IN-VITRO EVALUATION OF ANTICONVULSANT ACTIVITY OF RAPHANUS SATIVUS
HTML Full TextIN-VITRO EVALUATION OF ANTICONVULSANT ACTIVITY OF RAPHANUS SATIVUS
Anu Malik Yadav *, Nand Lal Singh, Vihangesh Dixit, Shashi Alok and Nitika Singh
Department of Pharmacy, Bundelkhand University Jhansi - 284128, Uttar Pradesh, India.
ABSTRACT: The literature survey reveals that the plant Raphanus sativus belongs to the family Cruciferae. It is widely used to treat the viral, bacterial infection, antihepatotoxicity anti lithiasis, Hypolipidaemic, inflammation, and cancer. The study focused on the phytochemical screening of the ethanolic extract of radish bulbs (Raphanus sativus). The molecular masses to provide probable structures and associated molecular properties for its constituents. Alkaloids, flavonoids, saponins, and tannins were analyzed. The following research shows the anticonvulsant activity of R. sativus. Anxiety is an overwhelming sense of uneasiness or discomfort. We have important plants reported anxiolytic activity, uses, models and doses for anxiolytic activity & results.
| Keywords: |
Anxiety, Anxiolytic, Cruciferae, Alkaloids, Saponins, Anticonvulsant, Raphanus Sativus, Flavonoids
INTRODUCTION: Radish, Raphanus sativus (Cruciferae Family) commonly known as muli is a common pungent ingredient used in various abdominal disorders. Almost all parts of the plant including leaves, seeds, and roots are utilized in medicine 1-3. The fresh juice obtained from leaves are diuretic, laxative, roots are used for urinary complaints, hemorrhoids, gastrodynia pains, and various gastric ailments. Radishes have been used as medicinal foods for a variety of ailments including liver dysfunction and poor digestion. Radish extracts have biological activity including antioxidant, antimutagenic and antiproliferative effects. Radishes are rich in ascorbic acid, folic acid, and potassium. They are a good source of vitamin B6, riboflavin, magnesium, copper and calcium 4-7.
They are low in saturated fat and are very low in Cholesterol. One cup of sliced red radish bulbs (116 g) provides approximately 20 cal, largely from carbohydrates. The present study deals with the anticonvulsant effect of Radish ethanolic extract on MES models in rats.
MATERIAL AND METHOD: The fresh radish was purchased from the local vegetable market and authenticated by NVRI a voucher specimen (21950) is deposited at the herbarium for reference 8-10.
Preparation of Extract: The R. sativus were cutting and air dried in the shade. Further coarsely made to prevent the loss of active phytoconstituents, samples were kept under constant observation to avoid any fungal growth.
Extract Preparation: Extracts of R. sativus niger root peel sample were prepared using organic solvents ethanol. The coarse powder root was packed in the Soxhlet apparatus & continuously extracted with ethanol at temp. at 60-80 °C till all the constituents were separated. The success of the extraction with ethanol is directly related to the extent that yellowish colors were removed into the solvent. When the tissues on repeated extraction were completely free of brown, it can be assumed that all the low molecular weight compounds have been extracted.
Drugs and Chemical: Phenytoin (Sain medicaments Pvt. Ltd., Hyderabad) and Polyethylene glycol.
Phytochemical Studies: Preliminary Phytochemical studies revealed the presence of Ethanolic extract of the Raphanus sativus Linn. root shows the presence of carbohydrates, proteins, amino acids, phenolic compounds, flavonoids, glycosides, alkaloids, anthraquinone glycosides, and cardiac glycosides.
Thin Layer Chromatography: The TLC studies of ethanolic extract of R. sativus. Shows 6 spots of same colors, with different Rf value in solvent system Benzene: Ethyl acetate: Formic acid (9:1:1) carried out in the lab. It indicates the presence of seven different components in the extracts. Detecting reagent used is 0.5% vanillin in dil. H2SO4 and iodine chamber for the better isolation & identification of the different components of an ethanolic extract of R. sativus Linn.11
HPTLC: The HPTLC of ethanolic extract of R.sativus Linn. was also carried out at PG Tech Private Ltd. Indore. The report of HPTLC indicates the presence of 9 spots with different Rf values which shows to occupy the different area with different Rf values. It shows that the compounds contain about 9 constituents 12.
Animals: Swiss albino mice (25-30 gm) of either sex (bred in D.R.D.O Gwalior, M.P.) were used. The animals were obtained from animal house of the Institute of Pharmacy, Bundelkhand University, Jhansi; India. The animals were housed in standard cages with free access to food (standard laboratory rodent’s chow) and water. The animal’s house temperature was maintained at 23 ± 3.0°C with a 12 h light / dark cycle. All the experimental procedures and protocols used in this study were reviewed by the Institutional Animal Ethics Committee (IAEC) of the Institute with reference no. BU / Pharm / IAEC / 09 / 014 (approved by CPCSEA Regd No. 718 / 02 / a / CPCSEA) 13-15.
Acute Toxicity Test: An acute toxicity study was performed for the extract according to the acute toxic classic method as per OECD guidelines (OECD Guidelines for the testing of chemicals, 2001). Swiss albino mice of either sex were used for acute toxicity study. The method of Up and Down was used to determine the dose. The animals were kept fasting for overnight providing only water, after which the extracts were, administered orally 100, 300, 500, mg/kg dose and percent mortality was observed 24 h then 72 h and after that once daily for 14 days. The ethanolic extract of root of Raphanus sativus (Linn.) did not cause any mortality up to 5000 mg/kg during the observation period of 24 hrs then 72 h and after that once daily for 14 days and was considered as safe. The oral LD50 of the ethanolic extract estimated in mice must be > 5000 mg/kg.
Assessment of Anticonvulsant Activity:
Maximal Electro Shock Model: For the assessment of anticonvulsant activity, the animals were divided into five groups of five animals each Swiss albino mice.
Group I received Normal saline.
Group II received Phenytoin.
Group III received 100 mg/kg of RSEE suspended in polyethylene glycol.
Group IV received 300 mg/kg of RSEE suspended in polyethylene glycol.
Group V received 500 mg/kg of RSEE suspended in polyethylene glycol Corneal electrodes were used for bilateral delivery of electrical stimulus. Electroconvulsive shock (50 mA for 0.2 sec) was delivered through the corneal electrode to induce Hind Limb Tonic Extensor (HLTE) phase in mice. There are five phases observed in mice after giving maximal electroshock. The five steps are
- Flexor
- Extensor
- Convulsion
- Stupor and
- Recovery or Death are noted and also the time spent by mice in each phase. Before delivery, the current output was checked by using a multimeter. The electrical stimulus was applied using a stimulator apparatus. (Biocraft Scientific System Pvt. Ltd., Agra, India) for five groups of five animals each. The orientation for the anticonvulsant effect was the abolition of HLTE within 10 sec. After delivery of the electroshock 16-20.
TABLE 1: ASSESSMENT FOR ANTICONVULSANT ACTIVITY OF CONTROL BY MES MODEL
| Treatment | Body Wt. (gm) | Dose
|
Duration (Sec.) in various phases of Convulsion | ||||
|
Control |
Flexor | HLTE | Convulsion | Stopper | R/D | ||
| 30 |
Normal Saline (10ml/kg) |
4.18 | 14.90 | 21.10 | A | D | |
| 25 | 4.85 | 14.30 | 20.10 | A | D | ||
| 30 | 4.55 | 14.08 | 24.55 | 33.05 | R | ||
| 28 | 4.18 | 18.50 | A | A | D | ||
| 28 | 4.49 | 15.75 | A | A | D | ||
R = Recovery, D = Death, A = Absent
TABLE 2: ASSESSMENT FOR ANTICONVULSANT ACTIVITY OF STANDARD DRUG (PHENYTOIN) BY MES MODEL
| Treatment | Body Wt. (gm) | Dose
|
Duration (Sec.) in various phases of Convulsion | ||||
|
Phenytoin |
Flexor | HLTE | Convulsion | Stupper | R/D | ||
| 30 |
25 mg/kg |
2.15 | 3.20 | 10.24 | 33.98 | R | |
| 28 | 2.50 | 3.75 | 10.28 | 34.05 | R | ||
| 28 | 2.78 | 3.10 | 10.59 | 37.75 | R | ||
| 25 | 2.12 | 3.45 | 10.84 | 39.19 | R | ||
| 28 | 2.42 | 3.95 | 11.41 | 23.53 | R | ||
R = Recovery, D = Death, A = Absents
TABLE 3: ASSESSMENT FOR ANTICONVULSANT ACTIVITY OF RAPHANUS SATIVUS ETHANOLIC EXTRACT BY MES MODEL
| Treatment | Body Wt.
(gm) |
Duration (Sec.) in various phases of Convulsion | ||||
|
RSEE (100 mg/kg) |
Flexor | HLTE | Convulsion | Stupper | R/D | |
| 28 | 1.58 | 10.49 | A | A | D | |
| 30 | 2.54 | 8.97 | 18.44 | 20.08 | R | |
| 30 | 2.21 | 7.8 | 35.22 | 38.05 | R | |
| 25 | 3.27 | 11.07 | 20.39 | A | R | |
| 28 | 2.37 | 10.18 | 17.39 | 35.11 | R | |
|
RSEE (300 mg/kg) |
28 | 2.28 | 8.93 | 19.43 | 33.90 | R |
| 25 | 2.58 | 8.95 | 22.95 | 31.30 | R | |
| 28 | 2.29 | 9.38 | 25.58 | 35.49 | R | |
| 28 | 2.83 | 8.59 | 21.34 | 28.40 | R | |
| 29 | 2.83 | 7.22 | A | A | D | |
|
RSEE (500 mg/kg) |
27 | 1.58 | 4.75 | 33.90 | 20.41 | R |
| 30 | 2.54 | 3.35 | 31.30 | 22.55 | R | |
| 27 | 2.21 | 3.80 | 25.58 | 24.51 | R | |
| 28 | 3.27 | 5.74 | 22.59 | A | R | |
| 28 | 2.37 | 4.41 | A | A | R | |
R = Recovery, D = Death, A = Absents
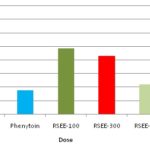
TABLE 4: EFFECT OF RAPHANUS SATIVUS EXTRACT ON MES INDUCED SEIZURES IN MICE
| S. No. | Treatment | Duration of HLTE | Mortality (%) | Recovery (%) |
| 1. | Control | 15.22 ± 0.45 | 80 | 20 |
| 2. | Phenytoin | 3.50 ± 0.15 | 0 | 100*** |
| 3. | RSEE-100 | 9.70 ± 0.58 | 20 | 80** |
| 4. | RSEE-300 | 8.81 ± 0.38 | 20 | 80** |
| 5. | RSEE-500 | 4.37 ± 0.42 | 0 | 100*** |
Values are mean ± SEM where ** P<0.01, *** P<0.001
FIG. 1: ANTICONVULSANT ACTIVITY OF RAPHANUS SATIVUS ON MAXIMAL ELECTROSHOCK (MES) INDUCED SEIZURES IN MICE.
Statistical Analysis: The results are expressed in mean ± S.E.M. (n=5) Statistical analysis was done by one way ANOVA, followed by Dunnett multiple comparison tests vs. control. P<0.05 was considered as statistically significant.
RESULT AND DISCUSSION: Ethanolic extract of Raphanus sativus Linn. in experimentally induced convulsion in rats. An acute toxicity study was performed for the extract according to the acute toxic classic method as per OECD guidelines. Swiss albino rats of either sex were used for acute toxicity study. The method of Up and Down was used to determine the dose. The animals were kept fasting for overnight providing only water, after which the extracts were, administered orally 100, 300, 500, 2000, 5000 mg/kg dose and percent mortality was observed 24 h then 72 h and after that once daily for 14 days.
The ethanolic extract of roots of R. sativus Linn. Did not cause any mortality up to 5000 mg/kg during the observation period of 24 hrs then 72 hrs and after that once daily for 14 days and were considered as safe. The oral LD50 of the ethanolic extract estimated in mice must be > 5000 mg/kg. Ethanolic extracts of Raphanus sativus Linn. Significantly reduced the elevated level of convulsion. The histopathological findings also show sign of improvement after treatment with extract. All these observations provided the basis for the conclusion that Raphanus sativus Linn L. root extract inhibit the convulsion induced by phenytoin treatment.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Alqasoumi, Saleh, Mohammed Al-Yahya, Tawfeq, Al-Howirini, Rafatulla: Gastroprotective Effect Of Radish “Raphanus Sativus” on Experimental Gastric Ulcer Models In Rats. Pharmacia 2008; VI(2): 204-214.
- Cassady J and Douros J: Anticancer agents based on natural products models. Academic Press, New York 1980; 94.
- Chaturvedi P and Akala H: effect of raphanus sativus root extract on glucose level in normal and diabetic rats. Journal of Applied Zoology Research 2001; 12: 172-177.
- Gurdeep RC and Sham KA: Instrumental methods of Chemical Analysis (Analytical Chemistry). Himalaya Publishing House Pvt. Ltd. Fifth Revised & Enlarged edition 2002; Reprint in 2008: 2.599-2.600.
- Franky RG: In-vitro antifungal activity of a radish (Raphanus sativus ) seed protein homologous to nonspecific lipid transfer proteins. Plant Physiol 1992: 100: 1055-1058.
- Gilani AH and Ghayur MN: Pharmacological basis for the gut stimulatory activity of Raphanus sativus J Ethnopharmacol 2004; 95: 169-172
- Goldman SA and Kennedy DL: FDA’s Medical Products Reporting program. A joint effort toward improved public health. Postgr. Med 1998; 103: 13-16.
- Gutierrez RM and Perez RL: Raphanus sativus (radish): their chemistry and biology. Sci World J 2004; 4: 811-837.
- Castro-Torres IG, Naranjo-Rodr´ıguez EB, Domı´nguez-Ortı´z MA, Gallegos-Estudillo J and Virginia Saavedra-V´elez M: Antilithiasic and Hypolipidaemic effect of Raphanus sativus Var. Niger on mice fed with lithogenic diet. Journal of Biomedicine and Biotechnology 2012; 1-8.
- Kasture AV, Mahadik KR, Wadodkar SG and More HN: Pharmaceutical Analysis (Instrumental Methods). Nirali Prakashan 2006; 15(2): 10-17.
- Kumar SK, Rao KS, Sridhar Y and Shankaraiah P: Effect of Raphanus sativus against gentamicin induced nephrotoxicity in rats. Journal of Advanced Pharmaceutical Sciences 2013; 3: 355-365.
- Kokate CK, Purohit AP and Gokhale SB: Pharmacognosy, Sixth edition 2010: 1.2-1.5.
- Kritikar RK and Basu BD: Indian Medicinal Plant.Vol.1. Ed. 2nd edition, Indian: International Book Distributor,9/B, Rajpur Road, Deharadun 1987; 1: 178.
- Kumar KA, Narayani M, Subanthini A and Jayakumar M: Antimicrobial activity and phytochemical analysis of citrus fruit peels utilization of fruit waste. Int J Environ Sci Tech, 2011; 3: 5415-5421.
- Meera R, Devi P, Muthumani P and JeyaSundari K: Phyto physic chemical evaluation of leaf of Raphanus sativus international Journal of Biological & Pharmaceutical Research 2010; 1(2): 61-64.
- Mohammed N, Abelgasim AI and Mohammed AH: Protective effect of Raphanus sativus against carbon tetrachloride-induced hepatotoxicity in wistar albino rats. J Pharmacol Toxicol 2008; 3: 272-278.
- =Pulak KM: Evaluation of Indian Traditional medicine,” Drug Information, USA 2001; 35: 631-640.
- Nakamura Y, Nakamura K, Asai Y, Wada T, Tanaka K, Mastuo T, Okamato S, Meijer J, Kitamura Y, Nishikawa A, Park EY, Sato K and Ohtsuki K: Comparison of the glucosinolate-myrosinase system among Daikon (raphanus sativus, Japanese white radish) varieties. J Agr Food Chem 2008; 56: 2702-2707.
- Parades SD: Ethnobotonica Mexicana: Plantas popularmente empleadas tertamiento de enfermedades hepaticas y vesiculares. Tesis Lic .Mexico D.F. Facultad de Ciencias. UNAM 1984.
- Donald LP, Gary ML and George SK: Introduction to spectroscopy a guide for students of organic chemistry. Thomson Learning, Inc. 2001; Third edition.
How to cite this article:
Yadav AM, Singh NL, Dixit V, Alok S and Singh N: In-vitro evaluation of anticonvulsant activity of Raphanus sativus. Int J Pharmacognosy 2015; 2(10): 514-18. doi: 10.13040/IJPSR.0975-8232.2(10).514-18.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
6
514-518
576
2093
English
IJP
A. M. Yadav *, N. L. Singh, V. Dixit, S. Alok and N. Gupta
Department of Pharmacy, Bundelkhand University Jhansi, Uttar Pradesh, India.
anumalik.yadav@gmail.com
25 August 2015
26 September 2015
19 October 2015
10.13040/IJPSR.0975-8232.IJP.2(10).514-18
31 October 2015