IN-VITRO ANTIBACTERIAL ACTIVITY OF ACACIA ETBAICA AGAINST STAPHYLOCOCCUS AUREUS AND ESCHERICHIA COLI
HTML Full TextIN-VITRO ANTIBACTERIAL ACTIVITY OF ACACIA ETBAICA AGAINST STAPHYLOCOCCUS AUREUS AND ESCHERICHIA COLI
Belayneh Getachew * 1, Samrawit Getachew 1, Berhan Mengiste 2 and Abebe Mekuria 3
College of Veterinary Medicine 1, Mekelle University, Ethiopia.
College of Health Science 2, Debre-Birihan University, Ethiopia.
College of Health Science 3, Arsi University, Ethiopia.
ABSTRACT: This study was conducted to determine the in-vitro anti-microbial activity of Acacia etbaica, native plant to east African countries, against Staphylococcus aureus and Escherichia coli. To achieve this, the methanol extract of leaf of Acacia etbaica was tested for its in-vitro antibacterial activity against Staphylococcus aureus and Escherichia coli using agar disc diffusion method at two different concentrations (500 µg/disc and 1000 µg/disc). The minimum inhibitory concentration (MIC) of the plant crude extract was also determined using the microdilution method in 96-well plates. Acacia etbaica showed significant antibacterial activity with the mean zone of inhibition of 13.34 ± 1.04 mm and 11.13 ± 1.04 mm in diameter at a concentration of 1000µg of plant extract per disc against S. aureus and E. coli respectively. The MIC of the crude extracts of Acacia etbaica was determined to be 0.039 mg/ml and 0.313 mg/ml against S. aureus and E. coli respectively. The results suggest that the methanol extract of Acacia etbaica could be a rich source of antibacterial compounds. The results also indicate merit in the Acacia etbaica ethnomedicine use by the local communities.
| Keywords: |
Antimicrobial activity, Disc diffusion, E. coli, Acacia etbaica, Minimum inhibitory concentration, S. aureus
INTRODUCTION: Antimicrobial resistance is a global challenge that makes effective treatment and control of infection difficult. It is an increasingly serious threat to the world public and animal health. Antimicrobial resistances have been documented in different species of bacteria in many countries of the world 1, 2, 3. There must be an effort to search for an option to tackle this global problem. One of the options is searching for alternative antimicrobials from different sources like plants.
Acacia etbaica is a medicinal plant which occurs in dry bushland, thickets, semi-desert scrub, and wooded grasslands. It is native to Eritrea, Ethiopia, Sudan, Somalia, Kenya, Uganda and Tanzania 4. It has been reported that the plant was used to treat swelling, ringworm infection, hemorrhoids, scabies, fire burn, eye infection of livestock and anthrax by the community of Kilte Awulaelo District of Tigray Region, Ethiopia 5.
The bark of the plant is also chewed as a stimulant and for the treatment of gonorrhea. Though the plant is used to treat various diseases of human and animal diseases by the local communities for centuries, still there is limited information on the antibacterial activity of the plant scientifically. Therefore, the objective of this paper was to determine the in-vitro antibacterial activity of Acacia etbaica.
MATERIALS AND METHODS:
Plant Collection & Crude Extract Preparation: The leaf of Acacia etbaica was collected in 2014 from Mekelle and around Mekelle city which is located in the Tigray region of Ethiopia. Then, the leaf of the plant was air-dried under a shed at room temperature before it was ground with a micro plant grinder machine. The crude extract of the plant was extracted using 80% methanol according to the procedure described by Shewit et al. 6
Disc Preparation for the Experiment: 6 mm of discs were prepared from filter paper (Whatman No 4 filter paper, Whatman Ltd., England). Then the discs were impregnated by the extract at two concentrations (1000 µg and 500 µg/disc). This was done by loading with 10µl of plant extract solution (100 mg of plant extract per 1 ml of DMSO) which results in 1000 µg of plant extract per disc, and the other group was loaded with 5 µl of the same solution which results in 500 µg of plant extract per disc. Finally, the prepared discs were sterilized under UV for 30 min.
Preparation of Test Bacteria: The test bacteria used to determine the antibacterial activity of Acacia etbaica were Staphylococcus aureus and Escherichia coli. These bacteria were obtained from the National Veterinary Institute, Ethiopia in lyophilized form. They were revived in the nutrient broth before culturing them in their respective selective media, i.e. Staphylococcus aureus, and Escherichia coli were cultured on mannitol salt agar and McConkey agar respectively for confirmation. Finally, the confirmed isolated were sub-cultured on nutrient agar aseptically, and separated colonies were suspended in sterile salt solution within a test tube until the turbidity matches with 0.5 McFarland standards. All these activities were conducted by following the standard laboratory procedures.
Antimicrobial Susceptibility Test:
Disc Diffusion Test: The disc diffusion method for antimicrobial susceptibility testing was performed according to EUCAST 7. Briefly, the inoculums adjusted to 0.5 McFarland turbidity standards were spread evenly over the entire surface of the plate containing Muller -Hinton agar using sterile swabs. Then discs were applied immediately on the surface using sterile forceps. We used five discs (3 discs loaded with crude extract of a plant with equal concentration, one disc loaded with DMSO as a negative control and a chloramphenicol disc as a positive control) per 90 mm diameter plate. The antimicrobial activity of each plant was tested at a concentration of 1000µg and 500 µg per disc. Then, the inoculated plates were incubated at 37 °C for 20 h. Finally, zones of inhibitions were measured using electronic digital caliper in mm.
Determination of Minimum Inhibitory Concentration (MIC): The minimum inhibitory concentration was determined by microdilution method in 96 well plates according to Andrews, 8 with slight modifications. Briefly, serial dilutions of the plant extracts were made in small test tubes. The first test tube was filled with 2 ml of DMSO, and the test tubes were filled with 1 ml of DMSO. 200mg of the plant extract was dissolved in the first tube, and 1 ml of the solution was transferred into the second test tube. After thorough mixing, again 1 ml of the solution was transferred into the third test tube. This procedure was repeated up to test tube 10. Then, 25 µl of the crude extracts were transferred from each test tube to wells of 96-well plates, i.e. the crude extracts of test tube 1, 2, 3,… were transferred to two wells of the first row, second row, third row,… of the 96 well plates respectively.
Each well of the plate was loaded with 25 µl of bacterial suspension (adjusted to 0.5 McFarland standards), and 200 µl of broth except wells left for checking sterility. Chloramphenicol was used as a positive control, inoculated wells of antibiotic-free broth were used as negative control, and un-inoculated wells of antibiotic-free broth were used to check sterility. Then the plates were covered with plate sealing tape and incubated at 37 °C for 20 h. Finally, the lowest concentration of the plant extract that showed no visible growth was taken as minimum inhibitory concentration.
Data Analysis: Data on the zone of inhibition produced by each disc on each bacteria were stored in an excel spreadsheet. Then mean values for the zone of inhibition and standard deviation were calculated using SPSS statistical software version 17. One way ANOVA was used to see any statistical differences among the mean values of the plant extracts at two concentrations and the negative control. Finally, post hock test (using Bonferroni) was used to compare the mean values of the negative control with that of the plant extract at different concentrations.
RESULTS AND DISCUSSION: For crude extraction from the leaf of Acacia etbaica, 80%methanol was used. 125.281gm of crude extract was found from 480gm of the plant powder which resulted in a total yield of 26.1%. The consistency of the crude extract was semi-solid and sticky. Using the agar disc diffusion method, Acacia etbaica showed mean zone of inhibition of 10.95 ± 1.49 mm and 13.34 ± 1.04 mm at a concentration of 500 µg and 1000 µg respectively against Staphylococcus aureus Fig. 1 and Table 1.
The plant also showed a mean zone of inhibition of 10.77 ± 1.18 mm and 11.13 ± 1.04 mm at a concentration of 500µg and 1000µg respectively against Escherichia coli Table 1.
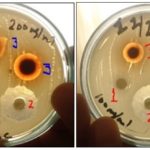
FIG. 1: ANTIMICROBIAL ACTIVITY OF ACACIA ETBAICA AGAINST STAPHYLOCOCCUS AUREUS. DISCS WERE LOADED WITH 1000µG (3 BLUE) AND 500µG (3 RED) OF 80% METHANOL EXTRACTS OF ACACIA ETBAICA. 1: Negative controls loaded with DMSO; 2: Positive controls which are standard chloramphenicol antibiotic discs
TABLE 1: MEAN ZONE OF INHIBITION OF 80% METHANOL CRUDE EXTRACT OF ACACIA ETBAICA AGAINST S. AUREUS AND E. COLI
| Concentration | Mean Zone of Inhibition in mm ± SD | |
| S. aureus | E. coli | |
| 500 µg/disc | 10.95 ± 1.49 | 10.77 ± 1.18 |
| 1000 µg/disc | 13.34 ± 1.04 | 11.13 ± 1.04 |
| Positive control (Discs loaded with
30µg of Chloramphenicol) |
21.19 ± 1.43 | 20.72 ± 1.62 |
| Negative control
(Discs Loaded with 10µl of DMSO) |
6 | 6 |
Acacia etbaica at a concentration of 500 µg/disc and 1000 µg/disc showed significant (p<0.05) antibacterial activity against Staphylococcus aureus and Escherichia coli when compared with that of the negative control. The Staphylococcus aureus bacteria that we used for this study was found to be resistant to penicillin while we conducted an antimicrobial susceptibility test for students in the laboratory. Thus, Acacia etbaica is potentially a promising plant for isolation of active compounds against resistant Staphylococcus aureus which is currently a big challenge in the globe. Possibly, the plant may also contain broad-spectrum antibacterial compounds, since it showed effect both on the gram negative and positive bacteria. Acacia etbaica is native to Eritrea, Ethiopia, Sudan, Somalia, Kenya, Uganda and Tanzania 4. There is no previous report on the antibacterial activity of this native plant which makes this work the first report on this aspect. To determine the minimum inhibitory concentration, microdilution method using 96 well plates were used. Using this method, the minimum inhibitory concentration of Acacia etibanica crude extract was found to be 0.039 mg/ml and 0.313mg/ml against Staphylococcus aureus and Escherichia coli respectively Table 2. Ceftriaxone and cefoxitin inhibit the growth of S. aureus ATCC® 25923 and E. coli ATCC® 25922 at a concentration of 0.008mg/ml 9. Even though Acacia etbaica showed higher MIC results than standard drug ceftriaxone and cefoxitin, it may be possible to get a comparable MIC result after separation and purification of the active compounds of the plant. In another study, Biswas, and Roymoj, 10 reported that MIC of cold methanol extracts of the leaf of Acacia arabica was 0.6 mg/ml against E. coli which is very closer to our result.
TABLE 2: MINIMUM INHIBITORY CONCENTRATION OF ACACIA ETBAICA CRUDE EXTRACT AGAINST STAPHYLOCOCCUS AUREUS AND E. COLI
| Test bacteria | Extract Concentration (mg/ml) | MIC
(mg/ml) |
||||||||||
| 10 | 5 | 2.5 | 1.25 | 0.625 | 0.313 | 0.156 | 0.078 | 0.039 | 0.019 | 0.009 | ||
| S. aureus | - | - | - | - | - | - | - | - | - | + | + | 0.039 |
| E. coli | - | - | - | - | - | - | + | + | + | + | + | 0.313 |
-: No growth of bacteria, +: There is the growth of bacteria
CONCLUSION: 80% methanol extract of leaf of Acacia etbaica showed antibacterial activity against Staphylococcus aureus and Escherichia coli. The results suggest that the methanol extracts of Acacia etbaica could be a rich source of antibacterial compounds against Staphylococcus aureus and Escherichia coli. The results also provide a scientific basis for the traditional use of Acacia etbaica by the local communities. Therefore, further studies should be conducted to isolate or fractionate the active components of the plant having an antibacterial effect. Moreover; in- vivo studies on this plant are needed to determine its effectiveness, toxicity, and side effects.
ACKNOWLEDGEMENT: This research partly received financial support from the Ministry of Education (Ethiopia) with Grant No. CVM/RB/10/ 2013. The authors are very grateful to College of Veterinary Medicine, Mekelle University for allowing us to use all the necessary facilities to conduct this research and College of Medicine, Department of Pharmacy, Mekelle University for the extraction of the plant.
CONFLICT OF INTEREST: Nil
REFERENCES:
- Ma P, Chan K and Chou C: Characterization of bacterial susceptibility isolates in sixteen dairy farms in Taiwan. Journal of Dairy Science 2006; 89: 4573-4582.
- Bedada A and Hiko A: Mastitis and antimicrobial susceptibility test at Asella, Oromia Regional state, Ethiopia. Journal of Microbiology and Antimicrobial 2011; 3(9): 228-232.
- Sharma D, Pradeep S and Malik A: Prevalence and Antimicrobial susceptibility of drug-resistant Staphylococcus aureus in raw milk of dairy cattle. International Research Journal of Microbiology 2011; 2(11): 466-470.
- Orwa A, Mutua R, Kind R, Jamnadass and Anthony: Agroforestree Database: a tree reference and selection guide version 4.0; 2009. (http://www.worldagroforestry .org/sites/treedbs/treedatabases.asp)
- Teklay A, Abera B and Gidey M: An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. Journal of Ethnobiology and Ethnomedicine 2013; 9:65.
- Shewit K, Mekonnen H, Gebremedhin G, Tsegay T, Samson S, Habtamu T and Mussie T: In-vitro antimicrobial activity screening of some ethnoveterinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray Region, Ethiopia. Asian Pacific Journal of Tropical Biomedicine 2012; 2(7): 512-522.
- European Committee on Antimicrobial Susceptibility Testing; Disc Diffusion Method for Antimicrobial Susceptibility Testing; European Society of Clinical Microbiology and Infectious Disease 2013;
- http://www.eucast.org/antimicrobial_susceptibility_testing
- Andrew M: Determination of minimum inhibitory concentrations. Journal of antimicrobial chemotherapy 2001; 48: 5-6.
- CLSI Clinical Laboratory Standards Institute, Performance standards for antimicrobial susceptibility testing, seventh information supplements 2007; 27(1): 32-44.
- Biswas D and Roymon G: Search for in-vitro antibacterial efficacy of phytoconstituents of Acacia arabica leaf extracts against various serogroups of coli associated with diarrheal infections in ruminants. Recent Research in Science and Technology. 2013; 5(2): 73-74.
How to cite this article:
Getachew B, Getachew S, Mengiste B and Mekuria A: In-vitro antibacterial activity of Acacia etbaica against Staphylococcus aureus and Escherichia coli. Int J Pharmacognosy 2015; 2(12): 577-81. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.2(12).577-81.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
4
577-581
514
2128
English
IJP
B. Getachew *, S. Getachew, B. Mengiste and A. Mekuria
College of Veterinary Medicine, Mekelle University, Ethiopia
belaygeta1999@yahoo.com
07 October 2015
26 November 2015
29 November 2015
10.13040/IJPSR.0975-8232.IJP.2(12).577-81
30 December 2015