IN-VITRO ANTIOXIDANT POTENTIAL, TOTAL PHENOLIC AND FLAVONOID CONTENT OF LAMIUM AMPLEXICAULE LEAF EXTRACT IN AQUA-METHANOL AND AQUA-ACETONE
HTML Full TextIN-VITRO ANTIOXIDANT POTENTIAL, TOTAL PHENOLIC AND FLAVONOID CONTENT OF LAMIUM AMPLEXICAULE LEAF EXTRACT IN AQUA-METHANOL AND AQUA-ACETONE
Gayatri Thakurathi, P. B. Rao and Kamlesh Kumar Bhakuni ⃰
Department of Botany, L. S. M Government Postgraduate College, Soban Singh Jeena University, Almora, Uttarakhand, India.
ABSTRACT: Lamium amplexicaule L. is a genus of the family Lamiaceae. This plant species has been used widely in traditional medicines to treat many disorders. Phytochemical screening (qualitative and quantitative), antioxidant capacity, total phenol, and total flavonoid content were analyzed in a crude extract of leaves. The extract was prepared in aqua-methanol and aqua acetone in 30:70. Antioxidant capacity was analyzed in both extracts through (2,2-diphenyl-1picrylhydrazyl (DPPH) Free Radical Scavenging Assay, FRAP (Ferric Ion Reducing Antioxidant Potential), FCA (Fe2+ Ion Chelating Activity), Phosphomolybdenum assay (TAA), Total Phenol Content (TPC) and Total Flavonoid Content (TFC). Both extracts showed the presence of tannins, alkaloids, flavonoids, proteins, phenols, saponins, terpenoids, carbohydrates, quinones, oils, and resins. The result for antioxidant assay (DPPH, FRAP, FCA and TAA) was comparatively higher in aqua-methanol than in aqua-acetone. TFC values were higher in aqua-acetone, while TPC in aqua-methanol extract. Thus, the plant species can be used as a potent source of ayurvedic medicine.
Keywords: Antioxidant, Lamiaceae, Lamium amplexicaule, Phytochemicals
INTRODUCTION: Plants are used globally to develop novel drugs and treat diseases through research 1. Traditional medicinal plants have acknowledged substantial attention because their bioactive components are responsible for new drug discoveries. The Lamium genus has been experimental in the encounter of natural medicinal products. Lamium amplexicaule L. genus is a perennial herb of family Lamiaceae. The genus Lamium comprises about 40 species distributed in Asia, Africa and Europe 2, 3.
More than 20,000 species of plants are potential reservoirs for new drugs and are used in traditional medicines 4, 5. Members of Lamium are used in folk medicines for the treatment of inflammation, trauma, hypertension, fracture, putrescence, paralysis, leucorrhoea, and many women disorders, such as menorrhagia, uterine haemorrhage, cervical / vaginal inflammations 4, 6, 7.
MATERIALS AND METHOD:
Plant Material: The leaves of the plant material Lamium amplexicaule L. were collected from PantnagarCampus (29⁰ 1′ 27.79″ N latitude and 79⁰ 29′ 22.47″ E longitude), Udham Singh Nagar, Uttarakhand. The plant was identified by Dr. D.S. Rawat, Assistant Professor, Department of Biological Sciences, College of Basic Sciences and Humanities, G. B. P. U. A. & T., Pantnagar. Leaves of the plants were brought to the laboratory, shade dried, grounded, and kept for further experimentation under the laboratory conditions.
Preparation of Extract: The plant material (leaves) was thoroughly washed with tap water to remove dirt and then shade-dried for approximately two weeks. The dried material was grounded by using an electric grinder and stored in sealed jam glass for extract preparation. Dried powder (10 g) of plant sample was mixed in aqua-methanol and aqua-acetone (30:70 v/v) in a conical flask and sealed with paraffin wax and aluminium foil. Plant samples were kept in Remi orbital shaker for 10 days at 150 rpm in triplicates. Extracts were then filtered using Whatmann no. 1 filter paper and supernatant was collected in Petri plates and kept undisturbed for evaporation. Then extracts stored immediately at 4⁰C for further experimentation.
Phytochemical Screening: The phytochemical analysis of selected plant species was performed by using standardized methods proposed by Harbone (1973) 8 and Sofowara (1993) 9. Phytochemical screening was performed to test for both extracts' proteins, carbohydrates, alkaloids, tannins, phenols, oil and resin, saponins, flavonoids, terpenoids, and quinones.
In-vitro Antioxidant Estimation:
DPPH (2,2-diphenyl-1 Picrylhydrazyl) Radical Scavenging Activity: The DPPH (2, 2-diphenyl-1 picrylhydrazyl) assay was used to measure the antioxidant potential of plant material. DPPH radical was assessed in accordance to Brand-Williams et al. (1995) with slight modifications 10.
The antioxidant potential of both extracts was compared with standard, Butylated hydroxytoluene (BHT). 1 ml of leaf extract of different concentrations (40, 80, 120, 160 and 200 µl) was added with 3 ml of DPPH solution, vortexed and then incubated for 60 min in dark. Absorbance of both samples was measured at 517 nm through UV-vis spectrophotometer. The DPPH discolouration percentage was calculated by the following formula:
DPPH inhibition (%) = 1 – At / Ac ×100
Where, At and Ac are the absorbance of the sample and control at 517 nm, respectively.
FRAP (Ferric Ion Reducing Antioxidant Potential): Frap was performed using Benzie and Strain's (1996) method with subtle modifications 11. Fresh FRAP reagent was prepared by mixing acetate buffer, TPTZ, and FeCl3. 6H2O in 10:1:1 (v/v) proportion, respectively and kept at 37 ⁰C. 3 ml reagent was added with 1 ml of BHT and plant leaf sample with varying concentrations (40, 80, 120, 160, and 200 µl) and incubated at 37 ⁰C for 30 min. The absorbance of the increasing blue-colored complex (ferrous tripyridyltriazine complex) was measured at 593 nm. FRAP values of plant samples were expressed as µg Butylated Hydoxytoluene Equivalent (BHTE)/mg extract.
Fe2+ ion Chelating Activity (FCA): Fe2+ chelating potential of plant extracts depended upon the formation of ferrous ion-ferrozine complex and was analyzed by the procedure of Hsu et al. (2003) 12. 1 ml of leaf extract with suitable concentrations of 40, 80, 120, 160 and 200 µl/gm was mixed with 0.1 ml of 2 mMFeCl2. 4H2O and 0.2 ml ferrozine (5 mM) and volume made up to 5 ml with aqua-methanol or aqua-acetone. The reaction mixture is incubated for 10 min and absorbance was read at 562 nm. A decrease in absorbance with increasing concentrations of plant extract indicated their high Fe2+ chelation capacity. FCA (%) was calculated with the following formula:
Chelating activity (%) = 1 – At / Ac ×100
Where, At and Ac are the respective absorbance of the sample and control at 562 nm.
Phosphomolybdenum Assay: Phospho-molybdenum assay was used to assess total antioxidant activity (TAA). The method applied in the present study is of Prieto et al. (1999)with minor modifications13.3 ml of freshly prepared reagent (0.6 M H2SO4+ 4 mM ammonium molybdate + 28 mM monobasic sodium phosphate) was mixed with 1 ml of varying concentrations of 40, 80, 120, 160 and 200 µl of leaf extract and incubated at 95 ⁰C for 90 min. After cooling, absorbance was measured at 695 nm of the resultant green-coloured complex (phosphomolybdenum) against blank.
TAA values were expressed as ascorbic acid equivalent (AAE)/mg weight of the extract.
Quantitative Phytoconstituent Assays:
Total Phenol Content (TPC): The total phenolic content in both aqua-methanol and aqua-acetone leaf extract was analyzed via Folin-Ciocalteu colorimetric method given by Wolfe et al. (2003) with slight modifications 14.
Folin-Ciocalteu reagent (0.2 ml) was mixed with 0.5 ml of varying concentrations of plant leaf extract (40, 80, 120, 160 and 200 µl) and neutralized with 7 % saturated NaCO3 (0.5 ml). The final volume was made up to 3ml with distilled water and vortexed. After 60 min of incubation, the absorbance of the resulting blue-colored complex was measured at 765 nm against blank. TPC values of plant samples were expressed as µg gallic acid equivalent (GAE)/mg extract.
Total Flavonoid Content (TFC): The total flavonoid content (TFC) of leaf extract was analyzed by the method of Djeridane et al. (2006) with required modifications 15. 2 ml of plant leaf extract was mixed with 2 ml of 2 % aluminium chloride and incubated for 60 min at room temperature. The absorbance of the yellow-colored solution was measured at 420 nm against the control. TFC values of plant samples were expressed as µg quercetin equivalent (QE)/mg extract.
RESULT AND DISCUSSION:
Phytoconstituents and Yield: Equal phytochemicals were present in aqua-methanol and aqua-acetone extract. The yield was higher in aqua-acetone Table 1.
TABLE 1: QUALITATIVE ANALYSIS IN AQUA-METHANOL AND AQUA-ACETONE LEAF EXTRACT OF LAMIUM AMPLEXICAULE
| Phytochemicals and Yield
|
Extract | |
| Aqua-methanol | Aqua-acetone | |
| Carbohydrate | + | + |
| Phenols | + | + |
| Flavonoids | + | + |
| Terpenoids | + | + |
| Saponins | - | - |
| Protein | + | + |
| Alkaloids | + | + |
| Tannins | + | + |
| Quinones | + | + |
| Oils and resins | + | + |
| Yield | 10.74 % | 15.39 % |
(+) = present and (-) = absent.
Antioxidant Capacity / Potential:
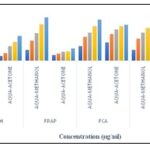
2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Assay: DPPH radical scavenging activity was increased in dose-dependent manner both in aqua-methanol and aqua-acetone leaf extract of L. amplexicaule. In aqua-methanolic extract ranging from 23.74±0.44 to 81.62±3.48 and in aqua-acetone ranging from 08.47±1.57 to 44.42±0.14 at varying concentration (40, 80, 120, 160 and 200) µg/ml Fig. 1. Kumar et al. (2013) 16 in Solanum torvum (23.0±0.8 to 50.6±0.9), Senna auriculata (18.2±0.8 to 55.2±0.3). The mean value of DPPH activity was 54.65 and 25.35 in aqua-methanol and aqua-acetone, respectively. Orhan and Aslan (2009) 17 reported DPPH activity (% inhibition) in three plant species of family Lamiaceae was: Salvia triloba (35.3 ± 1.1), Teucrium polium (22.9 ± 0.3) and Melissa officinalis (25.3 ± 0.9), Elfalleh et al. (2019) 18 in Stachys tmolea (50.88±1.55 mg Trolox equivalent (TE)/g dry plant) indicates that all the above values are more or less closer to the values obtained in the present study in both aqua-methanol and aqua-acetone leaf extracts due to the better extraction and more amount of polyphenols and flavonoids.
FRAP (Ferric Ion Reducing Antioxidant Potential): In the present study, the FRAP values (µg BHTE/mg of extract) or reducing the capacity of leaf extracts increased in a concentration-dependent manner (40 to 200 µg/ml). In aqua-methanolic extract ranging from 19.83±0.88 to 77.16±1.51 and in aqua-acetone ranging from 10.50±0.16 to 22.25±0.27 at varying concentrations (40, 80, 120, 160 and 200) µg/ml Fig. 1. However significantly lower FRAP values were reported by Seal (2012) 19 ranged from 3.19±0.09 in Gynocardia odorata and 7.14±0.18 in Aficus geniculate. The mean value of FRAP was 56.62 and 21.68 in aqua-methanol and aqua-acetone, respectively. In the present investigation, comparatively higher FRAP values were observed in aqua-methanol than in aqua-acetone extracts. The higher ferric ion-reducing power of aqua-methanol extracts may be due to higher polarity and can extract more bioactive components from plant samples than in aqua-acetone Tiwari et al., (2011) 20. FRAP values reported by Tan et al. (2014) 21 in Tradescantia pallida (0.9±0.1), Callisia fragrans (1.5±0.3), Rheo spathacea variegata (1.4±0.1), Rheo bermudensis (2.7±0.3) and Tradescantia zebrina (4.8±0.3) mg GAE/g in methanol; Alam et al. (2014) 22 in different solvents of Portulaca oleracea (7.39±0.08 to 104.2±6.34 mg TE/g DW) and Shanmugapriya et al. (2017) 23 in leaf extract of Gnaphalium polucaulon (0.74), stem (0.67) and flower (0.65) were more or less similar to present investigation.
Fe2+ Chelating Activity (FCA): The chelation capacity (%) in the present study in aqua-methanol and aqua-acetone extract of plant species was elicited at five different concentrations (40, 80, 120, 160, and 200 µg/ml). A dose-response (the chelating activity increased with increasing concentrations) relation was observed in metal chelation capacity in both plant extracts. The Fe2+ chelating activity (%) at varying concentrations (40, 80, 120, 160 and 200 µg/ml) ranged (26.24±3.25 to 72.96±0.67) and (33.03±1.56 to 64.21±1.82) in aqua-methanol and aqua-acetone extracts, respectively Fig. 1.
The mean (mean of all five concentrations) values of Fe2+ chelating activity (%) were 50.69 and 52.04 in aqua-methanol and aqua-acetone, respectively. In earlier studies, more or less similar values were also reported by Elfalleh et al. (2012) 18 in Stachys tmolea (40.58±3.45 a mg TEs/g dry plant ) and (27.90±0.28 b mg TEs/g dry plant) in aqueous and methanol extracts, respectively; Aktumsek et al. (2013) 23 in Centaurea kurdica (57.282±0.722) and C. amanicola (56.566±0.578); Govindan and Muthukrishnan (2013) 24 in Boerhavia erecta inethanol leaf (19.68 to 68.78 %); and Emmanuel et al. (2018) 25 in methanolic leaf extract of Alternanthera brasiliana (92.2±10.19). The variations in the ferrous chelating capacity (%) reported in previous studies compared to the present study may be due to different plant species, varying extraction procedures, experimental conditions, and solvents used.
FIG. 1: DPPH, FRAP, FCA AND TAA IN BOTH AQUA-METHANOL AND AQUA-ACETONE LEAF EXTRACT OF L. AMPLEXICAULE AT VARYING CONCENTRATIONS
Total Antioxidant Capacity: The total antioxidant capacity (µg VCE/mg extract) in aqua-methanol in selected plant species at different concentrations (40, 80, 120, 160 and 200 µg/ml) ranged from 19.36±1.25 to 78.32±1.00 Fig. 1. The mean (mean of all five concentrations) values of total antioxidant activity (µg AE/mg) in the aqua-methanol extract was 48.96. In aqua-acetone extract, the TAA was 9.08±2.77 to 66.67±3.29 while mean value of all concentrations was 37.19. Thus, the TAA value was higher in the aqua-methanol extract than aqua-acetone, which may be due to the more polar nature of the former solvent than the latter.
In earlier investigations, more or less similar TAA values were reported by Ambiga and Jeyaraj (2015) 26 in Ipomoea carnea flower alcoholic extract (15.47mgAAE/g); Saxena and Rao (2018) 27 in Malvastrum coromandelianum in both methanol (69.40±1.18) and acetone (59.89±0.64) leaf extracts. However higher values were reported by Haggag et al. (2011) 28 in Leucenia leucocephala 80 % n-butanol (550.66±7.64 mg AAE/g ext.), ethanol (600.34±4.72 mg AAE/g extract) and methanol (677.98±3.70 mg AAE/g ext.) leaf extracts; and Saeed et al. (2018) 29 in seeds methanol extracts of Chenopodium album (308.75±12.014 µg AAE/ml) and the variation among values may be due to different plant species and different experimental methods.
TABLE 2: IN-VITRO ASSAY OF ANTIOXIDANT CAPACITY IN AQUA-METHANOL AND AQUA-ACETONE LEAF EXTRACT OF L. AMPLEXICAULE
| In-vitro assay | Extracts (mean) | |
| Aquamethanol | Aquaacetone | |
| DPPH (%) | 54.65 | 25.35 |
| FRAP (µg BHTE/mg of extract) | 56.62 | 21.68 |
| FCA (%) | 50.69 | 52.04 |
| Total antioxidant activity (µg VCE/mg extract) | 48.96 | 37.19 |
Quantitative Phytochemical Analysis:
Total Phenol Content: These are low molecular weight secondary metabolites with a hydroxyl group attached to an aromatic hydrocarbon and act as a potential antioxidant, metal ion chelating agents, anti-carcinogenic and anti-mutagenic, etc. (Alam et al., 2014) 22. In the present study, the total phenol content in both aqua-methanol and aqua-acetone was analyzed using the Folin-ciocalteu reagent method, and results were expressed by providing a direct comparison of plant’s activity with standard antioxidant, i.e., gallic acid. The TPC (µg GAE/mg) in plant species at different concentrations showed a dose-response relation, i.e., it increases with increasing extract concentrations (40 to 200 µg/ml) in both aqua-methanol and aqua-acetone.
A dose-dependent relationship was also reported by John et al. (2014) 30 in Baliospermum montanum, Chukrasia tabularis, Atuna indica, and Soymida febrifuga; Chigayo et al. (2016) 31 in Kirkia wilmsiic and Adebiyi et al. (2017) 32 in Grewia carpinofolia. TPC in aqua-methanol extract ranged (from 11.41±1.64 to 35.46±0.64) and aqua-acetone (3.82±0.28 to 11.22±0.23) at different concentrations (40, 80, 120, 160 and 200) ug/ml. mean value for aqua-methanol and aqua-acetone was 27.59 and 7.10, respectively.
More or less similar values were also reported by Ahmed et al. (2013) 33 in O. corniculata leaves 20.91±4.26 (methanol), 28.23±2.08 (hexane), 34.50±2.62 (chloroform) and 49.15±4.45 (water) and Ramalingamet al. (2013) 34 in Leucas lanata (64.412±8.446 mg GAE/g). Gonçalves et al. (2012) 35 in Viola tricolor in butanolic fractions of flower (10.86±0.002 mg GAE/g) fraction and in leaves/roots fractions (6.58±0.011 mg GAE/g fraction); Khodja et al. (2014) 36 in Ajuga iva (26.86 ± 0.84 µg GAE/mg extract), Marrubium vulgare (20.07 ± 0.44 µg GAE/mg extract), Mentha pulegium (72.84 ± 1.46 µg GAE/mg extract) and Teucrium polium (45.65 ± 1.1 µg GAE/mg extract). TPC values were comparatively higher in aqua-methanol than in aqua-acetone, thus making it a better solvent for the extraction of phenolic compounds.
Total Flavonoid Content: Flavonoids are naturally occurring, most prevalent, and ubiquitous polyphenolic compounds formed of a 15-carbon skeleton structure with a heterocyclic and two phenyl rings in all plant tissues. Flavonoids can scavenge free radicals, chelate metal ions such as iron and copper, and inhibit enzymes responsible for free radical generation (Benavente et al., 1997) 37. In the present study, the total flavonoid content in leaf extracts of selected plant species was expressed as mg Quercetin equivalent (QE)/mg extract at 1 mg/ml concentration.
The total flavonoid content (TFC, mg QE/mg extract) was comparatively higher in aqua-acetone (15.62±0.41) than in aqua-methanol (13.08±0.53) at 200ug/ml. It may be due to larger size and somewhat non-polar nature of flavonoids that can be dissolved more frequently in less polar solvents (aqua-acetone). More or less similar values were also reported by Govindan and Muthukrishnan (2013) 24 in Boerhavia erecta (11.6±0.6 mg/mL of CE eq.); Amari et al. (2014) 38 in Thymelaea hirsute (flower, 5.70±0.06); Formisano et al. (2014) 39 in Calamintha origanifolia (26.50±0.07) and Micromeria myrtifolia (13.20±0.04); Merculieff et al. (2014) 40 in Elaeagnus kologa leaf (chloroform, 5.20±0.06 and petroleum ether, 21.19±0.45); Saha and Verma(2016) 41 in Terminalia chebula fruit (7.934 mg QE/g); and Bouterfas et al. (2016) 42 in methanolic extract of Marrubium vulgare (29.5). In the present study, both extracts exhibited the presence of different phytochemicals such as proteins, carbohydrates, phenols, saponins, flavonoids, alkaloids, terpenoids, tannins, quinones, oils, and resins. In-vitro antioxidant assays (DPPH, FRAP, FCA and TAA) were accomplished for both plant extracts, including TPC and TFC. Both aqua-methanol and aqua-acetone extracts exhibited scavenging activity against radicals catalyzed by copper and iron ions 27.
The aqua-methanol solvent was found to be good for the extraction of polyphenols. The plant species showing higher antioxidant activity have higher phenolic content, and the effective quality of flavonoids may be responsible for higher activity. Different experimental procedures, chemical reagents, and solvents used for antioxidant analysis may result in different values compared with previous literature. Further, variations may arise due to phytochemical composition present in various environmental conditions. Plant species can be a potential natural source of antioxidants and can be used as a substitute against synthetic drugs by the pharmaceutical, cosmetic, and nutraceutical industries.
CONCLUSION: The leaf extract of Lamium amplexicaule L. was rich in polyphenols, flavonoids, and other bioactive components and was found to be a potential source of natural antioxidants and capable of preventing degenerative diseases. Aqua-methanol solvent was found good for the extraction of large number of bioactive components as compared to aqua-acetone. Only phenols and flavones are not responsible for good antioxidant activity; other bioactive compounds are also responsible for it. Hence, research needs to be continued to evaluate the significance of other possible components other than polyphenols for better antioxidants. In-vitro analysis of plant leaf extract has little importance if there is no clear evidence of the efficacy of extracts in-vivo. Therefore, more in-vivo investigation of this plant is required before using it as a potential source of medicine in pharmaceuticals.
ACKNOWLEDGEMENT: Authors gratefully acknowledge Director Experimentation, Dean P. G. S, G. B. P. U. A & T, Pantnagar, for their prestigious support and reassurance during the study. We also acknowledge Dr. D. S. Rawat, Department of Biological Sciences, G.B. Pant Univ. of Ag & Tech., for the identification of plant species.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Alipieva KI, Taskova RM, Evstatieva LN, Handjieva NV and Popov SS: Benzoxazinoids and iridoid glucosides from four Lamium species. Phytochemistry 2003; 64: 1413-17.
- Willis JC: A Dictionary of the Flowering Plants and Ferns, Revised by HK Airy Shaw, Edition 1973; 8: 1245.
- Alipieva KI, Taskova RM, Jensen SR and Handjieva NV: Iridoid glucosides from Lamium album and Lamium maculatum (Lamiaceae). Biochemical Systematics and Ecology 2006; 1(34): 88-91.
- Akkol EK, Yalcin FN, Kaya D, CalisI, Yesilada E and Ersoz T: In-vivo anti-inflammatory and antinociceptive actions of some Lamium species. Journal of Ethnopharmacology 2008; 118(1): 166-172.
- Hamamouchi M: Medicinal plants in Morocco: Traditional use, marketing and strategies for conservation and increasing value. Esperance Med 2002; 9: 454-8.
- Amor ILB, Boubaker J, Sgaier MB, Skandrani I, Bhouri W, Neffati A, Kilani S, Bouhlel I, Ghedira K and Chekir-Ghedira L: Phytochemistry and biological activities of Phlomis species. J of Ethnopharma 2009; 125(2): 183-202.
- Ge X, Jing L, Zhao K, Su C, Zhang B, Zhang Q, Han L, Yu X and Li W: The phenolic compounds profile, quantitative analysis and antioxidant activity of four naked barley grains with different color. Food Chemistry 2021; 335: 127655.
- Harborne J: Phytochemical methods, a guide to modern techniques of plantanalysis, JB Harborne. Champman London GB 1973; 279.
- Sofowara A: Medicinal plants and traditional medicine in Africa. Ibadan: Spectrum Books Ltd 1993; 289-300.
- Brand-Williams W, Cuvelier ME and Berset CLWT: Use of free radical method to evaluate antioxidant activity. LWT Food Science and Technology 1995; 28(1): 25-30.
- Benzie IEF and Strain JJ: Ferric reducing abilities of plasma (FRAP) as ameasure of antioxidant power; the FRAP assay. Annals of Biochemistry 1996; 239(1): 70-76.
- Hsu CL, Chen T, Hu P, Yang J and Wang S: Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Food Chemistry 2003; 83(1): 85-92
- Prieto P, Pineda M and Aguilar M: Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical. Biochemistry 1999; 269(2): 337-341.
- Wolfe K, Wu X and Liu RH: Antioxidant activity of apple peels. J of Agricul and Food Chemi 2003; 51(3): 609-614.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P and Vidal N: Antioxidant activity of some Algerian medicinal plant extracts containing phenolic compounds. Food Chemistry 2006; 97(4): 654-660.
- Kumar A: Ethnobotanical study of wild vegetables used by rural communities of Kannauj district, Uttar Pradesh, India. Emirates J of Food and Agri 2013; 25(10): 760-766.
- Orhan I and Aslan M: Appraisal of scopolamine-induced antiamnesic effect inmice and in-vitro antiacetyl cholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. Journal of Ethnopharmacology 2009; 122(2): 327-332.
- Elfalleh W, Kirkan B and Sarikurkcu C: Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Industrial Crops and Products 2019; 127: 212-216.
- Seal T: Antioxidant activity of some wild edible plants of Meghalaya state of India: a comparison using two solvent extraction systems. IJNM 2012; 4(3): 51-56.
- Tiwari P, Kumar B, Kumar M, Kaur M, Debnath J and Sharma P: Comparative anthelmintic activity of aqueous and ethanol stem extract of Tinospora cordifolia. International Journal of Drug Development & Amp Research 2011; 3(1): 70-83.
- Tan JBL, Yap WJ, Tan SY, Lim YY and Lee SM: Antioxidant content, antioxidant activity and antibacterial activity of five plants from the Commelinaceae family. Antioxidants 2014; 3(4): 758-769.
- Alam M, Juraimi AS, RafiiMY, Abdul Hamid A, Aslani F, Hasan MM, Zainudin M, Asraf M and Uddin M: Evaluation of antioxidant compounds, antioxidant activities and mineral composition of 13 collected purslane (Portulaca oleracea) accessions2014; Biomed Research International Article ID 296063, http://dx.doi.org/10.1155/2014/296063
- Aktumsek A, Zengin G, Guler GO, Cakmak YS and Duran A: Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food and Chemical Toxicology 2013; 55: 290-296.
- Govindan P and Muthukrishnan S: Evaluation of total phenolic content and free radical scavenging activity of Boerhavia erecta. J of Acute Med 2013; 3(3): 103-109.
- Emmanuel UC, Monday SS, Chijioke DC, Ngozi OA and Simeon E: Phytochemical and in-vitro antioxidant activities of methanol leave extract of Alternanthera brasiliana. J of Plant Research 2018; 12(6): 835-839.
- Ambiga S and Jeyaraj M: Evaluation of in-vitro antioxidant activity of Ipomoea carnea International J of Current Microbiol and App Sci 2015; 4(5): 327-338.
- Saxena S and Rao PB: GC-MS screening of bioactive constituents and antioxidant profiling in an invasive weed, Malvastrum coromandelianum (L.) Garcke. Pharma Innovation 2018; 7(4): 738-746.
- Haggag EG, Kamal AM, Abdelhady MI, El-Sayed MM, El-Wakil EA and Abd-El-hamed SS: Antioxidant and cytotoxic activity of polyphenolic compounds isolated from the leaves of Leucenia leucocephala. Pharmaceutical Biology 2011; 49(11): 1103-1113.
- Saeed A, Marwat MS, Chohan AM, Shah AH, Naz R, Gul J, Bhatti MZ and Saeed A: Antioxidant activity in seeds of Avena fatua and Chenopodium album weeds associated with wheat crop. Pakistan Journal of Weed Science Research 2018; 24(3): 203-212.
- John BIJU, Sulaiman CT, George S and Reddy VRK: Total phenolics and flavonoids in selected medicinal plants from Kerala. International Journal of Pharmacy and Pharmaceuticals Sciences 2014; 6(1): 406-408.
- Chigayo K, Mojapelo PEL, Mnyakeni-Moleele S and Misihairabgwi JM: Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii Asian Pacific Journal of Tropical Biomedicine 2016; 6(12): 1037-1043.
- Adebiyi OE, Olayemi FO, Ning-Hua T and Guang-Zhi Z: In-vitro antioxidant activity, total phenolic and flavonoid contents of ethanol extract of stem and leaf of Grewia carpinifolia. Beni-Suef University Journal of Basic and Applied Sciences 2017; 6(1): 10-14.
- Ahmed D, Zara S and Baig H: In-vitro analysis of antioxidant activities of Oxalis corniculata fractions in various solvents. African J of Traditional Compl and Alternative Medicines 2013; 10(1): 158-165.
- Ramalingam R, Nath AR, Madhavi BB, Nagulu M and Balasubramaniam A: Free radical scavenging and antiepileptic activity of Leucas lanata. Journal of Pharmacy Research 2013; 6(3): 368-372.
- Goncalves AFK, Friedrich RB, Boligon AA, Piana M, Beck RCR and Athayde ML. Anti-oxidant capacity, total phenolic contents and HPLC determination of rutin in Viola tricolor (L) flowers. Free Radicals and Antioxidants 2012; 2(4): 32-37.
- Khaled-Khodja N, Boulekbache-Makhlouf L and Madani K: Phytochemical screening of antioxidant and antibacterial activities of methanolic extracts of some Lamiaceae. Industrial Crops and Products 2014; 61: 41-48.
- Benavente-Garcia O, Castillo J, Marin FR, Ortuno A and Del Rio JA: Uses and properties of citrus flavonoids. J of Agricultural and Food Chem 1997; 45(12): 4505-4515.
- Amari NO, Bouzouina M, Berkani A and Lotmani B: Phytochemical screening and antioxidant capacity of the aerial parts of Thymelaea hirsuta Asian Pacific Journal of Tropical Disease 2014; 4(2): 104-109.
- Formisano C, Oliviero F, Rigano D, Saab AM and Senatore F: Chemical composition of essential oils and in-vitro antioxidant properties of extracts and essential oils of Calamintha origanifolia and Micromeria myrtifolia two Lamiaceae from the Lebanon flora. Industrial Crops and Products 2014: 62: 405-411.
- Merculieff Z, Ramnath S, Sankoli SM, Venkataramegowda S, Murthy GS and Ceballos RM: Phytochemical, antioxidant and antibacterial potential of Elaeagnus kologa (Schlecht.) leaf. Asian Pacific Journal of Tropical Medicine 2014; 7: 599-602.
- Saha S and Verma RJ: Antioxidant activity of polyphenolic extract of Terminalia chebula Retzius fruits. J of Taibah University for Science 2016; 10(6): 805-812.
- Bouterfas K, Mehdadi Z, Elaoufi MM, Latreche A and Benchiha W: November. Antioxidant activity and total phenolic and flavonoids content variations of leaves extracts of white Horehound (Marrubium vulgare Linné) from three geographical origins. In Annales pharmaceutiques Francaises Elsevier Masson 2016; 74(6): 453-462.
How to cite this article:
Thakurathi G, Rao PB and Bhakuni KK: In-vitro antioxidant potential, total phenolic and flavonoid content of Lamium amplexicaule leaf extract in aqua-methanol and aqua-acetone. Int J Pharmacognosy 2022; 9(12): 206-12. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.9(12).206-12.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
206-212
500 KB
905
English
IJP
Gayatri Thakurathi, P. B. Rao and Kamlesh Kumar Bhakuni ⃰
Department of Botany, L. S. M Government Postgraduate College, Soban Singh Jeena University, Almora, Uttarakhand, India.
kamal.bot2011.pth@gmail.com
01 August 2022
10 December 2022
12 December 2022
10.13040/IJPSR.0975-8232.IJP.9(12).206-12
31 December 2022