane-3,8-diol (4%), citronellylacetae (3%) and eucalyptol (1%) were the most abundant bioactive components. HPLC analysis of the infusion and decoction extracts revealed the presence of chlorogenic acid, caffeic acid, rutin, and ferulic acid. The essential oil and extracts produced good antimicrobial activities against the selected pathogens. The leaves of E. citriodora have bioprospecting potential in drug discovery for respiratory tract infections.
Keywords: COVID-19, Respiratory tract infection (RTI), Essential oil, Eucalyptus citriodora, Antimicrobial
INTRODUCTION: Public health challenges due to respiratory tract infections and the catastrophic dawn of the COVID-19 pandemic, which has infected over 187 million people and killed over 4 million globally as of 13th July 2021 1 has necessitated vehement research of promising substances to at least reduce drastically or put an end to mortality and optimally ensure very low morbidity rate 2. COVID-19, an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been noted to cause respiratory tract infection 3. The risk of the severe disease marked by dyspnea, hypoxia and extensive lung involvement seen in COVID-19 cases is more severe in people with other underlying medical disorders, such as lung, heart, kidney, liver disease, diabetes, and compromised immunity. This can progress to respiratory failure necessitating mechanical ventilation, shock, multi-organ failure and death 3. There is no specific medicine recommended to prevent or treat SARS-CoV-2 3. Currently, some drugs are being tested for their potency and efficacy against SARS-CoV-2, but none has been clinically approved for prevention and treatment. The most promising is Remdesivir (GS-5734). This nucleoside analogue pro-drug showed inhibitory effects on pathogenic animals and human coronavirus, including SARS-CoV-2 but was not associated with statistically significant clinical benefits in the randomized, double-blind, placebo-controlled multicenter trial 4.
Favipiravir, chloroquine, hydroxychloroquine, and convalescent plasma treatments have also been tested. Given the symptoms of the COVID-19 disease, many people all around the globe have resorted to some of the few drugs still undergoing testing and some medicinal plants which have been used for decades to treat infectious diseases. Medicinal plants have been used as sources of drugs by mankind for several years. In fact, ancient man was dependent on plants for his needs of treatment, prevention, and other forms of medicaments, thus utilizing plants as drugs for millennia. For the past 3000 years, many plants have been used in health care practices, such as Traditional Medicine in China, India and Africa, most of which contain therapeutic values which Western standards have ascertained. Furthermore, several other plants have been employed for centuries by several cultures, which are less likely to be proven by Western standards 5.
Medicinal plants, however, remain a major source of novel drug discovery and development and have contributed greatly to human health 6. Much is known about their prophylactic and therapeutic benefits in alleviating acute and chronic ailments given their long-standing use in many cultures 7, 8. In the recent decade, given the increase in the incidence of some diseases such as cancer and resistance of most infections to antibiotics, thought to be associated with modern lifestyle and consumption of synthetic products, there has been a global move to revert back to nature via ‘organics’. Also, medicinal plant use has moved from fringe to mainstream use due to the increased efficacy of drugs derived from plants, growing interest in natural products, and rising concerns about the adverse effect of western medicine 9. Eucalyptus citriodora Hook, commonly known as lemon-scented gum, is a species of tall tree of the family Myrtaceae that is endemic to north-eastern Australia. It has smooth white to pink bark, narrow lane-shaped to curved adult leaves, flower buds in groups of three white flowers, and urn-shaped or barrel-shaped fruit. Lemon-scented gum consists of essential oil, mainly citronellal (80%). Unrefined oil from the lemon eucalyptus tree is used in insect repellents, especially against mosquitoes.
A study comparing mosquito repellents found that products using the oil of lemon eucalyptus were effective at driving mosquitoes away from a human hand 10. Research done by 11, on ‘Antibacterial and preliminary phytochemical analysis of Eucalyptus citriodora leaf showed that maximum extract was obtained using methanol; tannin and flavonoid were present while steroids and glycosides were absent; and the susceptible bacteria among those tested (Pseudomonas pseudoalcaligenes, Proteus vulgaris, Citobacterfreundii, Staphlococcus sublara, Bacillus megaterium and Enterobacter aerogenes) was C. freundii 11. Findings from work done by 12, on the ‘Antimicrobial activity of Eucalyptus citriodora essential oil’ showed that it may provide a valuable antimicrobial agent for drug-resistant infections. Analgesic, anti-inflammatory, and anti-microbial properties of eucalyptus citriodora have been reported from different parts of the world. Still, till the time of the research, no scientific work has been done to validate the claim of its use in folk medicine for RTIs.
The oil in the eucalyptus also plays a role in treating respiratory tract infections (RTIs) 13. Based on WHO data, Lower respiratory tract infection and chronic obstructive pulmonary disease (COPD) have remained the top major killers during the past decade. Although WHO has a well-organized global vaccine action plan against most bacteria or viruses causing respiratory tract infections, many people suffer from Influenza, pneumonia, or tuberculosis.
Without proper treatment, these diseases can kill many people worldwide. Essential oils may possess a preventive role in the treatment of RTIs. The application of essential oils via inhalation seems to be the most effective way to cure patients because of their volatile nature; they can reach the site intended to be treated 14.
Eucalyptus oil primarily includes treating cough, cold, bronchitis, and symptomatic relief of colds and catarrh of the upper respiratory tract. For inhalation, 12 drops per 150 ml of boiling water or a 1.5% V/V solution prepared from 1 tablespoon (15 ml) per litre of warm water can be applied, and the treatment may be repeated up to three times daily 15. Infections of the respiratory tract can be categorized into upper and lower respiratory tract infections. Upper respiratory tract infections (URTI) include laryngotracheobronchitis, epiglottitis, pharyngitis, peritonsillar abscesses and rhinitis. Although most URTIs are caused by viruses, including the novel coronavirus (SARS-Cov2), other pathogens, such as bacteria, can also be involved in these diseases. The most important diseases of the lower respiratory tract are acute or chronic bronchitis, bronchiolitis, and pneumonia. Different species of viruses and bacteria 16 as well cause these.
Scientific evidence has proved that microbial co-infection escalates the risk of disease severity in human beings 17. It has been found that, among other viral pneumonitis epidemics such as MERS and SARS, there were increased secondary bacterial and fungal infections associated with poor patient clinical outcomes. The pathogens implicated in the secondary infections were particularly Streptococcus pneumonia, Haemophilus influenza, Staphylococcus aureus and Aspergillus spp 18. In research carried out in Wuhan China by 17 on co-infection with respiratory pathogens among COVID-19 cases, a total of 24 out of 39 respiratory pathogens were detected among 242 patients out of 257 COVID-19 patients involved. Bacteria co-infection was noted to be dominant (96.2%), followed by bacterial-viral co-infection (33.3%), then viral-fungal and viral-bacterial-fungal co-infections (each 14.1%) in all the patients. The most common pathogens in descending order are Streptococcus pneumonia, Klebsiella pneumonia (KP), Haemophilus influenza (HI), Aspergillus, E. coli, Staphylococcus aureus, human rhinovirus (HRV), Pseudomonas aeruginosa, Moraxella catarrhalis (MC), human adenovirus (HAdV), Acinetobacter baumannii, Herpes simplex virus (HSV) and Chlamydia pneumonia (CP). The research on the co-infection rate between SARS-CoV-2 and other respiratory pathogens in northern California by 19 showed a higher rate of co-infection between SARS-CoV-2 and other respiratory pathogens with a human respiratory syncytial virus (RSV), HRV and non-SARS-CoV-2-Coronaviridae being more common out of 9 respiratory pathogens tested. There was a definitive conclusion that other co-infections caused by a respiratory pathogen cannot be ruled out by diagnosing SARS-CoV-2, nor can diagnosing non-SARS-CoV-2 rule out COVID-19 infection 19, 20.
The result of the respiratory pathogens detected by 20 in their study was similar to those obtained by 17. The bacteria co-infection in COVID-19 patients included S. pneumonia, S. aureus, KP, CP, Acinetobacter baumannii, also Legionella pneumonia and Mycoplasma pneumonia. The fungal co-infection pathogens detected are Candida species and Aspergillus flavus. At the same time, the viruses included Influenza, coronavirus, HIV, HRV, enterovirus, parainfluenza and meta pneumonia virus 18 have also demonstrated that there is bacteria co-infection in hospitalized confirmed COVID-19 patients which was more as the disease progressed 18. Also, 88.46% of the COVID-19 patients in Qingdao, China, tested positive for respiratory pathogen serology test conducted by 21 and the most common pathogens detected included IFV-A(73.08%), IFV-B (73.08%), MP (46.15%) and LP (42.31%).
69.23% of the patients have co-infection with at least two viral or bacterial pathogens. Due to the rapid spread and mortality rate of the novel coronavirus (Covid–19) pandemic, it has become necessary to continuously discover new drugs and alternative treatments from medicinal plants that can be used to manage the infection. Claims exist in social media that the leave of eucalyptus is taken as tea or infusion by Covid-19 patients as an alternative medicine to clear the airway. Yet, no scientific evidence exists to support or debunk this claim. Thus, the need for this research. This study aimed to evaluate the in-vitro antimicrobial effect of Eucalyptus citriodora leaf essential oil and extracts against selected pathogens implicated in respiratory tract infections as co-morbidity of the COVID-19 pandemic.
MATERIALS AND METHODS:
Plant Material: The leaves of eucalyptus citriodora were collected from NIPRD garden Abuja. The leaves were dried for 5 days at ambient temperature (25 - 30 oC).
Isolation of Essential Oils: The dried leaves of Eucalyptus citriodora were chopped into small pieces and hydrodistilled for four hours using Clevenger-type apparatus. The essential oil was collected and dried with anhydrous sodium sulphate and used within four hours for analysis. The aqueous extracts were obtained by hot infusion and decoction.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis: The essential oil was analyzed by GC-MS using Shimadzu QP-2010 GC with QP-2010 mass selective detector [MSD, operated in the EI mode (electron energy = 70 eV), scan range = 45-400 amu and scan rate = 3.99 scans/sec] and Shimadzu GCMS solution data system. The GC column was HP-5MS fused silica capillary with a (5% phenyl)-polymethylsiloxane stationary phase, length 30 m, internal diameter 0.25 mm, and film thickness 0.25 μm.
The carrier gas was helium with a flow rate of 1.61 ml/min. The program used for GC oven temperature was isothermal at 60 oC, followed by 60-180 oC at a rate of 10 oC/min, then held at 180 oC for 2 min followed by 180-280 oC at a rate of 15 oC/min, then again held at 280 oC for 4 minutes. The injection port temperature was 250 oC.
The ionization of sample components was performed in the E.I. mode (70eV). The injector temperature was 250 oC while the detector temperature was 280 oC. Helium was used as carrier gas at a flow rate of 1.61 ml/min. 1.0 µl of the diluted sample (1:100 in hexane, v/v) was injected using an autosampler and in the split mode. The split ratio was 10:90.
High-Performance Liquid Chromatography (HPLC) Analysis: The bioactive constituents of the aqueous extracts were analyzed by high-performance liquid chromatography. The HPLC consisted of Ultra-Fast LC-20AB equipped with SIL-20AC auto-sampler; DGU-20A3 degasser; SPD-M20A UV-diode array detector (UV-DAD) 180 – 800 nm wavelength; column oven CTO-20AC, system controller CBM-20Alite and Windows LC solution software (Shimadzu Corporation, Kyoto Japan); column, 5µm VP-ODS C18 and dimensions (4.6 x 150 mm). The chromatographic conditions included mobile phase: 0.2% v/v formic acid and acetonitrile (20:80); isocratic mode; flow rate 0.6 ml/min; injection volume 10 µl of 50 mg/ml solution of extract in water; detection wavelength was set to 254 nm. The HPLC operating conditions were programmed to give solvent B: 20%. The column oven temperature was 40 oC.
The total run time was 15 minutes. Flavonoid and phenolic acid standards such as rutin, caffeic acid, ferulic acid and chlorogenic acid were employed to identify the bioactive constituents of the extracts by comparing their retention time under similar experimental conditions 22.
Media: Muller Hinton agar (Oxoid Ltd, UK), Muller Hinton broth (Oxoid Ltd, UK), Ciprofloxacin disc 5 ug (Oxoid Ltd, UK).
Microorganisms: Typed isolates of Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Salmonella paratyphi ATCC 9150, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 10231; clinical isolates of Streptococcus pneumonia, Klebsiella pneumonia
Antimicrobial Susceptibility Tests: The cup plate agar diffusion method was adopted two-fold serial dilutions of the herbal suspension were made using sterile distilled water to achieve 100, 50 and 25 mg/mL concentrations.
A hundred microliters of the different concentrations were dispensed into bored holes on mullerhinton agar seeded with the test microorganisms and incubated at 35 -37 oC for 24 hours. Ciprofloxacin was used as a positive control, while sterile distilled water served as a negative control. The zones of inhibition were measured using a meter rule. The test was done in duplicate.
Minimum Inhibitory Concentrations (MIC) Determination: Micro broth dilution method was employed 12. Simply, 100 µL of the stock concentration of the sample (dependent on the outcome of the susceptibility study) was dispensed in the first row of the 96 well micro titre plate, followed by 50 µL of MHB into the remaining wells. Two for serial dilution of the samples will be done with the last row left as organism viability control.
Fifty microliters of suspension of microorganisms standardized to 105 cfu were then added to each well. The plate will be swirled for few seconds and incubated at 37 oC for 24 h. Twenty microliters of tetrazolium dye will be added to each hole.
The presence of purple coloration due to formazan production will be interpreted as the presence of microorganism. The MIC will be taken as the lowest concentration showing no dye reaction.
RESULTS AND DISCUSSION: The results revealed that all the extracts tested possessed antimicrobial activity, which was concentration dependent with diameter zones of inhibition ranging from 10 to 30 mm. The oil was shown to have slightly better antimicrobial activity, especially against Gram-positive organisms. The decoction showed stronger activity than the hot infusion.
This could be linked to the method of extraction as the decoction could have extracted out the extract's active components more than the infusion. Eucalyptus citriodora leaf essential oil contained citronellal and eucalyptol predominantly.
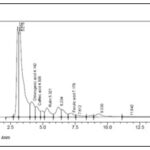
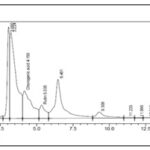
Eucalyptus citriodora leaf hot water infusion contained chlorogenic acid, caffeic acid, rutin and ferulic acid Fig. 2. Eucalyptus citriodora leaf decoction contained chlorogenic acid and rutin Fig. 3.
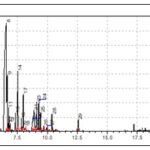
FIG. 1: GC-MS CHROMATOGRAM OF EUCALYPTUS CITRIODORA LEAF ESSENTIAL OIL
Fig. 1 showed that the major chromatogram peaks of Eucalyptus citriodora leaf essential oil are 8: citronellal (47%), 9: isopulegol (8%), 14: citronellol (8%), 17: tetradecan-3-ol (5%), 21: citronellic acid (4%), 23: p-methane-3,8-diol = 4%, 24: citronellylacetae (3%).
FIG. 2: HPLC CHROMATOGRAM OF EUCALYPTUS CITRIODORA LEAF INFUSION
FIG. 3: HPLC CHROMATOGRAM OF EUCALYPTUS CITRIODORA LEAF DECOCTION
TABLE 1: ANTIMICROBIAL SUSCEPTIBILITY OF EUCALYPTUS CITRIODORA EXTRACTS
| Organisms | Zone of Inhibition (mm) | |||||||
| Essential oil | Infusion | Decoction | Ciprofloxacin | |||||
| 50mg/ml | 25mg/ml | 50mg/ml | 25mg/ml | 50mg/ml | 25mg/ml | 5 μg/ml | ||
| S. aureus | 30.0 | 20.0 | 23.0 | 18.0 | 25.0 | 20.0 | 30.0 | |
| P. aeruginosa | 25.0 | 20.0 | 30.0 | 21.0 | 30.0 | 18.0 | 35.0 | |
| B. subtilis | 25.0 | 19.0 | 20.0 | 15.0 | 25.0 | 19.0 | 30.0 | |
| S. paratyphi | 22.0 | 18.0 | 21.0 | 15.0 | 25.0 | 19.0 | 30.0 | |
| E. coli | 24.0 | 17.0 | 14.0 | 10.0 | 15.0 | 12.0 | 30.0 | |
| K. pneumonia | 20.0 | 15.0 | 18.0 | 12.0 | 18.0 | 12.0 | 30.0 | |
TABLE 2: MINIMUM INHIBITORY CONCENTRATION (MIC) OF EUCALYPTUS CITRIODORA EXTRACTS
| Organisms | Minimum inhibitory Concentration(mg/ml) | |||
| Essential oil | Infusion | Decoction | Ciprofloxacin | |
| S. aureus | 12.0 | 25 | 12 | 0.016 |
| P. aeruginosa | 12.0 | 25 | 25 | 0.032 |
| B. subtilis | 25.0 | 25 | 25 | 0.016 |
| S. paratyphi | 6.25 | 12 | 6.25 | 0.016 |
| E. coli | 6.25 | 12 | 12 | 0.016 |
| K. pneumonia | 6.25 | 12 | 6.25 | 0.016 |
The HPLC chromatogram of Eucalyptus citriodora leaf extracts Fig. 1 & 2 showed the presence of chlorogenic acid, caffeic acid, ferulic acid, and rutin, which are reputable bioactive phenolic acids and flavonoids with strong antioxidant properties, amongst others.
From Table 2, the results of the extracts' minimum inhibitory concentration (MIC) ranged between 6.25 – 25 mg/ml. The essential oil showed a higher inhibitory action on the test organisms than the aqueous extracts, which was in tandem with the susceptibility test. The antimicrobial activity of the essential oil in Table 1 agrees with reports by several studies 11, 13 which documented the inhibitory activities of Eucalyptus oil on respiratory tract infections.
CONCLUSION: From the results, it can be concluded that the essential oil of E. citriodora leaf and aqueous extracts possess antimicrobial activity, which justifies using E. citriodora leaf in folklore as remedies for the management of respiratory tract infections. It can also serve as a remedy in the management of respiratory infections.
ACKNOWLEDGEMENT: The authors wish to thank the Department of Medicinal Plant Research and Traditional Medicine, National Institute for Pharmaceutical Research and Development, Idu Industrial Area, Abuja, Nigeria, for providing technical support for this study.
CONFLICTS OF INTEREST: We declare that we have no conflict of interest.
REFERENCES:
- Johns Hopkins Coronavirus Resource Center, 2021. COVID-19 United States cases by county. Johns Hopkins University.
- Boakye CA and Sakuya OK: WHO support scientifically proven traditional medicine. WHO Regional Office for Africa 2020; 4(5).
- Yang Y, Islam S, Jin W, Yuan L and Xin C: Traditional Chinese Medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. International Journal of Biological Sciences 2020; 16(10): 1708-1717.
- Yeming W, Dingyu Z, Guanhua D, Ronghui D, Jianping Z and Yang J: Remdesivir in adults with severe COVIC-19: a randomized, double-blind, placebo-controlled, multicenter trial. Lancet Journal 2020; 395(10236): 1569-1578.
- Akinyemi O, Oyewole SO and Jimoh KA: Medicinal plants and sustainable human health: a review. Horticulture International Journal 2018; 2 (4): 194-195.
- Aboh MI, Olayinka BO, Adeshina GO and Ibrahim K: Preliminary studies on antifungal activities of the successive extracts of Mitracarpus villosus (Sw.) Dc aerial parts obtained in Abuja, Nigeria. Malaysian Journal of Microbiology 2014; 10(2): 133-138.
- Suroowan S and Muhomoodally MF: Herbal medicine of the 21st century: a focus on the Chemistry, pharmacokinetics and toxicity of five widely advocated phytotherapies. Journal of Current topics in Medicinal Chemistry 2019; 19(29): 2718-2738.
- Chikezie PC and Ojiako OA: Herbal Medicine: Yesterday, Today and Tomorrow. Alternative and Integrative Medicine 2015; 4(3): 195.
- Dubey NK, Rajesh K and Pramila T: Global promotion of herbal medicine: Indian opportunity. Current Science 2003; 86(1): 37-41.
- Stacy DR, Lisa LD, David PP, John IH and Immo A: The efficacy of some commercially available insect repellents for Aedes aegypti and Aedes albopictus. Journal of Insect Science 2015; 15(1): 140.
- Vghasiya Y, Nair R and Chanda S: Antibacterial and preliminary phytochemical and physico-chemical analysis of Eucalyptus citriodora Hook leaf. National Product Research Journal 2008; 22(9): 754-762.
- Gyorgyi H and Kamilla A: Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: a review. Flavour and Fragrance Journal 2015; 30: 331-341.
- Harris B: In Handbook of Essential Oils. Science, Technology and Application, K. H. Can Baser, G. Buchbauer (eds). CRC Press, Taylor & Francis Group: New York 2010; 315-351.
- ESCOP Monographs. The Scientific Foundation for Herbal Medicinal Products, 2nd edn. Thieme, Stuttgart: New York 2003.
- Forbes BA, Sahm DF and Weissfeld AS: Bailey and Scott’s Diagnostic Microbiology, 12th edition. Mosby Elsevier: St. Louis 2007.
- Xiaojuan Z, Yiyue G, Tao W, Kangchen Z, Win C and Bin W: Co-infection with respiratory pathogens among COVID-19 cases. Journal of Elsevier Public Health Emergency Collection 2020; 285.
- Stephen H, Luke SM, Oliver T, Hugo D and Nabeela M: Bacterial and fungal co-infection among hospitalized patients with COVID-19: A retrospective cohort study in a UK secondary care setting. Journal of Microbiology and Infection 2020; 26(10): 1395-1399.
- David K, James Q and Benjamin P: Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. Journal of American Medical Association 2020; 325(20): 2085-2086.
- Chih-Cheng L and Po-Ren H: Co-infections among patients with COVID-19: The need for combination therapy with non-anti-SARS-CoV-2 agents. Journal of Microbiology, Immunology and Infection 2020; 53(4): 505-512.
- Xing Q, Li G, Xing Y, Chen T, Li W and Ni W: Precautions are needed for COVID-19 patients with co-infection of common respiratory pathogen. Diagn Microbiol Infect Dis 2020; 98(4): 115199.
- Krishna Murthy TP and Manohar B: Optimization of supercritical carbon dioxideextraction of phenolic compounds from mango ginger rhizome (Curcuma amada Roxb.) using response surface methodology. Biomedicine and Biotechnology 2014; 2(1): 14-19.
- Luqman S, Dioivedi GR, Darokar MP and Khanuga SPS: Antimicrobial activity of eucalyptus citriodora essential oil. International Journal of Essential oil Therapeutics 2008; 2(1): 69-75.
How to cite this article:
Okhale SE, Oladosu PO, Aboh MI, Imoisi C and Gana JJ: In-vitro evaluation of Eucalyptus citriodora leaf essential oil and extracts on selected pathogens implicated in respiratory tract infections. Int J Pharmacognosy 2022; 9(12): 195-01. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.9(12).195-01.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
2
195-201
528 KB
866
English
IJP
Samuel Ehiabhi Okhale *, Peters O. Oladosu, Mercy I. Aboh, Chinyere Imoisi and Josiah James Gana
Department of Medicinal Plant Research & Traditional Medicine , National Institute for Pharmaceutical Research & Development, P.M.B. 21, Garki, Abuja, Nigeria.
samuelokhale@gmail.com
01 August 2022
11 December 2022
12 December 2022
10.13040/IJPSR.0975-8232.IJP.9(12).195-01
31 December 2022