IN-VITRO ANTI-CANCER EFFECTS OF ETHANOLIC EXTRACT, LEUCAS ASPERA (WILLD.) LINK WHOLE PLANT AGAINST HELA, HEP G2 AND MCF-7 CELL LINES
HTML Full TextIN-VITRO ANTI-CANCER EFFECTS OF ETHANOLIC EXTRACT, LEUCAS ASPERA (WILLD.) LINK WHOLE PLANT AGAINST HELA, HEP G2 AND MCF-7 CELL LINES
S. Indira and V. Mahalakshmi *
Department of Pharmaceutics, S. A. Raja Pharmacy College, Tirunelveli, Tamil Nadu, India.
ABSTRACT: Leucas aspera (family-Lamiaceae) has great therapeutic significance in the Indian system of medicine due to its rich antioxidant activity. The main objective of the present work was to isolate the active constituent of ethanolic extract of Leucas aspera by column chromatography, Characterization of isolated compound by IR, 1H NMR, Mass spectroscopy and in-vitro anticancer activity evaluations against Hela, Hep G2 And MCF-7 Cell Lines by MTT assay. The EELA showed highest percentage of cell inhibition 64.18% in the concentration of 500 (µg/ml) on the HepG2 (Human liver cancer cell line) and significant activity than the HeLa & MCF-7. The present study concluded that the antioxidant potentials like flavonoids, saponins present in extract might be responsible for the anticancer activities.
Keywords: Leucas aspera, Anticancer activity, HepG2, MTT assay, Column chromatography, H NMR
INTRODUCTION: "Thumbai" is the popular name for the plant Leucas aspera (Wild) Linn, family Lamiaceae 1, 2. Leucas aspera is a plant that grows along pavement and in ruins in India. It is a member of the Lamiaceae family and the Leucas genus, is a widespread medicinal plant in tropical Asia and Africa 3. It has an appealing flavour and is commonly used in unrefined form as an antipyretic in south India. The entire plant is significant historically because it has several medicinal benefits. Leucas aspera flowers are used as stimulant, expectorant (treat coughs), as perients (relieve constipation), diaphoretic (inducing perspiration), insecticide and the flowers are mixed with honey and given to children to treat cold 4.
The juices from the leaves are thought to be a treatment for chronic rheumatism, psoriasis, and other skin eruptions 5. The leaves are also used as insecticides and mosquito repellant 6. The plant extract was administered with honey for bloating and indigestion 7. The juice from its leaves is administered topically to reduce painful swellings. Indigenous people use smashed leaves to cure snake bites 8, 9. This study's goal was to examine the ethanolic extract of Leucas aspera's in-vitro anticancer activities.
MATERIALS AND METHODS: Leucas aspera, a plant utilised for the study, was procured from a nearby farm in Vadakangulam, Tirunelveli District, Tamil Nadu, India.
Preparation of Plant Extracts: Leucas aspera (Willd.) Link entire plant was dried in the shade, all taken together, and then ground into a coarse powder using a mechanical grinder. The powder was placed in an airtight container after passing through sieve no. 40. To get rid of waxy materials and chlorophyll, which often obstruct the extraction of phytoconstituents, the dried powder material (150 gm) was defatted with petroleum ether (60°–80° C). The marc was dried and extracted with ethanol (90% v/v) in a Soxhlet extractor for 72 hours. The semisolid material that resulted after the solvent was removed was then dried in a vacuum evaporator, and the yield was determined 10, 11.
Phytochemical Analysis: The extracts of Leucas aspera (Willd.) Link was subjected to qualitative tests for the identification of various plant constituents 12, 13.
Thin Layer Chromotography (TLC): The chromatographic method of TLC is used to disperse non-volatile mixtures. On a piece of glass, plastic, or aluminium foil that has been covered with a thin layer of an absorbent substance-typically silica gel, aluminium oxide, or cellulose-thin-layer chromatography is carried out. The stationary phase refers to this adsorbent layer. Thin-layer chromatography can be used to assess a substance's purity, identify the chemicals present in a particular combination, and track the development of a reaction. To create the solvent, 5:5:1 ratios of chloroform, ethyl acetate, and formic acid were combined. A drop of plant extract was applied to the paper, which was then held in the solvent to allow the components to separate 14, 15.
Column Chromotagraphy: Natural products are frequently separated, isolated, and purified using the column chromatographic method. The separation of the chemicals is based on the adsorption at the solid-liquid interface. The interaction between the adsorbent and the component must be reversible for the solid support.
Therefore, the different components will travel down the column as the adsorbent is cleaned with new solvent until they are finally organised in order of their affinity for the adsorbent. The molecules with the lowest affinity elute from the end of the column first and travel down it more quickly than the molecules with the highest affinity for the adsorbent. By using column chromatography, the separation may be accomplished by altering the polarity of the mobile phase. Analytical methods including mass, IR, and NMR spectroscopy can be used to characterise the separated molecules 16, 17.
Spectral Characterization:
FT-IR Spectroscopy: Using the KBr pellet method, IR spectral investigations on an isolated chemical were conducted. In this procedure, a 1:100 mixture of the medication and KBr was used. These mixes were then formed into pellets via pressing. By adopting the KBr pellet approach, the FT-IR spectra for isolated compounds were captured in the 4000-400 cm-1 range 18.
NMR Spectroscopy: When using NMR to determine the structure of an unknown compound, three pieces of information must be taken into account: the position of the peak's resonance (or chemical shift), the number of hydrogen atoms responsible for the signal (integration), and the number of peaks that make up the signal (multiplicity). NMR data to determine the structure, although using MS or IR data to determine the unknown is also acceptable 19.
MASS Spectroscopyl: A method of analysis called mass spectrometry (MS) determines the mass-to-charge ratio of charged particles. It is used to determine particle masses, to ascertain a sample's or molecule's elemental makeup, and to clarify the chemical structures of molecules like peptides and other chemical compounds. The mass-to-charge ratios of the charged molecules or fragments produced by ionising chemical substances are measured in the MS principle 20, 21.
Pharmacological Studies:
In-vitro Anticancer Activity: The National Centre for Cell Science (NCCS), Pune provided the human breast cancer cell line (MCF-7) and cervical cancer cell line (HeLa). HeLa cells were cultivated in Eagles minimal essential medium (EMEM) with 10% foetal bovine serum (FBS), whereas (MCF-7) cells were grown in Dulbecco's modified Eagles' medium (DMEM). A constant 37°C, 5% CO2, 95% air, and 100% relative humidity were used for all cells. Weekly passages of maintained cultures took place, and the culture medium was switched out twice a week 22, 23.
Cell Treatment Procedure: Trypsin-EDTA was used to separate the monolayer cells into single cell suspensions, and viable cells were counted using a hemocytometer. The single cell suspensions were then diluted with media containing 5% FBS to provide a final density of 1x105 cells/ml. 10,000 cells were plated onto 96-well plates with 100 microliters of cell suspension per well and incubated to promote cell attachment at 37° C, 5% CO2, 95% air, and 100% relative humidity 24, 25.
The cells were exposed to successive concentrations of the extracts and fractions after 24 hours. To create five concentrations, they were first dissolved in dimethylsulfoxide (DMSO) and then further diluted in serum free media. To achieve final concentrations of 10, 20, 50, 100, and 200 g/ml on plates, 100 microliters of each concentration were applied to each well. The plates were incubated at 37°C, 5% CO2, 95% air, and 100% relative humidity for 48 hours. The final volume in each well was 200 µl. As a control, the medium containing no samples was used. All concentrations were maintained in triplicate 26.
MTT Assay: A yellow tetrazolium salt known as MTT is water soluble. The tetrazolium ring is broken by succinate dehydrogenase, a mitochondrial enzyme found in live cells, turning the MTT into the insoluble purple formazan. In light of this, the quantity of formazan generated directly correlates with the quantity of viable cells. Each well received 15 l of MTT (5 mg/ml) in phosphate buffered saline (PBS) after 48 hours of incubation, which was followed by a 4 hour incubation period at 37 °C. Next, the MTT-containing medium was turned off, the formazan crystals that had formed were dissolved in 100 l of DMSO, and the absorbance at 570 nm was then determined using a microplate reader. The formula below was used to calculate the percent cell inhibition 27, 28.
% cell Inhibition = (100- Abs (sample) / (Abs (control) × 100
Nonlinear regression graph was plotted between percent cell inhibition was determined using Microsoft Excel software 29.
RESULTS AND DISCUSSION: Leucas aspera (Willd) Link, a member of the Lamiaceae family, has been thoroughly studied using a systematic approach that included phytochemical characterisation and pharmacological research. Litrature analysis showed that this plant receives little maintenance. Therefore, it was deemed beneficial to conduct pharmacological and phytochemical investigations on this plant.
Phytochemical Studies:
Extraction of Leucas aspera (Willd.) Link Leaves: Leucas aspera (Willd.) Link's dried, crushed, entire plant was extracted using petroleum ether and ethanol (90% v/v) continually using a Soxhlet equipment. Table 1 Shows the findings.
TABLE 1: DATA SHOWING THE EXTRACTIVE VALUES OF WHOLE PLANT OF LEUCAS ASPERA (WILLD) LINK
| S. no. | Extract | Colour/ Physical nature | Percentage yield (% w/w) |
| 1 | Petroleum ether | Green/ Waxy Semisolid | 4.85 |
| 2 | Ethanol (90% v/v) | Brownish Green/ Solid | 6.42 |
Preliminary Phytochemical Screening of whole Plant of Leucas aspera (Willd.) Link. Extracts: Leucas aspera (Willd) Link. entire plant extracts were submitted to qualitative phytochemical screening to determine the phytoconstituents present, and the findings are shown in Table 2.
TABLE 2: DATA SHOWING THE PHYTOCHEMICAL SCREENING OF WHOLE PLANT OF LEUCAS ASPERA (WILLD) LINK
| S. No. | Chemical Test | Petroleum ether extract | Ethanol (90% v/v) extract |
| 1. | Alkaloids | ||
| a. | Mayer’s Test | - | + |
| b. | Dragendroff’s Test | - | + |
| c. | Wagner’s Test | - | + |
| d. | Hager’s Test | - | + |
| 2. | Carbohydrates | ||
| a. | Molisch’s Test | + | + |
| b. | Fehlings Test | + | + |
| c. | Barfoed’s Test | + | + |
| d. | Benedict’s Test | + | + |
| 3. | Glycosides | ||
| a. | Legal’s Test | - | + |
| b. | Keller-Killiani Test | - | + |
| c. | Borntrager’s Test | - | + |
| 4. | Fixed Oil & Fat | ||
| a. | Stain Test | + | - |
| b. | Saponification Test | + | - |
| 5. | Tanins & Phenolics | ||
| a. | Ferric Chloride Test | + | + |
| b. | Lead Acetate Test | + | + |
| c. | Gelatin Solution Test | + | + |
| 6. | Steroids | ||
| a. | Salkowsky’s Test | + | - |
| b. | Libermann Burchard’s Test | + | - |
| 7. | Saponins | ||
| a. | Foam Test | - | + |
| 8. | Proteins | ||
| a. | Million’s Test | - | - |
| b. | Ninhydrin Test | - | - |
| 9. | Flavonoids | ||
| a. | Aqueous NaOH Test | - |
+ |
| b. | Conc.Sulphuric Acid Test | - | + |
| c. | Shinoda Test | - | + |
| 10. | Gum & Mucilage | - | - |
| 11. | Triterpenoids | ||
| a. | Knoller’s Test | - | - |
Thin Layer Chromatography (TLC): Based on the polarity, TLC was carried out using a variety of solvent solutions using a trial-and-error methodology. Leucas aspera (Willd.) Link entire plant ethanol extract was submitted to thin layer chromatography on silica gel G, and the results demonstrated high resolution of the solvent system toluene: ethyl acetate: acetic acid (55: 45: 1). Using the appropriate detecting agent, two locations were located. Rf values were computed and displayed in Table 3 and Fig. 1.
FIG. 1: THIN LAYER CHROMATOGRAPHY OF EELA
TABLE 3: THIN LAYER CHROMATOGRAPHY OF EELA
| Solvent system | No. of spots | Spray reagent | Rf Values |
| Ethyl acetate : Chloroform (6:4) | 1 | Iodine vapour | 0.57 |
| Chloroform: Methanol (96:4) | 1 | Iodine vapour | 0.60 |
| Ethyl acetate: Formic acid: Water (50:4:10) | 1 | Iodine vapour | 0.62 |
| Toluene: Ethyl acetate: Acetic acid (55: 45: 1) | 2 | Iodine vapour | 0.63, 0.71 |
Column Chromatography: Based on phytochemical screening and TLC research, column chromatography was used to isolate the active components of EELA using the isocratic elution technique. The solvent system used was toluene, ethyl acetate, and acetic acid (55:45:1), as indicated in Table 4 and Fig. 2.
FIG. 2: COLUMN CHROMATOGRAPHY OF EELA
TABLE 4: COLUMN CHROMATOGRAPHY OF EELA
| Fraction No. | Nature of Residue | Analysis by TLC | Colour of the spot | Rf Value |
| 1-4 | No residue | --- | --- | --- |
| 5-8 | No residue | --- | --- | --- |
| 9-12 | Green | --- | --- | --- |
| 13-16 | Green | ---- | --- | --- |
| 17-20 | Light Green | --- | --- | --- |
| 21-24 | Greenish yellow | 2 spots with tailing effect | Yellow Greenish brown | 0.67, 0.70 |
| 25-28 | Light yellow | 2 spots with tailing effect | Yellow Brown | 0.71, 0.64 |
| 29-32 | Yellow | 1 | Yellow | 0.63 |
| 33-36 | No residue | --- | --- | --- |
| 37-40 | No residue | ---- | ---- | --- |
The isolated compound from the fraction 29-32 obtained by column chromatography was named as EELA I.
Analysis of the Isolated Compound:
Thin Layer Chromatography of the Isolated Compound EELA I: The isolated substance EELA I produced from column chromatography was examined chemically using thin layer chromatography and identified using iodine vapours while utilising the solvent system toluene: ethyl acetate: acetic acid (55:45:1).
Fig. 3 from EELA I displayed the presence of a single yellow colour spot with an Rf value of 0.63.
Characterization of EELA I: Leucas aspera (Willd.) Link's entire plant was used to make an isolated chemical, which underwent testing and revealed positive results for flavonoids. The compound's physicochemical characteristics are listed above in a Table 5 and 6.
TABLE 5: RESULTS OF CHEMICAL TESTS FOR EELAI
| Chemical Tests | EELAI |
| Aqueous NaOH Test | Positive |
| Conc.Sulphuric Acid Test | Positive |
| Shinoda Test | Positive |
TABLE 6: PHYSICAL CHARACTERS OF EELAI
| Parameters | EELAI |
| Yield | 48 mg |
| Physical state and color | Yellow amorphous powder |
| Solubility | Water, acetone and DMSO |
| Melting point | 136 ºC |
| TLC solvent system | Toluene: Ethyl acetate: Acetic acid (55:45:1) |
| Rf value | 0.63 |
FIG. 3: THIN LAYER CHROMATOGRAPHY OF EELA I
Table 2 provides a summary of the qualitative analysis of the entire plant of Leucas aspera (Willd.) Link ethanol extract. According to preliminary phytochemical analysis, petroleum ether extract included sugars, fixed oils and lipids, steroids, tannins, and phenolics. The presence of flavonoids, glycosides, tannins and phenolics, alkaloids, carbohydrates, and saponins was shown in the ethanol extract. The TLC research was conducted, and Table 3 provides an overview of the findings.
The highest number of spots using toluene: ethyl acetate: acetic acid (55:45:1) as the detecting agent were two, according to the TLC investigation of the ethanol extract of Leucas aspera (Willd.) Link. The spots' Rf values were computed and discovered to be 0.63 and 0.71, respectively. Table 4 lists the outcomes of column chromatography of an ethanol extract of Leucas aspera (Willd.) Link. The isolated compound EELA I has produced promising flavonoid results in phytochemical research.
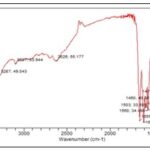
FIG. 4: IR SPECTRUM OF ISOLATED COMPOUND EELA I
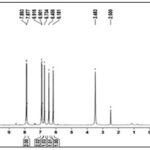
FIG. 5: 1H NMR SPECTRUM OF ISOLATED COMPOUND EELA I
FIG. 6: MASS SPECTRUM OF ISOLATED COMPOUND EELA I
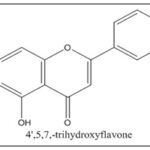
Compound EELA-I was produced as an amorphous powder, and its mass spectrum data revealed the [M-H]- ion at m/z 268.08, 183.53, 172.06, and 149.05. This allowed the chemical formula to be determined as C15H10O5. The IR and 1H NMR spectrum data for EELA-I further validated the chemical formula. The typical lines for aromatic-OH groups, C=O groups, C-O groups, and C=C groups were seen in the IR spectra at 3287.49 cm-1, 1611.29 cm-1, 1186.35 cm-1, and 1655.30 cm-1, respectively. Broad peaks at 10.36, 10.80, and 12.95 in the 1H NMR spectrum indicated the presence of three phenyl hydroxyl groups. The 1H NMR spectrum of EELA-I also showed the presence of two meta coupled aromatic doublets at δ6.18 and 6.45 corresponds to H-6 and H-8 protons, two doublets at δ6.95 (d,2H,J=6.4Hz) and 7.81 (d, 2H,J= 6.4Hz) for H-3'/H-5' and H-2'/H-6' protons of ring B, and a singlet at δ6.73 corresponding to H-3 proton; characteristic for a 5,7,4'-trisubstituted flavone. The substance was therefore identified as Apigenin based on the data and by comparison with the literature Fig. 7.
FIG. 7: STRUCTURE OF APIGENIN
Pharmacological Screening:
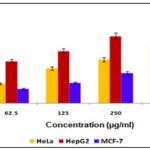
In-vitro Anticancer Activity: Using the MTT test technique, the anticancer activity of the ethanol extract of Leucas aspera was investigated on a variety of cell lines, including cervical cancer cell line HeLa, human liver cancer cell line Hep G2, and human breast cancer cell line MCF-7. With respect to the cancer cell lines HeLa, HepG2, and MCF-7, the EELA demonstrated a considerable anticancer activity at dosages of 62.5 g/ml, 125 g/ml, 250 g/ml, and 500 g/ml. The HepG2 (Human liver cancer cell line) demonstrated stronger inhibition and substantial activity than the HeLa & MCF-7, which were indicated in Table 7 & Fig. 8, on comparison of the percentage cell inhibition of HeLa, Hep G2, and MCF-7.
TABLE 7: % CELL INHIBITION OF EELA IN HELA, HEP G2 AND MCF-7 CELL LINES
| Concentration (µg/ml) | % Cell Inhibition | ||
| HeLa | HepG2 | MCF-7 | |
| 62.5 | 16.65 | 35.48 | 12.15 |
| 125 | 29.51 | 43.90 | 17.34 |
| 250 | 37.09 | 56.81 | 25.67 |
| 500 | 51.82 | 64.18 | 34.89 |
FIG. 8: % CELL INHIBITION OF EELA IN HELA, HEP G2 AND MCF-7 CELL LINES
Tumorigenic factors control the aberrant proliferation and differentiation of cells that result in cancer. The second leading cause of mortality in the world is cancer. Chemotherapy is one of the most effective therapeutic approaches for the treatment of cancer today 30. The negative effects and acquired drug resistance of synthetic small molecule drugs have raised more and more questions as their usage and knowledge have grown. This has led to a rise in interest in naturally occurring, palatable small compounds like flavones, which are predicted to have remarkable physiological effects, minimal toxicity, and non-mutagenic properties in the human body 31.
Chemically known as 4′, 5, 7-trihydroxyflavone, apigenin is a flavone subclass compound that is widely present in a variety of foods and drinks, including parsley, grapes, apples, chamomile tea, and red wine 32. One of the active components of Indian medicinal plants is apigenin. Because of its physiological roles as an antioxidant and anti-inflammatory, its part in decreasing blood pressure, and its antibacterial and antiviral activities, apigenin has been used as a traditional medicine for millennia 33, 34. In addition to aforementioned outcomes, apigenin has recently been shown to be effective at suppressing tumour growth 35. More and more data has been produced to suggest that apigenin has antitumor activity against many forms of cancer using both cell lines in-vitro and mice models in-vivo since 35. Originally revealed that it had anti-cancer actions in 1986 36. Numerous cancer types, including colorectal cancer, breast cancer, liver cancer, lung cancer, melanoma, prostate cancer, and osteosarcoma, have been shown to be sensitive to apigenin, which has been shown to exhibit wide anti-cancer properties. Through causing cell death, promoting autophagy, and modifying the cell cycle, this flavone prevents the development of cancer cells. Additionally, apigenin reduces the motility of cancer cells and prevents their migration and invasion. Apigenin has recently been found to have anti-cancer effects via triggering the immune system 37.
Apigenin affects a number of protein kinases and signalling pathways, including PI3K/AKT, MAPK/ERK, JAK/STAT, NF-B, and Wnt/-catenin, during those processes. In a variety of scientific fields, measuring cell viability and growth is an invaluable tool. HeLa, Hep G2, and MCF-7 cancer cell lines were used in the current study to examine the cytotoxicity of the ethanol extract of Leucas aspera (Willd.) Link due to their relevance as an in-vitro system for screening purposes involving natural products and their antimutagenic effects on these cells and their well-defined characteristics under experimental conditions. Tetrazolium salt reduction (MTT) is currently accepted as a reliable substitute for radiometric testing. Drug sensitivity, cytotoxicity, responsiveness to growth factors, and activation are just a few uses for the technique.HepG2 outperformed the other two cell lines in terms of anticancer activity in-vitro 38. Our research has demonstrated that the anticancer properties of the extract's flavonoids and saponins may be to blame. Leucas aspera (Willd.) Link as a whole plant has anti-cancer action, according to EELA I (Apigenin) spectral data.
CONCLUSION: Leucas aspera (Willd.) Link's whole plant ethanol extract was used to isolate the phytoconstituent, which was then thoroughly characterised using IR, H1 NMR, and mass spectrometry. The isolated compound's spectrum data revealed that EELA I had structural characteristics with apigenin. By using the MTT assay technique, the ethanol extract of Leucas aspera (Willd.) Link was tested for in vitro anticancer activities in three distinct cell lines, including HeLa, Hep G2, and MCF-7. In comparison to the other two cell lines, HepG2 generated much greater anticancer activity in-vitro. The results of the current investigation suggested that the anticancer properties of the extract's flavonoids and saponins may be related to their antioxidant capability. Based on the results of the investigations, which provide further scientific support for future research, we explore the lead chemicals found in the plant, assess its anticancer potential in in-vivo animal models, and propose a plan to conduct human trials.
ACKNOWLEDGEMENT: Nil
CONFLICT OF INTEREST: Nil
REFERENCES:
- Islam AM, Ohno O, Suenaga K & Kato-Noguchi H: Two novel phytotoxicsubstances from Leucas aspera. Journal of Plant Physiology 2014; 171(11): 877-883.
- Enjamoori VK, Nampalli A, Vasudha B, Gangarapu K & Boggula N: A review on Leucas aspera for phytopharmacological studies. INNOSC Theranostics and Pharmacological Sciences 2019; 2(1).
- Latha B, Rumaisa Y, Soumya CK, Shafeena S & Sadhiya N: Phytochemical studies on Leucas aspera. J of Chemical and Pharmaceutical Research 2013; 5(4): 222-228.
- Priya R, Nirmala M, Shankar T & Malarvizhi A: Phytochemical compounds of Leucas aspera L. Pharmacological Benefits of Natural Products 2018; 1: 19-35.
- Rai V, Agarwal M, Agnihotri AK, Khatoon S, Rawat AKS & Mehrotra S: Pharmacognostical Evaluation of Leucas aspera Link. Natural Product Sciences 2005; 11(2): 109-114.
- Kannappa Reddy M, Viswanathan S, Thirugnanasambantham P & Kameswaran L: Analgesic activity of Leucas aspera. Fitoterapia-Milano 1993; 64: 151-151.
- Das BK, Das B, Arpita FK, Morshed MA, Uddin A, Bhattacherjee R & Hannan JMA: Phytochemical screening and antioxidant activity of Leucas aspera. International Journal of Pharmaceutical Sciences and Research 2011; 2(7): 1746.
- Revathi P & Parimelazhagan T: Traditional knowledge on medicinal plants used by the Irula tribe of Hasanur Hills, Erode District, Tamil Nadu, India. Ethnobotanical Leaflets 2010; (2): 4.
- Alagesaboopathi C: Ethnomedicinal plants and their utilization by villagers in Kumaragiri hills of Salem district of Tamil Nadu, India. African Journal of traditional, complementary and alternative Medicines 2009; 6(3).
- Sairam TV: Home remedies: A handbook of herbal cures for common ailments (Vol. 2). Penguin Books India 1998.
- Jain S, Gupta A & Shukla K: In-vitro evaluation of Antibacterial and Antioxidant activity of ethanolic extract of Leucas aspera roots and leaves. International Journal of Pharmacy & Life Sciences 2020; 11(6).
- Chakraborty R, Pal D & Roy S: Characterization of Leucas aspera and evaluation of antioxidant activities before and after being subjected to digestion enzymes. International Journal of Vegetable Science 2020; 26(3): 302-320.
- Burranboina KK, Kumar KM, Reddy GM, Yogisharadhya R, Prashantha CN & Dhulappa A: GC-MS analysis, molecular Docking and pharmacokinetic studies of various bioactive compounds from methanolic leaf extracts of Leucas aspera (L) against anti-Capripox viral activity. Chemical Data Collections 2022; 39: 100873.
- Jangir M, Sharma S & Sharma S: Development of next-generation formulation against Fusarium oxysporum and unraveling bioactive antifungal metabolites of biocontrol agents. Scientific Reports 2021; 11(1): 22895.
- Sałdan A, Król M, Śmigiel-Kamińska D, Woźniakiewicz M & Kościelniak P: Development of the microemulsion electrokinetic capillary chromatography method for the analysis of disperse dyes extracted from polyester fibers. Molecules 2022; 27(20): 6974.
- Queiroz EF, Alfattani A, Afzan A, Marcourt L, Guillarme D & Wolfender JL: Utility of dry load injection for an efficient natural products isolation at the semi-preparative chromatographic scale. Journal of Chromatography A 2019; 1598: 85-91.
- Srivastava N, Singh A, Kumari P, Nishad JH, Gautam VS, Yadav M & Kharwar RN: Advances in extraction technologies: Isolation and purification of bioactive compounds from biological materials. In Natural bioactive compounds 2021; 409-433.
- Ahmed W, Azmat R, Mehmood A, Qayyum A, Ahmed R., Khan SU & Ahmad S: The analysis of new higher operative bioactive compounds and chemical functional group from herbal plants through UF-HPLC-DAD and Fourier transform infrared spectroscopy methods and their biological activity with antioxidant potential process as future green chemical assay. Arabian Journal of Chemistry 2021; 14(2): 102935.
- Dieterle F, Ross A, Schlotterbeck G & Senn H: Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Analytical Chemistry 2006; 78(13): 4281-4290.
- Patel RS, Roy M & Dutta GK: Mass spectrometry-A review. Veterinary World 2012; 5(3).
- Hosseinian H, Ortiz Ortega E, Rosales López MJ, Rodríguez Vera A & Hosseini S: Characterization Techniques for Mass Spectrometry Analysis. Material Characterization Techniques and Application 2022; 47-69.
- Ng WK, Saiful Yazan L, Yap LH, Wan NorHafiza WAG, How CW & Abdullah R: Thymoquinone-loaded nanostructured lipid carrier exhibited cytotoxicity towards breast cancer cell lines (MDA-MB-231 and MCF-7) and cervical cancer cell lines (HeLa and SiHa). Bio Med Research international 2015.
- Arung ET, Ramadhan R, Khairunnisa B, Amen Y, Matsumoto M, Nagata M & Shimizu K: Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi Journal of Biological Sciences 2021; 28(12): 7182-7189.
- Rafehi H, Orlowski C, Georgiadis GT, Ververis K, El-Osta A & Karagiannis TC: Clonogenic assay: adherent cells. JoVE (Journal of Visualized Experiments) 2011; (49): 2573.
- Sigaeva A, Morita A, Hemelaar SR & Schirhagl R: Nanodiamond uptake in colon cancer cells: The influence of direction and trypsin-EDTA treatment. Nanoscale 2019; 11(37): 17357-17367.
- Wanigasekara J, Barcia C, Cullen PJ, Tiwari B & Curtin JF: Plasma induced reactive oxygen species‐dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Processes and Polymers 2022; 19(4): 2100157.
- Benov L: Improved formazan dissolution for bacterial MTT assay. Microbiology Spectrum 2021; 9(3): 01637-21.
- Kamiloglu S, Sari G, Ozdal T & Capanoglu E: Guidelines for cell viability assays. Food Frontiers 2020 1(3): 332-349.
- Vivaudou M: eeFit: a Microsoft Excel-embedded program for interactive analysis and fitting of experimental dose–response data. Biotechniques 2019; 66(4): 186-193.
- Cao W, Chen HD, Yu YW, Li N & Chen WQ: Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chinese Medical Journal 2021; 134(07): 783-791.
- Malhotra L, Kaur P & Ethayathulla AS: Flavonoids as potential reactivators of structural mutation p53Y220C by computational and cell-based studies. Journal of Biomolecular Structure and Dynamics 2023; 1-12.
- Karpiński T, Adamczak A & Ożarowski M: Antibacterial activity of apigenin, luteolin, and their C-glucosides. In Proceedings of the 5th International Electronic Conference on Medicinal Chemistry 2019.
- Ghiƫu A, Pavel IZ, Avram S, Kis B, Minda D, Dehelean CA & Danciu C: An in-vitro in-vivo evaluation of the antiproliferative and antiangiogenic effect of flavone apigenin against SK-MEL-24 human melanoma cell line. Analytical Cellular Pathology 2021.
- Jang JY, Sung B & Kim ND: Role of induced programmed cell death in the chemopreventive potential of apigenin. International J of Molecular Sciences 2022; 23(7): 3757.
- Kim JK & Park SU: Recent insights into the biological functions of apigenin. EXCLI Journal 2020; 19: 984-991.
- Yan X, Qi M, Li P, Zhan Y & Shao H: Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell & Bioscience 2017; 7(1): 1-16.
- Imran M, Aslam Gondal T, Atif M, Shahbaz M, Batool Qaisarani T, Hanif Mughal M & Sharifi‐Rad J: Apigenin as an anticancer agent. Phytotherapy Research 2020; 34(8): 1812-1828.
- Bhattacharya S, Parihar VK & Prajapati BG: Unveiling the therapeutic potential of cabozantinib-loaded poly D, L-lactic-co-glycolic acid and polysarcosine nanoparticles in inducing apoptosis and cytotoxicity in human HepG2 hepatocellular carcinoma cell lines and in-vivo anti-tumor activity in SCID female mice. Frontiers in Oncology 2023’ 13: 1125857.
How to cite this article:
Indira S and Mahalekshmi V: In-vitro anti-cancer effects of ethanolic extract, Leucas aspera (Willd.) link whole plant against HELA, HEP G2 and MCF-7 cell lines. Int J Pharmacognosy 2023; 10(12): 562-70. doi link: http://dx.doi.org/10.13040/IJPSR.0975-8232.IJP.10(12).562-70.
This Journal licensed under a Creative Commons Attribution-Non-commercial-Share Alike 3.0 Unported License.
Article Information
1
562-570
812 KB
667
English
IJP
S. Indira and V. Mahalakshmi *
Department of Pharmaceutics, S. A. Raja Pharmacy College, Tirunelveli, Tamil Nadu, India.
mahakesavan2005@gmail.com
10 October 2023
19 December 2023
27 December 2023
10.13040/IJPSR.0975-8232.IJP.10(12).562-70
31 December 2023